Abstract
Influenza B virus Yamagata group strains, isolated in the 2000 to 2001 influenza epidemic season, reacted poorly to the polyclonal ferret sera prepared against strains isolated earlier. The results of genetic analysis clarified that a point mutation of the nucleotide at position 126 in the HA1 region and the corresponding one-amino-acid substitution altered viral antigenicity.
There are influenza epidemics every year. In recent years, diagnosis is rapidly confirmed in a clinic when the virus is detected by diagnosing kits, such as enzyme immunosorbent assays or direct immunofluorescence. In contrast, in a reference laboratory participating in influenza surveillance, the detailed antigenic characterization of isolates is required. In Japan, virus is isolated mainly in Madin-Darby canine kidney (MDCK) cells. Subsequently, it is subtyped by the hemagglutination inhibition (HI) test using the polyclonal ferret sera against influenza virus A or B. To subtype influenza A virus, the antibody has to be prepared continually, because the antigenic drift of hemagglutinin (HA) protein hampers its identification (2, 3, 6). On the other hand, the evolution of influenza B virus is characterized by a lower rate of antigenic change (7, 8). Recent isolates of influenza B virus are classified into two major phylogenetic trees: the influenza virus B/Victoria/2/87 subclass or the influenza virus B/Yamagata/16/88 subclass (4, 7, 8, 13, 15, 17). Group-specific antigens have been conserved among influenza virus B/Yamagata strains. In Japan, the standard ferret sera were prepared using influenza virus B/Mie/1/93 strain for the epidemic seasons in 1994 to 1995 through 1998 to 1999. It reacted well with all the 1998 to 1999 influenza virus B/Yamagata isolates in Osaka Prefecture by HI tests. However, their reactivities against monoclonal antibody (MAb) 5H4 were heterogeneous. MAb 5H4 had potent HI and neutralization activities against the influenza virus B/Yamagata strains isolated earlier. Approximately 6% of the isolates did not react against MAb 5H4, and a single amino acid substitution of Arg to Lys at position 149 in the HA1 region caused the phenomenon (12).
We studied the variation in antigenicity and nucleotide sequence of the 2000 to 2001 isolates of Kobe City, Japan.
MAbs were obtained by immunizing mice with influenza B virus strains as described previously (9-12). Ascitic fluid samples from mice injected with hybridoma cells were used as the sources of MAbs. The standard ferret sera were provided by the National Institute of Health, Tokyo, Japan: the sera against influenza virus B/Yamanashi/166/98 for influenza virus B/Yamagata and the sera against influenza virus B/Shangdong/7/97 for influenza virus B/Victoria. The methods for virus inoculation and visualization of infected cells by peroxidase-antiperoxidase (PAP) staining were described previously (9, 14, 16). Briefly, infected cells were treated successively with MAbs, rabbit anti-mouse immunoglobulin antibody, goat anti-rabbit immunoglobulin antibody, and PAP complex. The visualized cells were then observed under a light microscope. The results of HI tests are expressed as the reciprocal of antibody dilution (14). Direct sequencing of viral nucleotide was performed as described previously (10-12). Briefly, reverse transcriptase PCR products were sequenced with the DYEnamic ET terminator cycle sequencing kit (Amersham Pharmacia, Piscataway, N.J.) and analyzed by the ABI Prism 310 automatic sequencer (Perkin-Elmer, Foster City, Calif.).
