Abstract
In an attempt to characterize an unusual mycobacterial isolate from a 44-year-old patient living in France, we applied phenotypic characterizations and various previously described molecular methods for the taxonomic classification of mycobacteria. The results of the investigations were compared to those obtained in a previous study with a set of temporally and geographically diverse Mycobacterium ulcerans (n = 29) and Mycobacterium marinum (n = 29) isolates (K. Chemlal, G. Huys, P.-A. Fonteyne, V. Vincent, A. G. Lopez, L. Rigouts, J. Swings, W. M. Meyers, and F. Portaels, J. Clin. Microbiol. 39:3272-3278, 2001). The isolate, designated ITM 00-1026 (IPP 2000-372), is closely related to M. marinum according to its phenotypic properties, lipid pattern, and partial 16S rRNA sequence. Moreover, fingerprinting by amplified fragment length polymorphism (AFLP) analysis unequivocally classified this strain as a member of the species M. marinum, although it lacked two species-specific AFLP marker bands. However, PCR and restriction fragment length polymorphism analysis based on M. ulcerans-specific insertion sequence IS2404 showed the presence of this element in a low copy number in isolate ITM 00-1026. In conclusion, the designation of this isolate as a transitional species further supports the recent claim by Stinear et al. (T. Stinear, G. Jenkin, P. D. Johnson, and J. K. Davies, J. Bacteriol. 182:6322-6330, 2000) that M. ulcerans represents a relatively recent phylogenetic derivative of M. marinum resulting from the systematic acquisition of foreign DNA fragments.
Mycobacterium marinum disease and Buruli ulcer, caused by Mycobacterium ulcerans, are mycobacterial diseases with pathophysiologic similarities (both cause necrotizing skin lesions) and common (aquatic) reservoirs of infection (13, 14). M. marinum was first described in marine fish in an aquarium in Philadelphia, Pa., in 1926 and has been known as the cause of fish tank or swimming pool granulomas (1). The organism is an intracellular pathogen that causes small ulcers or nodules in humans, most commonly on the extremities (13, 28). The infection can usually be treated with antimycobacterial drugs. M. marinum can readily be identified by conventional mycobacterial characterization methods. It is a relatively rapid grower and is easily recognized by its photochromogenicity and its optimal growth at 33°C (27). M. ulcerans is an extracellular human pathogen that causes chronic necrotic skin lesions (18). In only a very few cases do the lesions respond favorably to antimicrobial therapy, making wide surgical excision and skin grafting the treatment of choice. M. ulcerans was first described in Bairnsdale, Australia, in 1948 (29) and was subsequently reported in numerous, mostly tropical, countries (15, 16, 22, 34, 36). The prevalence of this organism throughout West Africa has increased dramatically since the late 1980s (23, 30). This increased prevalence might be related to environmental and socioeconomic factors (36). M. ulcerans is sometimes difficult to isolate and, due to its long generation time, may require 6 to 8 weeks to produce visible growth in primary culture (35, 36, 51).
The marked differences in phenotypic properties and disease symptoms exhibited by M. marinum and M. ulcerans contrast strikingly with the high degree of genetic similarity between the two species. Lipid analysis shows that mycolates and species- or type species-specific phenolic glycolipids have identical compositions and structures (5, 7, 9). Sequence analysis of the hypervariable regions of the 16S rRNA gene reveals that the two taxa have identical signature sequences (24) and that within this locus the only sequence differences between the two taxa are at two nucleotides at the 3′ end of the gene (32). The nucleotide at one of these positions (positions 1450 to 1452) is different from that in M. marinum in only a limited number of M. ulcerans isolates (32). The pattern developed from these findings was confirmed by various DNA-based techniques, which demonstrated a strong taxonomic relationship between these two organisms (11, 24, 25, 33, 39). However, further analysis of the genomes of these two closely related species has revealed differences (4, 46, 49). Next to the separation of representatives of both species into two clusters by the amplified fragment length polymorphism (AFLP) analysis technique (4, 42), the finding that most clearly discriminates the two species is the presence of insertion sequence IS2404 in M. ulcerans in high copy numbers but a total absence of IS2404 in all M. marinum isolates from different geographic regions investigated (4, 46). Hence, this insertion sequence is believed to be highly characteristic for the species definition of M. ulcerans.
In one of our previous investigations we concluded that “the key to confirming the hypothesized recent divergence of M. ulcerans from M. marinum would be finding a ‘missing link’ between the two, e.g., a M. marinum with low IS 2404 copy number…indicating an evolving characteristic within the taxon” (4). In the present study, we determined the phenotypic and genotypic profiles of an isolate, designated ITM 00-1026 (IPP 2000-372), from a French patient. The isolate was previously identified by conventional tests as M. marinum. In an attempt to establish its taxonomic position by comparison with an extended collection of well-documented M. marinum and M. ulcerans strains, our findings led us to conclude that isolate ITM 00-1026 represents a strain intermediate between these two species.
MATERIALS AND METHODS
Strains used.
The 58 strains of M. ulcerans and M. marinum systematically analyzed in this study (Table 1) were from the Institute of Tropical Medicine (ITM) and Institut Pasteur de Paris (IPP) research collections and were identified as M. ulcerans or M. marinum by conventional biochemical methods (27). The geographic distributions of the strains involved in the study were diverse. All except one of the isolates were of animal and human origin; one isolate was from water. Fresh subcultures were made on Löwenstein-Jensen medium. Some strains were kindly provided by P. Lavalle (Centro Dermatologico Pascua, Mexico City, Mexico), W. R. Faber and P. H. G. van Keulen (Academic Medical Center, Amsterdam, The Netherlands), T. Tønjum (Institute of Microbiology, Oslo, Norway), P. L. Small (National Institutes of Health, Rocky Mountain, Montana), and H. F. A. K. Huchzermeyer (Veterinary Research Institute, Onderstepoort, South Africa). Two additional M. ulcerans isolates, one from an Australian patient (ITM 5142) and one from an aquatic bug from Benin (ITM 00-1441) (F. Portaels et al., unpublished data), were examined for their lipid contents.
TABLE 1.
