Abstract
Purpose
Surgery remains the primary treatment for early-stage colorectal and upper gastrointestinal (UGI) cancers. However, it can lead to postoperative complications and reduced functionality. Prehabilitation aims to improve functional reserves before surgery. We aimed to evaluate the implementation of a multimodal prehabilitation program in “real-world” patients undergoing gastrointestinal cancer surgery.
Methods
An implementation study evaluating prehabilitation in patients undergoing gastrointestinal (colorectal or UGI) cancer surgery at Concord Hospital. The prehabilitation program included supervised exercise, nutrition and nursing support delivered face-to-face or by telehealth (COVID-19 adaptations). Assessments: baseline, pre-surgery and 30 days after surgery. Primary outcome: implementation using the RE-AIM (Reach/Effectiveness/Adoption/Implementation/Maintenance) framework. Secondary outcomes: functional, nutritional and surgical outcomes, with comparisons to historical controls.
Results
Between January 2020 and December 2021, 181 patients were screened; 91 (50%) were eligible. Reach: 77/91 recruited (63 colorectal, 14 UGI). Median age, 70 years (IQR, 59–79); 60% were males. Median intervention duration, 16 days (IQR, 12.25–19.75). Effectiveness: quality of life, anxiety and functional capacity improved from baseline to pre-surgery (6-min walk test (+16.1 m, p=0.038) and 2-min step test (+10.0 steps, p<0.001)). Compared to historical controls, hospital length of stay was reduced by 2.1 days (p=0.010), with no differences in complications. Adoption: 91% of referrals came directly from surgeons. Implementation: 94% completed the intervention, with high adherence and satisfaction levels. Maintenance: after study completion, the program was incorporated into standard care with some modifications.
Conclusions
Prehabilitation can be implemented in a real-world setting, with a trend towards improving functional and surgical outcomes, but dedicated resources are necessary to implement and maintain the program.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-025-09496-5.
Keywords: Prehabilitation, Implementation, Gastrointestinal cancer, Surgery, Exercise, Nutrition
Introduction
Up to 80% of colorectal [1] and 10–20% of upper gastrointestinal (UGI) cancer patients (including cancers of the stomach, liver, oesophagus, pancreas and gallbladder) [2] will undergo elective surgery, providing a chance of cure and long-term survival. Despite advances in surgery, 30–45% of gastrointestinal cancer surgery patients experience major complications [3], leading to longer hospital stays, increased costs, delayed recovery, reduced quality of life (QOL) and survival rates [4]. Even without complications, many will experience a decline in functional capacity and fail to return to their previous level of function [5].
Prehabilitation aims to improve a patient’s physical condition and overall health before surgery to enhance surgical outcomes and recovery [6]. Initially focused on exercise only [6], prehabilitation has expanded into a multimodal approach consisting of exercise, nutrition and psychosocial interventions working synergistically together to produce more favourable outcomes [7]. While controlled trials in gastrointestinal cancer patients have shown promising results [8], little is known about its integration in the “real world” with factors such as uptake, acceptance, adherence and resources often not explored.
Traditionally, randomised controlled trials are considered the highest level of evidence to guide clinical practice [9]. However, only a small proportion (14%) of published evidence are successfully incorporated into clinical care [10]. Implementation frameworks, such as RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) help to identify the barriers and facilitators necessary to integrate research findings into practice [11], and can be instrumental in determining how interventions become integrated into real-world clinical care by documenting current practices, challenges and enablers. This study aims to evaluate the implementation of a multimodal (supervised exercise, nutrition and nursing support) prehabilitation program into standard care for patients undergoing curative intent gastrointestinal (colorectal or UGI) cancer surgery using the “RE-AIM” framework—evaluating Reach, Effectiveness, Adoption, Implementation and Maintenance [11].
Materials and methods
The study design, intervention and outcomes are described in the study protocol [12]; a summary is presented below.
Study design and setting
This is a prospective, single-centre implementation study using a before-and-after intervention design. Patients undergoing curative intent colorectal or UGI cancer surgery at Concord Repatriation General Hospital, a tertiary teaching hospital in Sydney, Australia, between January 2020 and December 2021, were invited to participate. Due to COVID-19 disruptions, recruitment was paused in March 2020 and resumed in August 2020 with telehealth adaptations. The study end date was extended from December 2020 to December 2021. The study was approved by the Sydney Local Health District Human Ethics Review Committee and conducted following the principles of the Declaration of Helsinki (2013). It was prospectively registered on the Australian and New Zealand Clinical Trials Registry (ACTR:12620000409976).
