Abstract
Background
Chitosan, a cationic polysaccharide, exerts hemostatic activity by promoting platelet adhesion and aggregation. This clinical study aimed to evaluate the effectiveness of chitosan-based dental dressing in achieving early local hemostasis, in comparison to gauze packs, after dental extractions in patients with deranged coagulation profiles.
Methods
This study included 102 patients (204 extraction sites), of whom 86 were on anticoagulant therapy,15 had liver cirrhosis, and one with thrombocytopenic purpura required two or more tooth extractions. These sites were randomly divided into test and control sites. Patients with deranged coagulation profiles, including an international normalized ratio of 1.5–4, altered prothrombin time, activated partial thromboplastin time, and decreased platelet counts, were selected. Hemostasis was assessed at 10, 30, and 60 minutes post-extraction. Patients were evaluated on days 1, 3, and 7 for dry sockets and other adverse effects.
Results
Hemostasis was achieved in 83.1% of test sites within 10 minutes, compared to only 18.8% of control sites. By 30 minutes, an additional 16.8% of test sites had achieved hemostasis versus an additional 16.7% of control sites. By 60 minutes, a further 5.9% of test sites had achieved hemostasis, compared to 63.7% of control sites. The mean postoperative hemostasis times were 15.10± 12.88 minutes for test sites and 45.20± 20.62 minutes for control sites. Dry socket incidence was slightly higher in test sites, but this tendency was not statistically significant (p>0.05).
Conclusion
The study suggests that chitosan-based dental dressing facilitates early local hemostasis after tooth extraction in anticoagulated patients or patients with bleeding disorders.
Keywords: Anticoagulants, Bleeding, Chitosan, Gauze, Hemostatics, Tooth extraction
INTRODUCTION
Dental extractions must often be performed in patients with deranged coagulation profiles. Such patients are prone to excessive post-extraction bleeding, which can cause discomfort, increase the risk of infection, and delay wound healing [1]. Bleeding during minor procedures can also compromise visibility, prolong operating time, and create challenges for the dental surgeon. Managing patients with bleeding disorders who are on anticoagulants requires careful preoperative, intraoperative, and postoperative measures to control bleeding. Various local hemostatic agents, such as cellulose, gelatin foam, Ostene, fibrin glue, aluminum solution, and tranexamic acid, are available, each with its advantages and limitations. Recently, chitosan-based wound and dental dressings, derived from shrimp shell chitin, have been developed. These dressings were initially used by the US military to control bleeding and infections [2], and they have shown promise in dental applications.
Studies have demonstrated that chitosan is a clinically effective hemostatic agent that mechanically seals wounds and significantly reduces bleeding following dental extractions and minor oral surgery in patients with deranged coagulation profiles [3,4]. Chitosan is a biocompatible material with anti-inflammatory properties that supports blood clot formation in tooth sockets post-extraction [3-5]. In this prospective study, we used a chitosan-based dressing made from freeze-dried molds and chitosan, which forms a highly electropositive, spongy, soft material. When it is applied to a bleeding site, its bio-adhesive properties result in the creation of a mechanical seal, preventing blood loss. Its porous structure quickly absorbs plasma, concentrating cellular and protein components at the wound site. The cationic charge of chitosan immobilizes negatively charged blood cells, promoting platelet aggregation and clot formation [5]. Importantly, active bleeding is necessary for it to function, making it particularly useful during dental extractions.
The primary objective of this study was to evaluate the effectiveness of chitosan-based dental dressing in achieving early hemostasis, reducing dry socket incidence, minimizing infection, and swelling after tooth extraction in patients with bleeding disorders on anticoagulant therapy.