Sources and origins of mycobacterial strains
| Species | Strain no. | Source | Provider (other strain designation)a | Geographical origin |
|---|---|---|---|---|
| M. ulcerans (n = 29) | ITM 7922 | Human | V.V. (IPT 141090018) | French Guiana |
| ITM 842 | Human | V.K. 701357 | Suriname | |
| ITM 8756 | Human | ATCC 33728 | Japan | |
| ITM 5147 | Human | ATCC 19423T | Australia | |
| ITM 5114 | Human | P.L. | Mexico | |
| ITM 94-1330 | Human | J.S. (143150) | Australia | |
| ITM 94-1325 | Human | J.S. (187859) | Australia | |
| ITM 94-1324 | Human | J.S. (176862) | Australia | |
| ITM 94-1326 | Human | J.S. (93160339) | Australia | |
| ITM 5122 | Human | F.P. | Democratic Republic of Congo | |
| ITM 94-662 | Human | F.P. | Ivory Coast | |
| ITM 94-339 | Human | F.P. | Australia | |
| ITM 94-1327 | Human | F.P. | Australia | |
| ITM 94-1329 | Human | F.P. | Australia | |
| ITM 94-886 | Human | F.P. | Benin | |
| ITM 97-111 | Human | F.P. | Benin | |
| ITM 97-104 | Human | F.P. | Benin | |
| ITM 9146 | Human | F.P. | Benin | |
| ITM 94-815 | Human | F.P. | Ivory Coast | |
| ITM 97-684 | Human | F.P. | Benin | |
| ITM 97-490 | Human | F.P. | Benin | |
| ITM 96-658 | Human | F.P. | Angola | |
| ITM 97-680 | Human | F.P. | Angola | |
| ITM 95-1112 | Human | F.P. | Australia | |
| ITM 9114 | Human | F.P. | Benin | |
| ITM 9550 | Human | D.D. (17679) | Australia | |
| ITM 9540 | Human | D.D. (11098) | Australia | |
| ITM 9537 | Human | D.D. (11878) | Papua New Guinea | |
| ITM 8849 | Human | D.D. (8471/69) | Australia | |
| M. marinum (n = 29) | ITM 94-996 | Fish | K.H. | South Africa |
| ITM 94-979 | Fish | K.H. | South Africa | |
| US H35392/93 | Human | P.S. | United States | |
| US M6 | Fish | P.S. | United States | |
| US LS | Fish | P.S. | United States | |
| ITM 7732 | Fish | ATCC (ATCC 927T) | United States | |
| ITM 00-1026 | Human | V.V. (IPP 2000372) | France | |
| IPP 99000876 | Human | V.V. | France | |
| IPP 99/890 | Human | V.V. | France | |
| IPP 2000449 | Human | V.V. | France | |
| IPP 99000843 | Human | V.V. | France | |
| IPP 2000355 | Human | V.V. | France | |
| IPP 031038 | Human | V.V. | France | |
| IPP 99/363 | Human | V.V. | France | |
| IPP 99000821 | Human | V.V. | France | |
| IPP CCUG533 | Human | V.V. | France | |
| ITM 8022 | Human | F.P. | Belgium | |
| ITM 94-56 | Human | F.P. | Belgium | |
| ITM 97-1321 | Axolotl | F.P. | Belgium | |
| ITM 98-852 | Human | F.P. | Italy | |
| ITM 00-533 | Human | F.P. | Belgium | |
| ITM 99-822 | Human | F.P. | Belgium | |
| ITM 99-2570 | Human | F.P. | Belgium | |
| ITM 99-3021 | Human | F.P. | Belgium | |
| ITM 1717 | Armadillo | F.P. | United States | |
| ITM 1726 | Armadillo | F.P. | United States | |
| ITM 97-1320 | Axololt | F.P. | Belgium | |
| TON T25/84 | Water | T.T. | Norway | |
| TON F106/91 | Human | T.T. | Norway |
Abbreviations: V.V., V. Vincent, IPP, Paris, France; F.P., F. Portaels, ITM, Antwerp., Belgium; P.L., P. Lavalle, Centro Dermatologico Pascua, Mexico City, Mexico; P.L., P. L. Small, National Institutes of Health; Rocky Mountain, Mont.; T. T., T. Tonjum, Institute of Microbiology, Oslo, Norway; V.K., P. H. J. van Keulen; Academic Medical Center, Amsterdam, The Netherlands; D.D., D. Dawson, Laboratory of Microbiology and Pathology, Queensland Health, Brisbane, Australia; J. S., J. Stanford, School of Pathology, London, United Kingdom, K. H., H. F. A. K. Huchzermeyer, Veterinary Research Institute, Onderstepoort, South Africa; ATCC, American Type Culture Collection.
Clinical history of the patient.
In January 1999, a 44-year-old French woman presented with a 2-month history of acute joint pain and swelling in a finger of the left hand. Incipient arthropathy was diagnosed. Injections of anti-inflammatory drugs initially reduced the pain, but shortly afterwards the pain and swelling increased, suggesting the formation of an abscess. Four months later, in May 1999, the abscess was drained surgically, and it was found that the exudate contained whitish granulations. In October 1999, the swelling and deformation of the finger increased. During this period, the patient disclosed that she had injured the finger while attending to her aquarium at home in France, raising the question of an atypical mycobacterial infection. The lesion was again drained in November 1999, and smears of the exudate revealed acid-alcohol-resistant bacilli. Clinically, the lesion resembled one that would be caused by M. marinum infection. Isoniazid, rifampin, and ethambutol treatment was started empirically while awaiting the results of bacteriologic tests. The PCR test (AMPLICOR; Roche) for tubercle bacilli was negative. Treatment with the three antibiotics was maintained for 3 months. In April 2000, the mycobacterial isolate from the patient, designated IPP 2000-372 (ITM 00-1026), was identified at the Institut Pasteur, Paris, France, as M. marinum.
Phenotypic properties of isolate ITM 00-1026.
The cultured organism was acid fast by the Ziehl-Neelsen technique. The bacterium was further characterized by its rate of growth, colonial morphology, pigmentation, and biochemical properties. The bacterium was tested for salt tolerance on Löwenstein-Jensen medium supplemented with 5% NaCl, Tween 80 hydrolysis, urease activity, semiquantitative catalase activity, nitrate and phosphate reduction, and niacin accumulation. All these tests were conducted by standard methods (27).
Lipid analysis.
Wet cells were extracted with organic solvents as described previously (9). The resulting lipid extracts and bacterial residues were saponified with a mixture of 5% KOH in methoxymethanol at 100°C in a screw-cap tube (7). To distinguish the multimethyl-branched fatty esters from other esters, e.g., phospholipids, acylglycerols, and mycolate derivatives, a saponification time of 3 h was used. Under these conditions, the former compounds are only partially hydrolyzed, whereas the latter substances are completely saponified (8). After acidification, the fatty acids were extracted with diethyl ether and methylated with an ethereal solution of diazomethane (7). The mycolate patterns of the strains were determined by analytical thin-layer chromatography on Silica Gel 60 (Macherey-Nagel) with petroleum ether-diethyl ether (9:1 [vol/vol]; five runs). Lipid spots were revealed by spraying the plates with molybdophosphoric acid (10% [wt/vol] in ethanol), followed by charring. To detect the multimethyl-branched fatty acyl-containing glycolipids, the lipid extracts were analyzed by thin-layer chromatography developed with chloroform-methanol (95:5 vol/vol) and visualized with an anthrone spray (0.2% [wt/vol] in sulfuric acid), followed by heating.
Lipids were structurally analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (in the positive mode) as described previously (26). Mass spectra were acquired on a Voyager-DE STR mass spectrometer (PerSeptive Biosystems, Framingham, Mass.) equipped with a pulsed nitrogen laser with emission at 337 nm. The samples were analyzed in the Reflectron mode by using an extraction delay time set at 100 ns and an accelerating voltage of 20 kV, with operation in the positive ion mode. Samples (1 mM in chloroform) were directly applied to the sample plate as 1-μl droplets, followed by the addition of 0.5 μl of matrix solution (2,5-dihydroxybenzoic acid [10 mg/ml] in CHCl3-CH3OH [1:1; vol/vol]), and the samples were allowed to crystallize at room temperature.
Gas chromatography (GC) of fatty methyl esters was performed on a Hewlett-Packard 5890 series II apparatus equipped with an OV1 capillary column (0.30 mm by 25 m) with helium gas. The temperature program consisted of an increase from 100 to 300°C at a rate of 5°C/min, followed by 10 min at 300°C.
Partial 16S rRNA gene analysis.