Participants
Patients were referred by their surgeon or screened in the hospital’s preadmission clinic. Patients were eligible if they were scheduled to undergo curative intent colorectal or UGI cancer surgery at Concord Repatriation General Hospital, were ≥18 years of age, had at least 14 days before surgery, were agreeable to the exercise and nutrition interventions and were medically cleared to exercise. A professional healthcare interpreter was provided to assist non-English-speaking patients to ensure inclusivity. Patients who were unable to provide informed consent, follow instructions due to cognitive impairment or were currently receiving neoadjuvant chemotherapy or radiotherapy were ineligible. Written informed consent was obtained prior to enrolment.
Multimodal prehabilitation intervention
Participants received a multimodal intervention for a minimum of 2 weeks before surgery, incorporating the following:
Exercise
The exercise program included supervised 60-min sessions, one to two times per week, at the Concord Hospital Sydney Cancer Survivorship Gym, delivered by exercise physiologists. Attending the second weekly session was optional but encouraged. Sessions were face-to-face or by telehealth (COVID-19 adaptation), individually or in small groups. Each session consisted of 20–30 min of individualised moderate-high intensity aerobic exercise (cycle ergometer, treadmill, rowing ergometer, elliptical trainer or boxing), followed by two sets of 8–12 repetitions of resistance exercise (body or hand weights, resistance bands or cable machines). Home-based aerobic and resistance exercises were prescribed for at least 30 min, 5 days/week. Exercise type, frequency and intensity were recorded in a daily diary.
Nutrition
Following a nutrition assessment, a dietitian provided individualised dietary education based on healthy eating principles [13], with a protein intake of 1.2–2 g/kg/body weight/day (or adjusted in overweight/obese participants) [14]. Additional dietary support was provided to manage nutrition-impact symptoms. High-protein nutritional supplements (Fresubin Protein Energy Drink, Fresenius Kabi) containing 20 g of protein were provided; one bottle was recommended daily within 60 min after exercise to support muscle synthesis [15]. Protein supplement intake was recorded in the daily diary.
Nursing support
Specialist cancer nurses provided weekly support, either in person or by phone, using semi-structured questions focusing on reassurance and adherence to the exercise and nutrition intervention. If necessary, referrals were made to general practitioners for medical optimisation, and smoking cessation was encouraged.
Miscellaneous
Distress was measured using the single-item distress thermometer [16]. Participants with a baseline score >4, indicating high psychological distress, were offered a referral to a clinical psychologist.
Resources
Additional resources were needed for the program, including a 1-year, part-time (3 days/week) dietitian position coordinating the program and a part-time (1 day/week) exercise physiologist position. Existing hospital facilities and nursing resources were utilised.
Postoperative care
After surgery, participants received standard postoperative care determined by their clinical team, including an enhanced recovery after surgery (ERAS) pathway for colorectal patients.
Outcomes
The primary outcome was implementation based on the RE-AIM evaluation framework: Reach, Effectiveness, Adoption, Implementation and Maintenance [11] (Table 1).
Table 1.
RE-AIM framework outcomes adapted to the PREHAB-GI study
| Domain | Description |
|---|---|
| Reach | Number (and proportion) of eligible patients having gastrointestinal cancer surgery who participated in the study compared to eligible patients who did not consent, and patient characteristics |
| Effectiveness | Changes in functionality, nutrition and well-being from pre- to post-intervention. Postoperative outcomes including complications and length of hospital stay |
| Adoption | Number (and proportion) of surgeons who referred, and surgeon characteristics compared to non-participating surgeons. Total number of referrals, referral sources, barriers to referrals. Clinician perceptions |
| Implementation | Delivery of the intervention as per the protocol and adaptations made. Overall completion rates, withdrawals and reasons, adherence to exercise sessions (attendance), protein supplement consumption (recorded in daily diary) and nurse support calls completed recorded in the attendance logs or daily diary. Program evaluations |
| Maintenance | Status of the program 12 months after study completion and adaptions made |
Secondary outcomes included changes in functional capacity (including the 6-min walk test (6MWT) and 2-min step test), nutritional status, body composition, anxiety/depression scores, QOL, and post-surgical outcomes, including hospital length of stay and complications graded using the Clavien-Dindo classification [17]. Major complications were defined as a Clavien-Dindo grade ≥3.