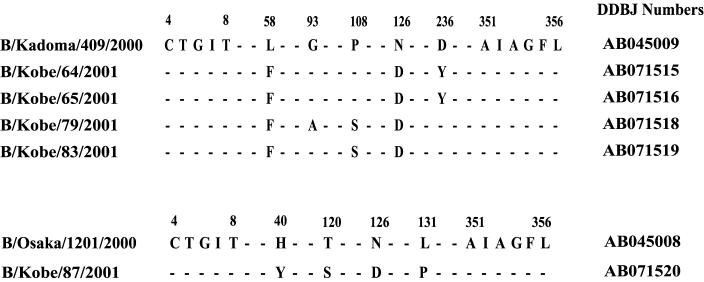
The reactivities of the 2000 to 2001 isolates against anti-influenza virus B/Yamagata sera in HI tests were as low as 20 to 40, while the reactivities of the earlier influenza virus B/Yamagata isolates ranged from 160 to 320 (Table 1). The isolates were PAP stained and identified as influenza virus B/Yamagata group strains with MAbs, NP-specific 9F3 and HA-specific 7H11. An influenza virus B/Victoria group HA-specific MAb, MAb10B8, did not react with these isolates. Nucleotide sequences and deduced amino acid sequences of the HA gene indicated that strains with two different origins were isolated in the 2000 to 2001 influenza season (Fig. 1), as observed in the previous two seasons (12). Four strains had 99% similarity to influenza virus B/Kadoma/409/2000, which was derived from influenza virus B/Shiga/T30/98, while influenza virus B/Kobe/87/2001 had 98% similarity to influenza virus B/Osaka/1201/2000, which was derived from influenza virus B/Tokyo/942/96. In both groups, there was an Asn-to-Asp substitution at position 126.
TABLE 1.
Results of HI tests and PAP staining
| Strain | HI test resulta
|
PAP stainingb with MAb:
|
|||||
|---|---|---|---|---|---|---|---|
| Anti-Yamagata sera | Anti-Victoria sera | MAb 5H4 | 9F3 | 10B8 | 7H11 | 5H4 | |
| B/Shiga/T30/98 lineage | |||||||
| B/Shiga/T30/98 | 320 | <10 | 25,600 | + | − | + | + |
| B/Kadoma/122/99 | 320 | <10 | 25,600 | + | − | + | + |
| B/Kadoma/409/2000 | 160 | <10 | <100 | + | − | + | − |
| B/Tokyo/942/96 lineage | |||||||
| B/Tokyo/942/96 | 320 | <10 | 51,200 | + | − | + | + |
| B/Kadoma/506/99 | 320 | <10 | <100 | + | − | + | − |
| B/Osaka/1201/2000 | 160 | <10 | <100 | + | − | + | − |
| 2000-2001 isolates | |||||||
| B/Kobe/64/2001 | 20 | <10 | <100 | + | − | + | − |
| B/Kobe/65/2001 | 20 | <10 | <100 | + | − | + | − |
| B/Kobe/69/2001 | 40 | <10 | <100 | + | − | + | − |
| B/Kobe/79/2001 | 20 | <10 | <100 | + | − | + | − |
| B/Kobe/84/2001 | 20 | <10 | <100 | + | − | + | − |
| B/Kobe/87/2001 | 20 | <10 | <100 | + | − | + | − |
The results are expressed as the reciprocal of antibody dilution.
+, stained; −, did not stain.
FIG. 1.
Comparison of the amino acid sequences of the A1 region. Sequences from amino acid residues 4 to 356 of the 2000 to 2001 isolates are shown and compared with those of influenza virus B/Kadoma/409/2000 (top) or influenza virus B/Osaka/1201/2000 (bottom). The sites where amino acid substitutions occurred in B/Yamagata group strains as compared with B/Kadoma/409/2000 (top) or influenza virus B/Osaka/1201/2000 (bottom) are shown. The DDBJ accession numbers of the sequences are shown to the right.