Sequencing of the 3′ end of the 16S rRNA gene (474 bp) was performed by EUROGENTEC Laboratories (Liège, Belgium) with an automated nucleic acid sequencer (Applied Biosystems, Foster City, Calif.).
Phylogenetic data analysis.
To determine the approximate phylogenetic relationship of isolate ITM 00-1026, the sequence of the isolate was initially compared to the sequences in the available databases by using the BLAST program. The sequence of the isolate was aligned with the sequences of the 3′ ends of the 16S rRNA genes of Mycobacterium tuberculosis (GenBank accession no. Z83862), M. marinum (GenBank accession no. X52920), and M. ulcerans (GenBank accession nos. X58954, X88926, and Z13990). A phylogenetic tree was constructed by the neighbor-joining method. The relative confidence of each phylogenetic analysis was estimated by bootstrap analysis, which included 1,000 replicates.
IS2404-specific PCR.
For all isolates, lysates were obtained by resuspending a loopful of bacterial cells in 100 μl of TE (10 mM Tris, 1 mM EDTA [pH 8.0]) containing 1% Triton X-100 (vol/vol) and heating at 100°C for 15 min. Ten microliters of lysate was added to 50 μl of a PCR mixture, which contained 50 pmol of each primer (primer PGP3 [5′-GGCGCAGATCAACTTCGCGGT-3′] and primer PGP4 [5′-CTGCGTGGTGCTTTACGCGC-3′]), 1 U of Ampli Taq DNA polymerase (Roche Molecular Systems, Brussels, Belgium), each deoxyribonucleotide triphosphate at a concentration of 200 μM, 1.5 mM MgCl2, 0.1% Triton X-100, and 10 mM Tris-HCl (pH 8.4). The mixture was then overlaid with mineral oil. Primers PGP3 and PGP4 target a 219-bp fragment of insertion sequence IS2404, specific for M. ulcerans. Cycling was performed as follows: denaturation at 94°C for 5 min and amplification for 30 cycles at 94°C for 45 s, 64°C for 45 s, and 72°C for 45 s, with a final extension at 72°C for 7 min. Subsequently, 7 μl of amplified DNA was electrophoresed through a 2% agarose gel, and bands were detected by ethidium bromide staining and UV transillumination.
PCR restriction profile analysis.
PCR restriction profile analysis was performed as described previously (4). Briefly, the lysates from all isolates were obtained by resuspending a loopful of bacterial cells in 100 μl of TE (10 mM Tris, 1 mM EDTA [pH 8]) containing 1% (vol/vol) Triton X-100 and heating at 100°C for 15 min. The PCR is performed with primers P11 and P61 and the same cycling conditions described previously (4). Restriction analysis of the amplification product was carried out for 2 h at 37°C in 20 μl of incubation buffer containing 15 U of a restriction enzyme (RsaI, DraI, and EcoNI) and 8 μl of the PCR product. The restriction fragment patterns were analyzed by gel electrophoresis of the restriction enzyme mixture at 50 V for 1.5 h in a 3% small-fragment agarose gel (Eurogentec).
Southern blotting and preparation of IS2404 probe.
The IS2404 probe was prepared by chemical labeling of a 219-bp PCR product as described in the protocol of van Embden and coworkers (52) for the preparation of the IS6110 probe. Primers PGP3 and PGP4 were used as described previously (3).
For Southern blot analysis, M. ulcerans genomic DNA was digested with a restriction enzyme (PvuII) and was allowed to separate overnight by electrophoresis on a 0.8% agarose gel (52). The DNA was transferred to a Hybond N+ nylon membrane (Amersham Biosciences, Roosendaal, The Netherlands) for 1 h in 0.4 M NaOH with a vacuum blotter system (Appligene-oncor, Illkirch, France). Hybridizations were performed at 42°C with high-stringency posthybridization washes (52). Detection of DNA was achieved with an enhanced chemiluminescence direct system according to the protocol of the manufacturer (Amersham Biosciences).
AFLP analysis.
The DNA was isolated and purified as described before (52). All protocols relating to the preparation of DNA templates for AFLP analysis were performed essentially as described previously (21). AFLP templates were prepared from ∼1 μg of high-molecular-weight genomic DNA through double enzymatic digestion with the endonucleases ApaI and TaqI, followed by restriction half-site-specific ligation of double-stranded oligonucleotide adapters and selective precipitation by the method of Janssen et al. (21). The adapters were prepared by mixing equimolar amounts of partially complementary oligonucleotides 5′-TCGTAGACTGCGTTACAGGCC-3′ and 5′-TGTACGCAGTCTAC-3′ (for ApaI) and partially complementary oligonucleotides 5′-GACGATGAGTCCTGAC-3′ and 5′-CGGTCAGGACTCAT-3′ (for TaqI). For fingerprinting by AFLP analysis, ApaI-TaqI restriction fragments tagged with specific adapters were used as the template DNA for selective PCR amplification directed by primers A02 and T02. The oligonucleotide sequences, amplification procedures, electrophoresis conditions, and data capture and analysis have been described elsewhere (20).
RESULTS
Biochemical characterization.
The phenotypic characteristics of the isolate designated ITM 00-1026 (IPP 2000-372) are summarized in Table 2. Visible growth was observed after 1 to 2 weeks at an optimum temperature of 33°C on Löwenstein-Jensen medium, with no growth observed at 37°C. The isolate was photochromogenic. The biochemical reactions of the isolate were compared with the known characteristics of M. ulcerans and M. marinum included in the study. The isolate was positive for niacin production and did not grow on hydroxylamine at 250 μg/ml, p-nitrobenzoate at 500 μg/ml, or 5% sodium chloride. The strain was negative for Tween 80 hydrolysis, acid phosphatase activity, nitrate reduction, and urease activity. A foam column >45 mm was not produced by the semiquantitative catalase test. The organism grew on thiophene-2-carboxylic acid hydrazide at 2 μg/ml.
TABLE 2.
Phenotypic characteristics of the different geographic subgroups of M. ulcerans, M. marinum, and ITM 00-1026
| Characteristic | Result for the following M. ulcerans subgroup or organisma
|
|||||||
|---|---|---|---|---|---|---|---|---|
| AF | AUS | MEXb | S. AM. | CHINAb | JAPANb | ITM 00-1026 | M. marinum | |
| Pigmentation in dark | +c | − | − | + | − | + | − | − |
| Pigmentation in light | +c | − | − | + | − | + | + | + |
| Growth at 37°C | − | − | − | − | − | − | − | − |
| Growth on peptone agar | − | − | − | − | − | − | − | − |
| Growth in presence of: | ||||||||
| Isoniazid (10 μg/ml) | + | M | + | − | − | − | − | M |
| Thiophene-2-carboxylic hydrazide (2 μg/ml) | + | + | + | + | + | − | + | + |
| Hydroxylamine (250 μg/ml) | − | + | + | M | + | + | − | + |
| p-Nitrobenzoate (500 μg/ml) | − | − | + | − | P | − | − | M |
| NaCl (5%) | − | − | − | − | − | − | − | − |
| Enzymatic properties | ||||||||
| Catalase activity >45 mm of foam | − | − | − | − | − | + | − | − |
| Tween 80 hydrolysis (10 days) | − | − | − | − | − | − | − | + |
| Urease activity | − | − | − | − | − | − | − | + |
| Niacin production | − | − | − | − | − | − | + | − |
| Nitrate reduction | − | − | − | − | − | − | − | − |
| Acid phosphatase activity | M | − | − | − | − | − | − | + |
| Colonial morphology | R | R | R | R | R | R | SmK | SmK-R |
Abbreviations and symbols: AF, African subgroup; AUS, Australian subgroup; S. AM, South American subgroup; CHINA, Chinese subgroup; JAPAN, Japanese subgroup (M. shinshuense); R, Rough; SmK, smooth M. kansasii (31); +, >85% of the strains were positive; − <15% of the strains were positive; P, partial; M, 50 to 85% of strains were positive.