Historical control group
To evaluate the effectiveness of the prehabilitation intervention, post-surgical outcomes were compared to a historical control group of similar patients who underwent elective gastrointestinal cancer surgery at the same hospital 1 year earlier (in 2019). The control group did not receive a prehabilitation intervention. Data for the control group was collected from the hospital’s colorectal cancer database or medical records (Supplementary Table 1). Functional, nutritional or psychological data were unavailable.
Study assessments
Data was collected at three time points—baseline, pre-surgery (1–4 days before hospital admission or surgery) and 30 days (±7 days) after surgery—and managed using a project-specific Research Electronic Data Capture Database (REDCap) (Vanderbilt University) [18], hosted by Sydney Local Health District. The following variables were collected (Supplementary Table 1):
Participant demographic and clinical data
Demographic and clinical data (surgical procedures, hospital length of stay, 30-day readmission and complications) were collected from hospital medical records and study questionnaires.
Functional data
Functional capacity was measured using the 6MWT [19], 2-min step test [20], 30-s sit-to-stand [20] and handgrip strength (JAMAR hydraulic hand dynamometer).
Anthropometry and nutritional indicators
Height (metres) was measured using a stadiometer. Body composition (weight, fat mass, skeletal muscle mass and visceral adiposity) was assessed using bioimpedance analysis (Seca mBCA 515 Analyser (Seca, Hamburg, Germany)). Nutritional status was determined using the validated Patient-Generated Subjective Global Assessment (PG-SGA) [21] tool and categorised as well-nourished (PG-SGA A) or malnourished (PG-SGA B or C).
Patient-reported outcomes
QOL was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [22]. Psychological well-being was evaluated using the Hospital Anxiety and Depression Scale (HADS) [23] and a single-item distress thermometer [24]. Self-reported exercise was measured using the modified Godin Shephard Leisure Time Physical Activity Questionnaire [25].
Satisfaction
Participants completed an investigator-developed satisfaction survey after the prehabilitation intervention (1–4 days before surgery) and 30 days after surgery. Surgical staff, including gastrointestinal surgeons, surgical ward nurses and anaesthetists, completed a post-study evaluation survey. Semi-structured qualitative interviews were conducted by an independent researcher on a subset of participants; these findings will be presented separately.
Adherence
Adherence to the prehabilitation program was measured by recording the number of supervised exercise sessions attended, protein supplements consumed and nursing support sessions received in the daily diary or attendance logs.
Telehealth adaptions
The program was modified to include telehealth services in response to the COVID-19 pandemic (Table 2).
Table 2.
Telehealth adaptations
| Outcome or intervention | Telehealth amendments |
|---|---|
| Consent | Electronically completed consent forms |
| Baseline, pre-surgery and 30 days after surgery assessments | Assessments conducted virtually over videoconferencing/zoom appointments with links emailed to patients |
| Nutrition assessment | Anthropometric measures self-measured: weight, height and girths (waist, hip and mid-arm; standardised tape measure provided) |
| Body composition analysis omitted | |
| Exercise assessment | Remote functional capacity assessment: |
| 6-min walk test (6MWT) and handgrip test omitted | |
| Continuation of 30-s chair stand, 2-min step test, 3-m timed up and go (to measure cardiovascular fitness) | |
| Supervised exercise intervention | Home-based individual supervised exercise sessions conducted virtually via videoconferencing/zoom |
| Principles of the original protocol followed in terms of frequency, intensity, time and types of exercise | |
| Nutrition intervention | Dietary education material and high-protein oral nutritional supplements mailed to patients |
| Patient-reported outcome/s questionnaires | Completed by hard copy (mailed out) or online/electronically using REDCap a |
aREDCap, Research Electronic Data Capture Database
Statistical analysis
Descriptive statistics were used to analyse demographic and clinical data and presented as means and standard deviations, medians and interquartile ranges or counts and percentages. Generalised Estimating Equations (GEE) [26] were used to model changes from baseline for patient-reported outcome measures. A Gaussian distribution with an identity link to estimate mean differences (MD) was used for continuous outcomes, while a Poisson distribution with a log link was used to estimate relative risks (RR) for dichotomous outcomes. Robust standard errors and an independent working correlation structure were used in the GEE analyses. The GEE framework was chosen for its ability to effectively accommodate missing observations for participants under the assumption that data are missing at random.