METHODS
A prospective, split-mouth randomized controlled trial was conducted on 102 patients who met the inclusion criteria, including bleeding disorders or anticoagulant therapy and the need for two or more tooth extractions. Ethical clearance (NK/4797/Study/57 dated 21/12/2018) was obtained from the institutional ethics committee, and written informed consent was obtained from all participants. Patients’ coagulation profiles, including the international normalized ratio, activated partial thromboplastin time, prothrombin time, and platelet counts, were assessed. Those with deranged coagulation profiles and an international normalized ratio of 1.5–4 were selected and continued to take their prescribed medications without alteration. In each patient, one extraction site was designated as the test site, and the contralateral or same-quadrant site served as the control. Single-rooted teeth were compared with other single-rooted teeth, and multirooted teeth were likewise compared with other multirooted teeth. Teeth toward the midline were considered as controls, while lateral teeth were considered as test sites. In the test group, chitosan dental dressing was placed in the extraction socket and pressed with a finger, followed by standard gauze pressure. In the control group, only standard gauze pressure pack was applied (Fig. 1). The dressing was removed the next day according to the manufacturer’s guidelines.
Fig. 1.
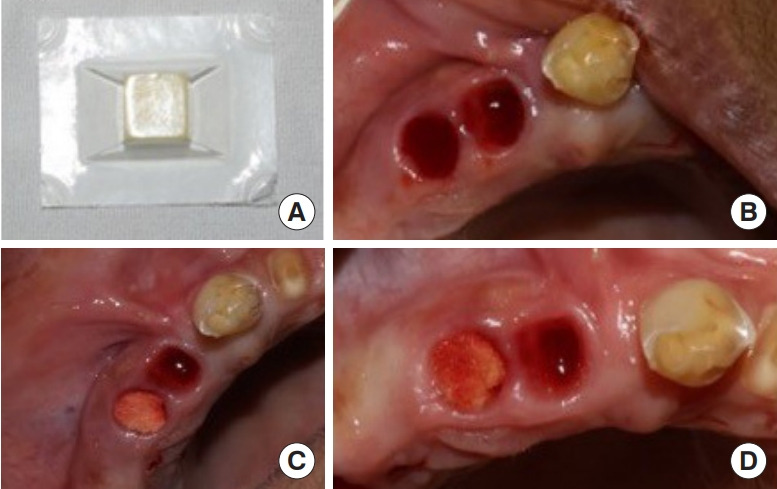
Procedure detail. (A) Chitosan dressing used in the study. (B) Extraction sockets after tooth extraction. (C) Dressing placed inside the socket. (D) Sockets after 10 minutes.
RESULTS
The study included 102 patients who received therapeutic anticoagulation or had various bleeding disorders and required the extraction of more than two teeth. Of these, 86 patients were on anticoagulant therapy for cardiac diseases, 15 had liver cirrhosis, and one had thrombocytopenic purpura (Table 1, Fig. 2). In the test group, hemostasis was achieved within 1 minute in 60 patients (58.8%), compared to 11 patients (10.8%) in the control group. After 10 minutes, an additional 25 patients (24.5%) in the test group achieved hemostasis, versus nine additional patients (8.8%) in the control group. By 30 minutes, hemostasis was achieved in 17 additional patients (16.8%) in the control group and 11 additional patients (10.8%) in the test group. At 60 minutes, six more patients (5.9%) in the test group had achieved hemostasis, versus 65 patients (63.7%) in the control group. The test and control groups exhibited a statistically significant difference in hemostasis (Table 2, Fig. 3). Dry socket was observed in six patients (5.9%) in the test group and one patient in the control group, though this difference was not statistically significant (Table 3). No infections were reported in either group. Swelling was significantly lower in the test group, occurring in four patients (3.92%), compared to 16 patients (15.68%) in the control group (Table 4, Fig. 4). The study concluded that chitosan-based dressings mechanically seal wounds and achieve hemostasis earlier than standard gauze, attenuating patient discomfort, reducing swelling, and promoting healing.
Table 1.
Distribution of patients according to anticoagulant use and bleeding disorders
| Condition | No. of patients |
|---|---|
| Taking anticoagulants for cardiac disease | 86 |
| Liver cirrhosis | 15 |
| Thrombocytopenic purpura | 1 |
Fig. 2.