Results are for only one strain.
Light yellow pigment.
Genotypic analysis.
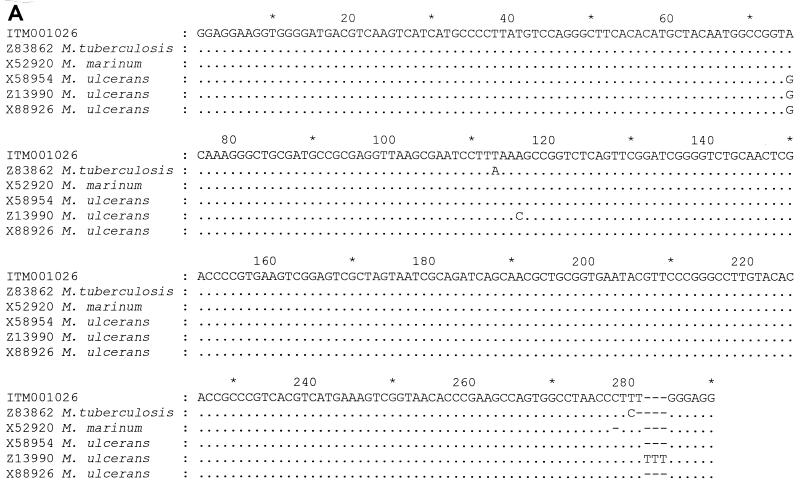
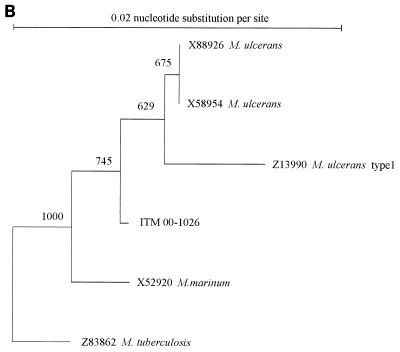
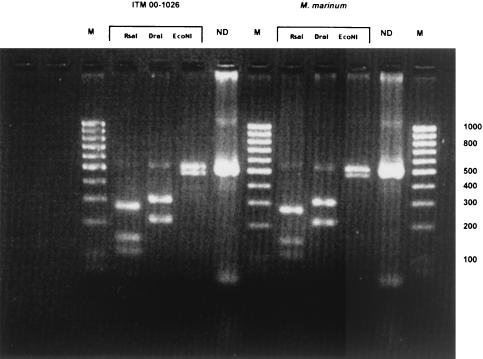
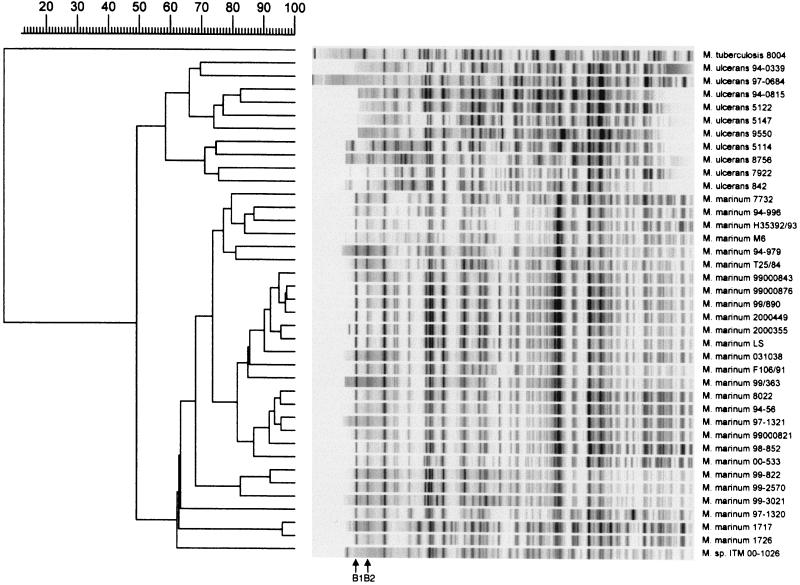
Analysis of the 3′ end of the 16S rRNA sequence revealed a sequence highly similar (99%) to that of M. marinum (Fig. 1a). The phylogram placed this isolate close to M. marinum and M. ulcerans (Fig. 1b). This was confirmed by PCR restriction profile analysis, by which the banding pattern for isolate ITM 00-1026 was the same as that for M. marinum (Fig. 2). Restriction fragment length polymorphism (RFLP) analysis with IS2404 showed that ITM 00-1026 had a low number of copies of this repetitive element (Fig. 3a). A PCR targeting the same insertion sequence was positive (Fig. 3b). In addition, a fingerprint was obtained by AFLP analysis with the combination of primers A02 and T02 (20); following numerical analysis by use of the Pearson product-moment correlation coefficient, the strain was assigned to the AFLP cluster representing M. marinum (Fig. 4). Strikingly, two bands (bands B1 and B2) appearing in the fingerprints of all other M. marinum strains were not present in the AFLP band pattern of isolate ITM 00-1026.
FIG. 1.
(A) Alignment of the 3′ end of the 16S rRNA sequence of ITM 00-1026 with selected closely related mycobacterial 16S rRNA. The numbering of the region in the 16S rRNA corresponds to that of the M. ulcerans sequence (base pairs 1110 to 1522) (GenBank reference sequence). Only nucleotides that differ from the reference sequence are shown. Dashes indicate deletions or absent nucleotides. (B) Phylogenetic tree based on the alignments of the partial 16S rRNA gene sequences illustrating the position of isolate ITM 00-1026 in relation to those of other closely related mycobacteria. The tree was constructed by using the neighbor-joining method. Bootstrapping (1,000 replicates) was used to assess support for particular nodes in the tree, and the values are shown above the nodes.
FIG. 2.
PCR restriction profiles obtained for ITM 00-1026 and M. marinum with three restriction enzymes (RsaI, DraI, and EcoNI). Lanes M, 100-bp DNA ladder; lanes ND, no digested PCR product. The numbers on the right of the gel are molecular sizes (in base pairs).
FIG. 3.
(A) Representative Southern blot obtained with nine M. ulcerans isolates (lanes 1 to 9, respectively) from different geographic areas and isolate ITM 00-1026. Lane 1, reference strain ATCC 19423; lane 2, an isolate from the Democratic Republic of Congo; lane 3, an isolate from Papua New Guinea; lane 4, an isolate from China; lane 5, an isolate from Japan; lane 6, an isolate from Suriname; lane 7, an isolate from French Guiana; lane 8, an isolate from Mexico; lane 9, an isolate from Benin; lane 10, isolate ITM 00-1026. Note the low copy number of IS 2404 bands for isolte ITM 00-1026 compared to the numbers for the M. ulcerans isolates. Molecular sizes (in kilobases) are shown on the left. (B) Image of the amplification product (219 bp) from the culture of ITM 00-1026 obtained by PCR with primers specific for IS2404. Lane 1, ITM 00-1026; lane 2, negative control; lane 3, positive control (M. ulcerans); lanes M, molecular size marker (100-bp DNA ladder).