Influenza virus B/Kobe/69/2001 had high similarity to influenza virus B/Kadoma/409/2000; however, two nucleotide residues were not determined in direct sequencing, suggesting that the virus was a mixture of more than one strain. Therefore, the virus was cloned, and 21 clones were obtained. When the ferret sera were used in HI tests, 8 clones had HI titers as low as 20, while the remaining 13 clones had titers of 160. HA1 regions of four representative strains were sequenced (Table 2). Among 1,060 nucleic acids in the HA1 region, only two points were different, which correspond to two amino acid substitutions. The strains with low reactivities had Phe at position 58 and Asp at position 126, while those with high reactivities had Leu and Asn at these two sites, respectively. Therefore, amino acid substitutions at position 126 strongly influenced the virus reactivity to the ferret sera. Amino acids in HA1 polypeptides of the influenza B virus strains were numbered according to the numbering used for the influenza virus A/Aichi sequence for the structural reference of the H3 HA protein of influenza A virus (1, 5). The amino acid at position 126 corresponds to the amino acid at position 129 of AH3 virus by this method. Position 126 is located near the receptor region.
TABLE 2.
Results of HI tests and nucleotide and amino acid sequencing of the clones of B/Kobe/69/2001
| Influenza virus strain or clone | HI test resulta
|
Sequencing result
|
DDBJ accession no. | ||
|---|---|---|---|---|---|
| MAb 5H4 | Ferret sera | Nucleotides at positions 172 and 376 | Amino acids at positions 58 and 126 | ||
| B/Kadoma/409/2000 | <100 | 160 | C, G | Leu, Asn | AB045009 |
| B/Kobe/69/2001 | <100 | 40 | |||
| Clone 1 | <100 | 20 | T, A | Phe, Asp | AB071521 |
| Clone 2 | <100 | 20 | T, A | Phe, Asp | AB071522 |
| Clone 3 | <100 | 160 | C, G | Leu, Asn | AB071523 |
| Clone 4 | <100 | 160 | C, G | Leu, Asn | AB071524 |
The results are expressed as the reciprocal of antibody dilution.
The amino acid residues Asn at position 126 and Arg at position 149 had been conserved in influenza virus B/Yamagata group strains for 10 years (7). Influenza virus B/Taiwan/2027/99 was the first strain found with Lys at position 149, while influenza virus B/Taiwan/1265/2000 was the first strain found with Asp at 126, according to the DDBJ Data Base. In Japan, substitution at position 149 was initially observed only with strains of the B/Tokyo/942/96 lineage. In the following seasons, it was also detected in those of influenza virus B/Shiga/T30/98 lineage (12) (Table 1). They became the major strains in the 2000 to 2001 influenza season, because the Kobe isolates in this period did not react to MAb 5H4 in HI tests or PAP staining (Table 1). The substitution at position 126 was observed with strains of both lineages in the 2000 to 2001 influenza season. Influenza virus B/Shiga/T30/98 and influenza virus B/Tokyo/942/96 are classified in different phylogenic trees, which diverged from their origins around 1993 (7). It is significant that the same amino acid substitutions at these positions created new strains with lower reactivity to the antibodies. This finding is compatible with the idea that the new antigenic variants are generated to escape from the existing human immunity and displace the old variants (6, 7, 11).
Henceforth, influenza virus B/Yamagata group viruses might emerge from the antigenic variants of the influenza virus B/Victoria group (11) and new group strains may appear from the variants of influenza virus B/Yamagata strains. This information will benefit the management of public health (foretelling the scale of future epidemics and selecting suitable strains for vaccines).
Nucleotide sequence accession numbers.
The nucleotide sequences of influenza B viruses from the 2000 to 2001 influenza season were deposited in DDBJ database under accession numbers as follows: influenza B/Kobe/64/2001, AB071515; B/Kobe/65/2001, AB071516; B/Kobe/79/2001, AB071518; B/Kobe/83/2001, AB071519; B/Kobe/87/2001, AB071520; and B/Kobe/69/2001 clones 1 (AB071521), 2 (AB071522), 3 (AB071523), and 4 (AB071524).
Acknowledgments
We thank T. Iwamoto for valuable advice on nucleotide sequencing.