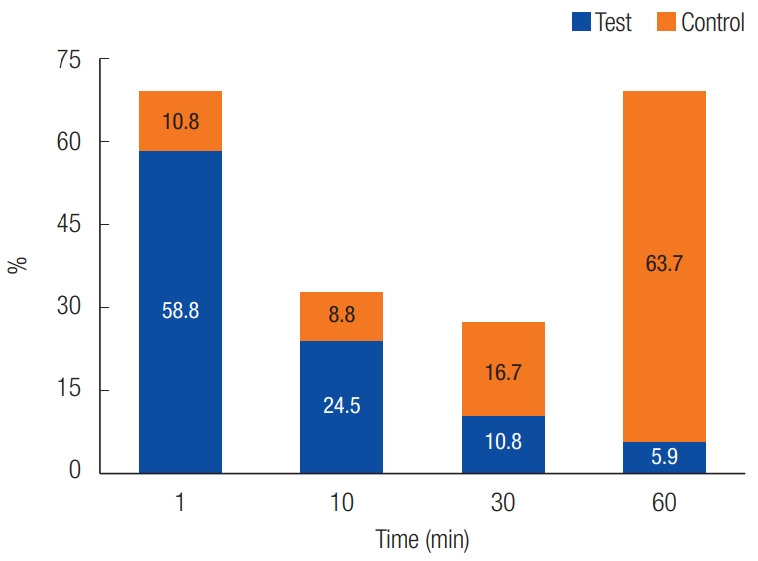
Comparison of hemostasis at the test and control sites.
Table 2.
Comparison of hemostasis at test and control sites
| Variable | Test site, No. (%) | Control site, No. (%) | p-value |
|---|---|---|---|
| Duration (min) | |||
| 1 | 60 (58.8) | 11 (10.8) | |
| 10 | 25 (24.5) | 9 (8.8) | |
| 30 | 11 (10.8) | 17 (16.7) | |
| 60 | 6 (5.9) | 65 (63.7) | |
| Total | 102 (100) | 102 (100) | |
| Mean ± SD (min) | 9.80 ± 15.49 | 44.23 ± 22.41 | 0.001a) |
There was a statistically significant difference between the test and control sites for hemostasis (Mann-Whitney U test).
Fig. 3.

Comparison of dry socket as complication between test and control sites.
Table 3.
Distribution of dry socket complications
| Dry socket | Test site, No. (%) | Control site, No. (%) | p-value |
|---|---|---|---|
| Present | 6 (5.88) | 1 (0.98) | |
| Absent | 96 (94.11) | 101 (99.02) | |
| Total | 102 (100) | 102 (100) | 0.110a) |
There was no statistically significant difference between the test and control sites for dry socket (Fisher exact test).
Table 4.
Distribution of swelling as a complication
| Swelling | Test site, No. (%) | Control site, No. (%) | p-value |
|---|---|---|---|
| Present | 4 (3.92) | 16 (15.68) | |
| Absent | 98 (96.08) | 86 (84.32) | |
| Total | 102 (100) | 102 (100) | 0.008a) |
There was a statistically significant difference between the test and control sites for swelling (Fisher exact test).
Fig. 4.

Distribution of swelling as a complication.
DISCUSSION
The study included patients in India who either received therapeutic anticoagulation or had various bleeding disorders. Over the past decade, there have been ongoing efforts to find a biomaterial that enhances clot formation and improves healing in extraction sockets for these patients. Patients who take anticoagulant medication or have bleeding disorders face a risk of bleeding. Chitosan-based dressings are used to control bleeding and enhance healing in both external and internal wounds [1]. Malette et al. [5], in a clinical study of chitosan solution, observed that it formed a coagulum upon contact with blood and proved to be an effective alternative for hemostasis in skin grafts due to the agglutination of red blood cells. Chou et al. [6] tested chitosan for its ability to increase platelet activity in rabbit platelet suspensions and found that chitosan significantly promotes platelet adhesion and aggregation. Azad et al. [7] used a chitosan membrane as a dressing in a clinical study for wound healing, applying it to 50% of the wounds, with the other half treated with chlorhexidine acetate-impregnated tulle grass as a control. They concluded that chitosan dressings facilitate effective adherence, hemostasis, re-epithelialization, and wound healing. Malmquist et al. [8] evaluated the efficacy of a chitosan-based dental dressing on hemostasis and healing following minor oral surgical procedures. Their results indicated that the chitosan dressing was clinically effective as a hemostatic agent, significantly reducing post-surgery bleeding time in patients on oral anticoagulant medications. ElShiha et al. [1] assessed the effectiveness of a chitosan-based hemostatic oral dressing compared with an absorbable gelatin sponge in terms of hemostasis and wound healing after tooth extraction. They found the dressing to be very successful in achieving hemostasis without the need for additional hemostatic measures. Kale et al. [9] demonstrated that chitosan-based dental dressing improved postoperative healing with minimal complications compared to standard bleeding control measures. Pogorielov et al. [10], in their review, concluded that chitosan is a biocompatible hemostatic agent and is an effective hemostatic material in powder or sponge forms. Kumar et al. [11], in a split-mouth randomized study of 30 patients with a control group, concluded that chitosan-based