FIG. 4.
Numerical analysis of normalized AFLP bands pattern generated from M. ulcerans (n = 10) and M. marinum (n = 27) strains and from isolate ITM 00-1026 with the A02-T02 primer pair. The dendrogram was constructed by the unweighted pair group method with arithmetic averages, with correlation levels expressed as percentages of the Pearson product-moment correlation coefficient. B1 and B2, two AFLP marker bands specific to all M. marinum strains evaluated.
Lipid analysis.
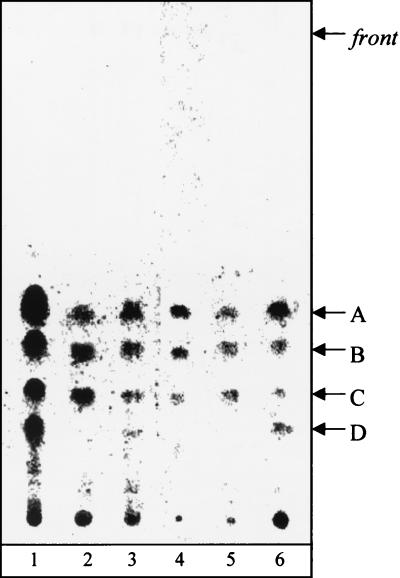
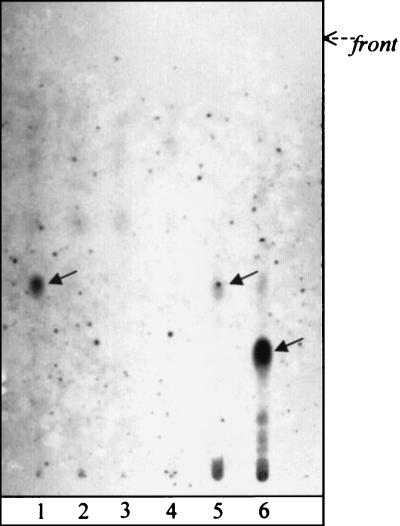
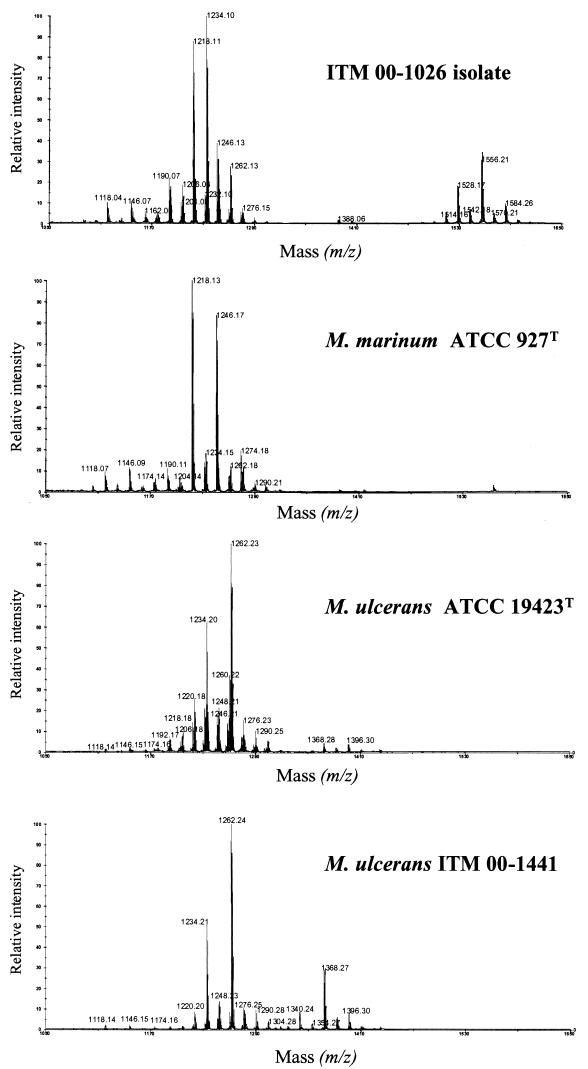
To further characterize isolate ITM 00-1026, the lipid pattern of the strain was compared to those of related mycobacterial species. When the mycolate profile of isolate ITM 00-1026 was determined, it appeared that the strain shared a pattern similar to those of several slow growers (e.g., M. tuberculosis-M. ulcerans and M. marinum) and had three types of mycolates, i.e., α-, methoxy-, and keto-mycolates (compounds A, B, and C, respectively, Fig. 5). GC analysis showed that isolate ITM 00-1026 was more related to M. ulcerans and M. marinum than to M. tuberculosis because the pyrolytic cleavage products of mycolates were identified as tetracosanoate (7). In addition, while a 2,4,6-trimethyltetracosanoate appeared to be a major constituent of the fatty methyl esters from the M. ulcerans strains, only a small amount of this compound was observed in the GC profiles of isolate ITM 00-1026 and the M. marinum strains tested (Table 3). The origin of this fatty acid was proved to be a phenolic glycolipid, formerly known as mycoside G (6, 9), which characterizes most strains of M. marinum and a few strains of M. ulcerans (Fig. 6). These data were confirmed by analysis of the matrix-assisted laser desorption ionization mass spectrum of the saponification products of isolate ITM 00-1026 (Fig. 7), which showed a series of pseudomolecular peak (M + Na) ions at m/z 1528, 1556, and 1584; these correspond to mycoside G. This mass spectrum also showed a difference between the profiles of peaks corresponding to the three types of mycolates from M. marinum, M. ulcerans, and isolate ITM 00-1026. While the major peaks observed in the mass spectra of the M. ulcerans strains corresponded to methoxymycolates at m/z 1234 and 1262 (26), the mass spectra of M. marinum (Fig. 7) showed intense signals at m/z 1218 and 1246, which corresponded to ketomycolates. Interestingly, the mass spectrum of isolate ITM 00-1026 exhibited an intermediate pattern, with similar contents of ketomycolates (m/z 1218) and methoxymycolates (m/z 1234). Altogether, data from the lipid analysis indicated that the lipid profile of the isolate was more like that of M. marinum than that of M. ulcerans.
FIG. 5.
Thin-layer chromatography profiles of mycolates. Fatty acid methyl esters were from M. ulcerans ITM 5142 (lane 1), isolate ITM 00-1026 (lane 2), M. ulcerans ATCC 19423T (lane 3), M. tuberculosis H37Ra (ATCC 25177) (lane 4), M. marinum ATCC 927T (lane 5), and M. ulcerans ITM 00-1441(lane 6). A, α-mycolates; B, methoxymycolates; C, ketomycolates; D, phenolphthiodiolone diphthioceranates. The developing solvent was petroleum ether-diethyl ether (9:1 [vol/vol]; five runs). Visualization was done by spraying with molybdophosphoric acid (10% [wt/vol]) in ethanol and charring.
TABLE 3.