REFERENCES
- 1.Berton, M. T., C. W. Naeve, and R. G. Webster. 1984. Antigenic structure of the influenza B virus hemagglutinin: nucleotide sequence analysis of antigenic variants selected with monoclonal antibodies. J. Virol. 52:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitch, W. M., R. M. Bush, C. A. Bender, and N. J. Cox. 1997. Long term trends in the evolution of H(3) HA1 human influenza type A. Proc. Natl. Acad. Sci. USA 94:7712-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhard, W., and R. G. Webster. 1978. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (H0N1) influenza virus with monoclonal antibodies. J. Exp. Med. 148:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanegae, Y., S. Sugita, A. Endo, M. Ishida, S. Senya, K. Osako, K. Nerome, and A. Oya. 1990. Evolutionary pattern of the hemagglutinin gene of influenza B viruses isolated in Japan: cocirculating lineages in the same epidemic season. J. Virol. 64:2860-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krystal, M., R. Elliot, E. W. Benz, Jr., J. F. Young, and P. Palese. 1982. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc. Natl. Acad. Sci. USA 79:4800-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstrom, S. E., Y. Hiromoto, R. Nerome, K. Omoe, S. Sugita, Y. Yamazaki, T. Takahashi, and K. Nerome. 1998. Phylogenetic analysis of the entire genome of influenza A (H3N2) viruses from Japan: evidence for genetic reassortment of the six internal genes. J. Virol. 72:8021-8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindstrom, S. E., Y. Hiromoto, H. Nishimura, T. Saito, R. Nerome, and K. Nerome. 1999. Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. J. Virol. 73:4413-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCullers, J. A., G. C. Wang, S. He, and R. Webster. 1999. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J. Virol. 73:7343-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa, N., A. Maeda, T. Kase, R. Kubota, and Y. Okuno. 1999. Rapid detection and identification of two lineages of influenza B strains with monoclonal antibodies. J. Virol. Methods 79:113-120. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa, N., R. Kubota, A. Maeda, T. Nakagawa, and Y. Okuno. 2000. Heterogeneity of influenza B virus strains in one epidemic season differentiated by monoclonal antibodies and nucleotide sequences. J. Clin. Microbiol. 38:3467-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa, N., R. Kubota, T. Nakagawa, and Y. Okuno. 2001. Antigenic variants with amino acid deletions clarify a neutralizing epitope specific for influenza B virus Victoria group strains. J. Gen. Virol. 82:2169-2172. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa, N., R. Kubota, S. Morikawa, T. Nakagawa, K. Baba, and Y. Okuno. 2001. Characterization of new epidemic strains of influenza B virus by using neutralizing monoclonal antibodies. J. Med. Virol. 65:745-750. [DOI] [PubMed] [Google Scholar]
- 13.Nerome, R., Y. Hiromoto, S. Sugita, N. Tanabe, M. Ishida, M. Matsumoto, S. E. Lindstrom, T. Takahashi, and K. Nerome. 1998. Evolutionary characteristics of influenza B virus since its first isolation in 1940: dynamic circulation of deletion and insertion mechanism. Arch. Virol. 143:1569-1583. [DOI] [PubMed] [Google Scholar]
- 14.Okuno, Y., K. Tanaka, K. Baba, A. Maeda, N. Kunita, and S. Ueda. 1990. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J. Clin. Microbiol. 28:1308-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rota, P., T. R. Wallis, M. W. Harmon, J. S. Rota, A. P. Kendal, and K. Nerome. 1990. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175:59-68. [DOI] [PubMed] [Google Scholar]
- 16.Ueda, M., A. Maeda, N. Nakagawa, T. Kase, R. Kubota, H. Takakura, A. Ohshima, and Y. Okuno. 1998. Application of subtype-specific monoclonal antibodies for rapid detection and identification of influenza A and B viruses. J. Clin. Microbiol. 36:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita, M., M. Krystal, W. M. Fitch, and P. Palese. 1988. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology 163:112-122. [DOI] [PubMed] [Google Scholar]