HemCon Dental Dressing (HemCon Medical Technologies) significantly reduced bleeding time and pain, and promoting better healing compared to the control group. Sharma et al. [12] reviewed 40 patients on anticoagulants with international normalized ratio between 1 and 3.5, where the Axiostat chitosan-based dental dressing (Advamedica) was used to control post-extraction bleeding. The patient group had a mean clot formation time of 1.30 minutes compared to a control mean of 14 minutes. The mean pain score was 1.93 compared to 3.63, which was statistically significant, and healing was also better than in the control group. They recommended the use of chitosan-based dental dressing to control bleeding in patients on anticoagulants and with bleeding disorders. Sinha et al. [13] studied 50 cardiac patients on antiplatelet therapy who required extractions and used Axiostat dental dressing to control post-extraction bleeding, finding an average hemostatic time of 1.5 minutes. They concluded that Axiostat dental dressing was highly effective as a hemostatic material and could be particularly useful in patients on anticoagulants undergoing extractions, as the use of local dressing significantly reduced the risk of thromboembolism without discontinuing anticoagulant therapy. Additional benefits of chitosan dressing, such as antimicrobial and anti-inflammatory properties, lead to better post-extraction healing [13]. Pippi et al. [14], in a randomized control trial, compared chitosan hemostatic dressing with a gelatin sponge and concluded that chitosan dressing is safe and superior for controlling post-extraction bleeding in patients on anticoagulants. Gupta et al. [15], in their prospective study of 27 patients after third molar extraction, found a decrease in pain score and better wound healing in the chitosan group compared to the non-chitosan group. Cicciu et al. [16], in their systematic review, found better healing and hemostasis without side effects with the use of chitosan as a dressing material for dental and oral surgery procedures. Radhakrishna et al. [17] also found better bleeding control in patients on single or dual antithrombotic therapy managed with chitosan dressing compared to simple gauze packing (96± 4 seconds vs. 797± 23 seconds, p< 0.001).
Our study, in line with other studies, also demonstrated control of inadvertent post-extraction bleeding in patients on anticoagulant therapy and with various bleeding disorders using chitosan-based dressing. It also helped decrease pain scores and postoperative swelling. The observations from randomized controlled trials provide evidence of the hemostatic and antiinflammatory properties of chitosan dental dressing in controlling excessive bleeding and inflammation of the socket after tooth extraction in patients on anticoagulant medications and with bleeding disorders.
We recommend chitosan dental dressing as a useful material for dental surgeons, as it facilitates the control of post-extraction bleeding in patients on oral anticoagulants and with bleeding disorders. It is also cost-effective with minimal complications (minimal swelling, dry socket, infection, etc.), good patient tolerability, and can be easily removed from the socket after hemostasis without interfering with the healing of the socket.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Ethical approval
The study was approved by the Institutional Review Board of Post Graduate Institute of Medical Education and Research (IRB No. NK/4797/Study/57) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Author Contributions
Conceptualization; Data curation; Formal analysis: Satnam Singh Jolly. Methodology: Satnam Singh Jolly, Vidya Rattan. Project administration: Satnam Singh Jolly. Writing - original draft: Satnam Singh Jolly. Writing - review & editing: Satnam Singh Jolly, Vidya Rattan. Investigation; Supervision; Validation: Vidya Rattan.