Lipid patterns of M. marinum, M. ulcerans, and related organisms
| Mycobacterial strain | Patterna
|
|||||
|---|---|---|---|---|---|---|
| Mycolate type
|
Phenolphthiodiolone diphthioceranatesb | Phenolic glycolipidc | Multimethyl-branched fatty estersd | |||
| α | Methoxy | Keto | ||||
| ITM 00-1026 | + | + | + | − | + | (+) |
| M. marinum ATCC 927T | + | + | + | − | − | (+) |
| M. ulcerans ITM 5142 | + | + | + | + | − | + |
| M. ulcerans ITM 00-1441 | + | + | + | + | − | + |
| M. ulcerans ATCC 19423T | + | + | + | + | − | + |
(+), minor component; +, major constituent; −, not detected.
Phenol phthiodiolone diphthioceranates (compound D in Fig. 5, lanes 1, 3, and 6) exhibit characteristic mass peaks at m/z 1340, 1368, and 1396 (Fig. 7).
Phenolic glycolipid (mycoside G; Fig. 6) exhibits characteristic pseudomolecular [M + Na]+ ions at m/z 1528, 1556, and 1584 (Fig. 7).
Multimethyl-branched fatty esters consist mainly of 2,4,6-trimethyltetracosanoate.
FIG. 6.
Thin-layer chromatography profiles of glycolipids. Lane 1, isolate ITM 00-1026; lane 2, M. ulcerans ATCC 19423T; lane 3, M. marinum ATCC 927T; lane 4, M. ulcerans 00-1441; lane 5, M. marinum CIPT 14012 0006; lane 6, M. tuberculosis Canetti. The developing solvent was CHCl3-CH3OH (95:5 [vol/vol]). Visualization was done by spraying with anthrone (0.2% [wt/vol] in H2SO4) and heating. Arrows indicate anthrone-positive spots corresponding to the phenolic glycolipids of isolate ITM 00-1026, M. marinum (mycoside G), and M. tuberculosis Canetti (PGL-Tb 1) in lanes 1, 5, and 6, respectively.
FIG. 7.
Matrix-assisted laser desorption ionization-time of flight mass spectra of lipids obtained after saponification of whole mycobacterial cells. The series of peaks between m/z 1118 and 1290 correspond to α-, methoxy-, and ketomycolates (26). In the higher masses, peaks corresponding to alkali-stable lipids were detected (phenolphthiodiolone diphthioceranate, m/z 1340, 1368, and 1396; phenol glycolipid, m/z 1528, 1556, and 1584). Samples were dissolved in chloroform (final concentration, 1 mM) and were applied to the sample plate as 1-μl droplets. 2,5-Dihydroxybenzoic acid was used as the matrix. The accelerating voltage was 20 kV in the positive mode.
The more polar lipid (Fig. 5, compound D) that was observed in the three M. ulcerans strains examined but that was absent from strains of M. marinum and isolate ITM 00-1026 was identified by mass spectrometry (Fig. 7) as phenolphthiodiolone diphthioceranates (8), which showed peaks at m/z 1368 and 1396 identical to those seen in the mass spectrum of purified compound D (data not shown). The abundance of this compound probably explains the presence of large amounts of 2,4,6-trimethytetracosanoate in the GC profiles of the fatty esters from all strains of M. ulcerans examined (Table 3).
DISCUSSION
Recent studies have highlighted an apparent paradoxical relationship between M. marinum and M. ulcerans, in that their high degree of genetic similarity would not be expected given their striking phenotypic differences and the unique diseases that they cause (5, 12, 24, 25, 39-41, 44, 48, 53). By analyzing eight housekeeping and structural genes of a set of M. marinum and M. ulcerans isolates, Stinear et al. (46) confirmed the close genetic relationship of the 16S rRNA genes from the two species, with nucleotide sequence identities ranging from 98 to 99.7%. To date, we have successfully used two whole-genome analysis techniques (RFLP analysis with IS2404 and AFLP analysis) to differentiate between these two species (4). The current investigation focused on the phenotypic and molecular characteristics of an isolate originating from a 44-year-old French patient admitted to the Centre Hospitalier Universitaire de Lille (Lille, France) with a chronic lesion of the hand clinically typical of the lesions caused by M. marinum infection. The etiologic agent was identified at the Institut Pasteur as M. marinum by conventional biochemical reactions. This preliminary identification was confirmed by analysis of the 3′ end of the 16S rRNA gene sequence and PCR restriction profile analysis (Fig. 1a). However, the detection of IS2404 by both PCR and RFLP analysis indicated that the isolate did not fit the molecular pattern of M. marinum because IS2404 is a defining sequence for M. ulcerans (Fig. 3a and b). Ongoing studies in our laboratory suggest that the copy number of this insertion sequence is related to the geographic origin and virulence of M. ulcerans isolates (K. Chemlal et al., unpublished data). African strains appear to be more virulent than other strains and exhibit high IS2404 copy numbers (more than 50 copies). Compared to the profiles of the other M. ulcerans strains tested, isolate ITM 00-1026 had the lowest number of copies of this insertion sequence (33 bands). In an attempt to understand the taxonomic affiliation of this strain better, we applied an AFLP analysis technique of demonstrated usefulness for the identification and typing of M. ulcerans and M. marinum isolates (4). The dendrogram generated by this technique classifies isolate ITM 00-1026 as an M. marinum strain which lacks two major bands, i.e., markers B1 and B2, which were detected in the AFLP fingerprints of all M. marinum strains investigated in this study (Fig. 4). Most likely, the acquisition of low numbers of copies of IS2404 has caused new DNA polymorphisms in the genome of isolate ITM 00-1026. The absence of bands B1 and B2 may simply reflect the fact that this genetic rearrangement has led to the disappearance of one or more restriction sites for the enzymes ApaI and/or TaqI (20). In turn, the AFLP profile of isolate ITM 00-1026 also comprises bands that are unique to this strain and absent in other M. marinum strains (Fig. 4). Lipid analysis also indicated that isolate ITM 00-1026 is more related to M. marinum than to M. ulcerans (Fig. 5, 6, and 7). The isolate differs from M. ulcerans by the absence of phenolphthiodiolone diphthioceranates (Table 3).
On the basis of comparisons of the phenotypic and molecular characteristics provided by partial 16S rRNA sequencing, restriction profiling, RFLP analysis with IS2404, IS2404-specific PCR, lipid analysis, and AFLP analysis, we conclude that isolate ITM 00-1026 is an intermediate form between M. marinum and M. ulcerans. This suggestion strongly supports the recent claim of Stinear et al. (46) that M. ulcerans phylogenetically evolved from M. marinum by acquiring foreign DNA from the environment.
Insertion sequences are emerging as excellent tools in studies of the genetics and pathogenesis of mycobacteria. They have been identified as markers that can be used for diagnosis and epidemiologic studies because the majority of them are species specific or are present over a narrow host range, making them useful as reliable strain-specific probes (10, 37). The transposition of IS6110 in M. tuberculosis can rapidly generate new subclones (43). In the Mycobacterium avium complex, the presence of IS900 and IS901-IS902 is a defining characteristic for M. avium subsp . paratuberculosis and M. avium subsp. silvaticum, respectively (17, 46). M. ulcerans has acquired at least two insertion sequence elements, IS2404 and IS2606 (45). Interestingly, the transposase associated with IS2404 has 31% amino acid identity with that associated with IS1629, an insertion sequence linked to genetic mobilization in strains of various Streptomyces spp. (19, 46). Notably, the transposition of an insertion sequence from a Streptomyces sp. into a mycobacterial genome has been demonstrated (2). Moreover, transformation and transposition of the genome of M. marinum have been accomplished with IS1096 (an insertion sequence isolated from Mycobacterium smegmatis) (47). Ramakrishnan et al. (38) succeeded in performing studies with M. marinum using basic genetic tools, including genetic transformation and random transposon mutagenesis. These observations suggest that M. marinum is a robust environmental species that might be subject to genetic rearrangements by the integration of mobile DNA that produces variations at the phenotypic and physiologic levels, giving rise to progenitors that have adapted to selected ecologic environments (with particular sensitivities to UV light, particular oxygen requirements, etc.).
In our case, isolate ITM 00-1026 shows a phenotypic profile exhibiting three major characteristics associated with three closely related species: M. marinum (photochromogenicity), M. ulcerans (positivity for Tween hydrolysis and acid phosphatase activity, negativity for urease activity), and M. tuberculosis (niacin positivity). By analyzing the fatty acid profile, levels of DNA-DNA hybridization, and 16S rRNA sequences, Tønjum et al. (49) have mapped the relationships of M. marinum and M. ulcerans to M. tuberculosis and have shown their close taxonomic relationship to each other compared to their relationships to the other pathogenic mycobacterial species, suggesting their usefulness as a model for studying the pathogenesis of M. tuberculosis. Isolate ITM 00-1026 is of particular interest in this regard because the genotypic and phenotypic characteristics of this isolate combine the characteristics of three important pathogenic mycobacteria. Analysis of the 3′ end of the 16S rRNA gene of this isolate showed 99% similarity to the 3′ end of the 16S rRNA gene of M. marinum, 99% similarity to the 3′ end of the 16S rRNA gene of M. ulcerans, and 98% similarity to the 3′ end of the 16S rRNA gene of M. tuberculosis (Fig. 1a). M. marinum and M. ulcerans are the mycobacteria that are the most closely related phylogenetically to the members of the M. tuberculosis complex (49). This does not imply that M. marinum is a surrogate for M. tuberculosis; rather, it suggests that these two pathogenic mycobacteria most likely share a common ancestor (49) and use similar strategies to replicate in macrophages and persist in granulomas. Perhaps the most striking similarity is that, at the histopathologic level, M. marinum infection of the human dermis is virtually indistinguishable from some forms of human cutaneous tuberculosis (50). By contrast, the histopathologic changes that occur as a result of M. ulcerans disease (Buruli ulcer) differ vastly from those that occur as a result of M. marinum infection.
In conclusion, by analyzing different molecular aspects of isolate ITM 00-1026, we speculate that this strain presents sufficient characteristics to be designated a missing link between M. marinum and M. ulcerans. This concept is compatible with previous findings demonstrating the close relationship between these two taxa. Additionally, this finding also opens new perspectives for future studies of the circulation of mobile DNA through mycobacteria and studies of the implication of this finding in redefining the ecological niche of these microorganisms and the mechanisms that promote mycobacterial speciation.
Acknowledgments
We thank D. Dawson, P. Lavalle, P. H. J. van Keulen, J. L. Stanford, P. L. C. Small, T. Tønjum, and F. A. K. Huchzermeyer for providing the M. ulcerans and M. marinum isolates. We also thank R. Kotlowski and J. C. Palomino for assistance and advice and K. Fissette for excellent technical work.
This study was partially supported by the Damien Foundation (Brussels, Belgium), the Belgian Agency for Development (Project Buruli Ulcer in Benin), and the Fund for Scientific Research of Flanders (Brussels, Belgium) (F.W.O.-Vlaanderen; contract G.0368.98). G.H. is a postdoctoral fellow of the Fund for Scientific Research of Flanders (F. W. O.-Vlaanderen).
REFERENCES
- 1.Aronson, J. D. 1926. Spontaneous tuberculosis in salt water fish. Infect. Dis. 39:315-320. [Google Scholar]
- 2.Bhatt, A., and T. Kieser. 1999. Transposition of IS117 of Streptomyces coelicolor A3(2) in Mycobacterium smegmatis. Microbiology 145:1201-1207. [DOI] [PubMed] [Google Scholar]
- 3.Chemlal, K., K. De Ridder, P. A. Fonteyne. W. M. Meyers, J. Swings, and F. Portaels. 2001. The use of IS2404 restriction fragment length polymorphism suggests the diversity of Mycobacterium ulcerans from different geographical areas. Am. J. Trop. Med. Hyg. 64:270-273. [DOI] [PubMed] [Google Scholar]
- 4.Chemlal, K., G. Huys, P.-A. Fonteyne, V. Vincent, A. G. Lopez, L. Rigouts, J. Swings, W. M. Meyers, and F. Portaels. 2001. Evaluation of PCR restriction profile analysis, IS2404 restriction fragment length polymorphism and amplified fragment length polymorphism fingerprinting for identification and typing of Mycobacterium ulcerans and M. marinum. J. Clin. Microbiol. 39:3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daffé, M., M.-A. Lanéelle, and C. Lacave. 1991. Structure and stereochemistry of mycolic acids of Mycobacterium marinum and Mycobacterium ulcerans. Res. Microbiol. 142:397-403. [DOI] [PubMed] [Google Scholar]
- 6.Daffé, M., and M.-A. Lanéelle. 1988. Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria. J. Gen. Microbiol. 134:2049-2055. [DOI] [PubMed] [Google Scholar]
- 7.Daffé, M., M.-A. Lanéelle, C. Asselineau, V. Lévy-Frébault, and H. David. 1983.. Intérêt taxonomique des acides gras des mycobactéries: proposition d'une méthode d'analyse. Ann. Microbiol. (Paris) 134B:241-256. [PubMed]
- 8.Daffé, M., M.-A. Lanéelle, J. Roussel, and C. Asselineau. 1984.. Lipides spécifique de Mycobacterium ulcerans. Ann. Microbiol. (Paris) 135A:191-201. [PubMed]
- 9.Daffé, M., A. Varnerot, and V. V. Lévy-Frebault. 1992. The phenolic mycoside of Mycobacterium ulcerans: structure and taxonomic implications. J. Gen. Microbiol. 138:131-137. [DOI] [PubMed] [Google Scholar]
- 10.Dale, J. W. 1995. Mobile genetic elements in mycobacteria. Eur. Respir. J. 8:S633-S648. [PubMed] [Google Scholar]
- 11.De Beenhouwer, H., Z. Liang, P. de Rijk, C. van Eekeren, and F. Portaels. 1995. Detection and identification of mycobacteria by DNA-DNA amplification and oligonucleotide-specific capture plate hybridization. J. Clin. Microbiol. 33:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devalois, A., K. H. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobos, K. M., F. D. Quinn, D. A. Ashford, C. R. Horshburgh, and C. H. King. 1999. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg. Infect. Dis. 5:367-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein, H. 1994. Mycobacterium marinum skin infections. Report of 31 cases and review of the literature. Arch. Intern. Med. 154:1359-1364. [DOI] [PubMed] [Google Scholar]
- 15.Flood, P., A. Street, P. O'Brien, and J. Hayman. 1994. Mycobacterium ulcerans infection on Philip Island, Victoria. Med. J. Aust. 160:160.. [PubMed] [Google Scholar]
- 16.Goutzamanis, J. J., and G. L. Gilbert. 1995. Mycobacterium ulcerans infection in Australian children: report of eight cases and review. Clin. Infect. Dis. 21:1186-1192. [DOI] [PubMed] [Google Scholar]