REFERENCES
- 1.ElShiha HY, Tawfik HAM, AbouSamrah NK, Marzouk HE, ElMagid Marzouk HA. Efficacy of chitosan and absorbable gelatin sponge on hemostasis and wound healing following tooth extraction “A Comparative Study”. Egypt Dent J. 2012;58:1–5. [Google Scholar]
- 2.Al-Mubarak S, Al-Ali N, Abou-Rass M, Al-Sohail A, Robert A, Al-Zoman K, et al. Evaluation of dental extractions, suturing and INR on postoperative bleeding of patients maintained on oral anticoagulant therapy. Br Dent J. 2007;203:E15. doi: 10.1038/bdj.2007.725. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan RC, Tirschwell DL, Longstreth WT, Jr, Manolio TA, Heckbert SR, Lefkowitz D, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. 2005;65:835–42. doi: 10.1212/01.wnl.0000176058.09848.bb. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart PB, Gibson J, Pond SH, Leitch J. Dental management considerations for the patient with an acquired coagulopathy. Part 1: Coagulopathies from systemic disease. Br Dent J. 2003;195:439–45. doi: 10.1038/sj.bdj.4810593. [DOI] [PubMed] [Google Scholar]
- 5.Malette WG, Quigley HJ, Gaines RD, Johnson ND, Rainer WG. Chitosan: a new hemostatic. Ann Thorac Surg. 1983;36:55–8. doi: 10.1016/s0003-4975(10)60649-2. [DOI] [PubMed] [Google Scholar]
- 6.Chou TC, Fu E, Wu CJ, Yeh JH. Chitosan enhances platelet adhesion and aggregation. Biochem Biophys Res Commun. 2003;302:480–3. doi: 10.1016/s0006-291x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 7.Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69:216–22. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 8.Malmquist JP, Clemens SC, Oien HJ, Wilson SL. Hemostasis of oral surgery wounds with the HemCon Dental Dressing. J Oral Maxillofac Surg. 2008;66:1177–83. doi: 10.1016/j.joms.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Kale TP, Singh AK, Kotrashetti SM, Kapoor A. Effectiveness of Hemcon Dental Dressing versus conventional method of haemostasis in 40 patients on oral antiplatelet drugs. Sultan Qaboos Univ Med J. 2012;12:330–5. doi: 10.12816/0003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogorielov MV, Sikora VZ. Chitosan as a hemostatic agent: current state. Eur J Med Ser B. 2015;1:24–33. [Google Scholar]
- 11.Kumar KR, Kumar J, Sarvagna J, Gadde P, Chikkaboriah S. Hemostasis and post-operative care of oral surgical wounds by Hemcon Dental Dressing in patients on oral anticoagulant therapy: a split mouth randomized controlled clinical trial. J Clin Diagn Res. 2016;10:ZC37–40. doi: 10.7860/JCDR/2016/17275.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S, Kale TP, Balihallimath LJ, Motimath A. Evaluating effectiveness of axiostat hemostatic material in achieving hemostasis and healing of extraction wounds in patients on oral antiplatelet drugs. J Contemp Dent Pract. 2017;18:802–6. doi: 10.5005/jp-journals-10024-2130. [DOI] [PubMed] [Google Scholar]
- 13.Sinha N, Mazumdar A, Mitra J, Sinha G, Baunthiyal S, Baunthiyal S. Chitosan based axiostat dental dressing following extraction in cardiac patients under antiplatelet therapy. Int J Oral Health Med Res. 2017;3:65–7. [Google Scholar]
- 14.Pippi R, Santoro M, Cafolla A. The use of a chitosan-derived hemostatic agent for postextraction bleeding control in patients on antiplatelet treatment. J Oral Maxillofac Surg. 2017;75:1118–23. doi: 10.1016/j.joms.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Rattan V, Rai S. Efficacy of Chitosan in promoting wound healing in extraction socket: a prospective study. J Oral Biol Craniofac Res. 2019;9:91–5. doi: 10.1016/j.jobcr.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicciu M, Fiorillo L, Cervino G. Chitosan use in dentistry: a systematic review of recent clinical studies. Mar Drugs. 2019;17:417. doi: 10.3390/md17070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishna S, Shukla V, Shetty SK. Is Chitosan dental dressing better than cotton gauze in achieving hemostasis in patients on antithrombotics? J Oral Maxillofac Surg. 2023;81:224–31. doi: 10.1016/j.joms.2022.10.011. [DOI] [PubMed] [Google Scholar]