- 17.Green, E. P., M. L. Tizard, M. T. Moss, J. Thompson, D. J. Winterbourne, J. J. McFadden, and J. Hermon-Taylor. 1989. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 17:9063-9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infections. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy, F. G., R. A. Bukhalid, and R. Loria. 1999. Characterization of an insertion sequence element associated with genetically diverse plant-pathogenic Streptomyces spp. J. Bacteriol. 181:1562-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huys, G., L. Rigouts, K. Chemlal, F. Portaels, and J. Swings. 2000. Evaluation of amplified fragment length polymorphism analysis for inter- and intraspecific differentiation of Mycobacterium bovis, M. tuberculosis, and M. ulcerans. J. Clin. Microbiol. 38:3675-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, P. D., M. G. Veitch, D. E. Leslie, P. E. Flood, and J. A. Hayman. 1996. The emergence of Mycobacterium ulcerans in Melbourne. Med. J. Aust. 164:76-78. [DOI] [PubMed] [Google Scholar]
- 23.Josse, R., A. Guédénon, J. Aguiar, S. Anagonou, C. Zinsou, C. Porst, J. Foundohou, and J. E. Touze. 1994. L'ulcère de Buruli, une pathologie peu connue au Bénin. A propos de 227 cas. Bull. Soc. Pathol. Exot. 87:170-175. [PubMed] [Google Scholar]
- 24.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F. C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 31:2882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kox, L. F. F., J. van Leeuwen, S. Knijper, H. M. Jansen, and A. H. Kolk. 1995. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J. Clin. Microbiol. 33:3225-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laval, F., M.-A. Lanéelle, C. Déon, B. Monsarrat, and M. Daffé. 2001. Accurate molecular mass determination of mycolic acids by MALDI-TOF mass spectrometry. Anal. Chem. 73:4537-4544. [DOI] [PubMed] [Google Scholar]
- 27.Lévy-Frébault, V., and F. Portaels. 1992. Proposed minimal standards for the genus Mycobacterium and for the description of new slowly growing Mycobacterium species. Int. J. Syst. Bacteriol. 42:315-323. [DOI] [PubMed] [Google Scholar]
- 28.Linnell, F., and A. Norden. 1954. Mycobacterium balnei. A new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc. Scand. 33(Suppl. 1):26-42. [PubMed] [Google Scholar]
- 29.MacCallum, P., J. C. Tolhurst, G. Buckle, and H. A. Sissons. 1948. A new mycobacterial infection in man. J. Pathol. Bacteriol. 60:93-122. [PubMed] [Google Scholar]
- 30.Marston, B. J., M. O. Diallo, C. R. Horsburgh, Jr., I. Diomande, M. Z. Saki, J. M. Kanga, et al. 1995. Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am. J. Trop. Med. Hyg. 52:219-224. [DOI] [PubMed] [Google Scholar]
- 31.Pattyn, S. R., and F. Portaels. 1972. Identification and clinical significance of Mycobacteria. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. Reihe A 219:114-140. [PubMed] [Google Scholar]
- 32.Portaels, F., P.-A. Fonteyne, H. de Beenhouwer, P. de Rijk, A. Guédénon, J. Hayman, and W. M. Meyers. 1996. Variability in 3′ end of 16S rRNA sequences of Mycobacterium ulcerans is related to geographic origin of isolates. J. Clin. Microbiol. 34:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portaels, F., J. Aguiar, K. Fissette, P.-A. Fonteyne, H. deBeenhouwer, P. de Rijk, A. Guédénon, R. Lemans, C. Steunou, C. Zinsou, J. M. Dumonceau, and W. M. Meyers. 1997. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J. Clin. Microbiol. 35:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portaels, F. 1995. Epidemiology of mycobacterial diseases. Clin. Dermatol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 35.Portaels, F., K. Chemlal, P. Elsen, P. D. R. Johnson, J. A. Hayman, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals, p. 252-264. In M. T. Collins and B. Manning (ed.), Scientific and technical review, vol. 20. Office International des Epizooties, Paris, France. [DOI] [PubMed]
- 36.Portaels, F. Basic microbiology, p. 23-30. In Buruli ulcer: Mycobacterium ulcerans infection. Report WHO/CDS/CPE/GBUI/2000. World Health Organization, Geneva, Switzerland.
- 37.Poulet, S., and S. T. Cole. 1995. Repeated DNA sequences in mycobacteria. Arch. Microbiol. 163:79-86. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, B. 1997. Molecular and immunological studies of Mycobacterium ulcerans. Ph.D. thesis. James Cook University, Cairns, Australia.
- 40.Rogall, T., J. Wolters, T. Floher, and E. C. Böttger. 1990. Towards a phylogeny and definition of the species at the molecular level within the genus Mycobacterium. Int. J. Syst. Bacteriol. 40:323-330. [DOI] [PubMed] [Google Scholar]
- 41.Sander, P., F. Alcaide, I. Richter, K. Frischkorn, E. Tortoli, B. Springer, A. Telenti, and E. Böttger. 1998. Inteins in mycobacterial GyrA are a taxonomic character. Microbiology 144:589-591. [DOI] [PubMed] [Google Scholar]
- 42.Savelkoul, P. H. M., H. J. M. Aarts, J. de Haas, L. Dijkshoorn, B. Duin, M. Otsen, J. W. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified fragment length polymorphism analysis: the state of the art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sreevastan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahl, D. A., and J. W. Urbance. 1990. The division between fast- and slowly growing species corresponds to natural relationships among the mycobacteria. J. Bacteriol. 172:116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stinear, T., B. C. Ross, P. D. R. Johnson, L. Marino, R. M. Robins-Browne, F. Oppedisano, A. Sievers, and J. K. Davies. 1999. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 37:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stinear, T., G. Jenkin, P. D. Johnson, and J. K. Davies. 2000. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J. Bacteriol. 182:6322-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talaat, A. M., and M. Trucksis. 2000. Transformation and transposition of the genome of Mycobacterium marinum. Am. J. Vet. Res. 61:125-128. [DOI] [PubMed] [Google Scholar]
- 48.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tøonjum, T., D. B. Welty, E. Jantzen, and P. L. Small. 1998. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Travis, D. W., L. B. Travis, G. D. Roberts, D. W. Su, and L. W. Weiland. 1985. The histopathologic spectrum in Mycobacterium marinum infection. Arch. Pathol. Lab. Med. 109:1109.. [PubMed] [Google Scholar]
- 51.Tsang, A. Y., and E. R. Faber. 1973. The primary isolation of Mycobacterium ulcerans. Am. J. Clin. Pathol. 59:688-692. [DOI] [PubMed] [Google Scholar]
- 52.van Embden, J. D. A., M. D. Cave, J. T. Crawford, W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wayne, L. G., R. C. Good, M. I. Krichevsky, Z. Blacklock, H. L. David, D. Dawson, W. Gross, J. Hawkins, I. Juhlin, W. Käppler, H. H. Kleeberg, V. Lévy-Frebault, McDurmont, E. E. Nel, F. Portaels, S. Rüsch-Gerdes, K. H. Schröder, V. A. Silcox, I. Szabo, M. Tsukamura, L. van Den Breen, B. Vergmann, and M. A. Yakrus. 1989. Third report of cooperative, open-ended study of slowly growing mycobacteria by International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Microbiol. 39:267-278. [Google Scholar]