Differences between the prehabilitation intervention and historical control group were assessed using linear regression (reporting MD and 95% confidence intervals (CI)) for continuous outcomes and logistic regression (reporting RR and 95% CI) or chi-square (χ2) for dichotomous variables. All models were adjusted for age, gender and surgeon. Statistical analysis was performed using STATA version 18 (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.). A p<0.05 was considered statistically significant.
Results
Between January 2020 and December 2021, 77 patients participated in the study (Fig. 1). Recruitment was paused in March 2020 due to COVID-19 restrictions and restarted in August 2020, with telehealth adaptations.
Fig. 1.
Study consort. +Intervention or assessment unable to be completed due to staff illness/leave, COVID staff redeployment, etc. Abbreviations: CRC, colorectal cancer; UGI, upper gastrointestinal cancer
Participant characteristics
Demographic and clinical data are presented in Table 3. Sixty-three (82%) had colorectal and 14 (18%) had UGI cancer. The median age was 70 years (IQR, 59–79). Age, gender and cancer type did not differ between the prehabilitation participants (n=77) and eligible patients who could not participate or declined (n=14) (Supplementary Table 2).
Table 3.
Baseline characteristics
| Characteristic | Prehab-GI (n=77) |
|---|---|
| Age, median (IQR), years | 70 (59–79) |
| Sex, male, n (%) | 46 (60) |
| NESB requiring an interpreter, n (%) | 21 (27) |
| Smoking status, n (%) | |
| Non-smoker | 47 (61) |
| Ex-smoker | 26 (34) |
| Current smoker | 4 (5) |
| Comorbidities, n (%) | |
| Diabetes | 16 (21) |
| Hypertension | 39 (51) |
| Cardiovascular | 5 (6.5) |
| Pulmonary | 2 (2.5) |
| Charlson Comorbidity Index Score a, median (IQR) | 5 (3.5–6) |
| ECOG performance status, n (%) | |
| 0 | 70 (91) |
| 1 | 7 (9) |
| 3 | 0 (0) |
| Cancer type, n (%) | |
| Colorectal | 63 (82) |
| Upper gastrointestinal | 14 (18) |
| Received neoadjuvant therapy | 9 (12) |
| Surgical approach, n (%) b,c | |
| Open surgery | 16 (22) |
| Laparoscopic | 56 (78) |
| Type of surgery, n (%) b,c | |
| Colon | 42 (58.3) |
| Rectal | 18 (25) |
| Gastric | 6 (8.3) |
| Oesophageal | 1 (1.4) |
| Pancreas | 1 (1.4) |
| Liver | 4 (5.6) |
| Stoma created, n (%) b,c | 12 (16.7) |
IQR interquartile range, NESB non-English speaking background, ECOG Eastern Co-operative Oncology Group
a Scores range from 0 to 24. Higher scores indicate a higher mortality risk within one year and more severe comorbid conditions
b Measured at 30 days
c Excludes participants who withdrew (n=5)
The median prehabilitation intervention (time between baseline and pre-surgery assessment) was 16 days (IQR, 12.25–19.75; range, 8–35). Four participants had an intervention period of <14 days because the surgery date was moved forward (n=1), work commitments (n=1) or interpreter unavailablity (n=2).
Results relating to each domain of the RE-AIM framework [11] are presented below.
Reach
Over 16 months, 181 (131 colorectal, 50 UGI) patients were screened (Fig. 1). Of these, 90 (50%) patients were ineligible; reasons included inadequate time before surgery (<14 days) (n=64, 71%) and not medically cleared to exercise (n=6, 7%). Among the 91 (50%) eligible patients, 10 (11%) declined to participate. Four eligible patients could not participate due to staffing issues (e.g., illness/leave, COVID-19 redeployment). Age, gender and language did not affect participation; however, colorectal cancer patients were more likely to participate.
Effectiveness
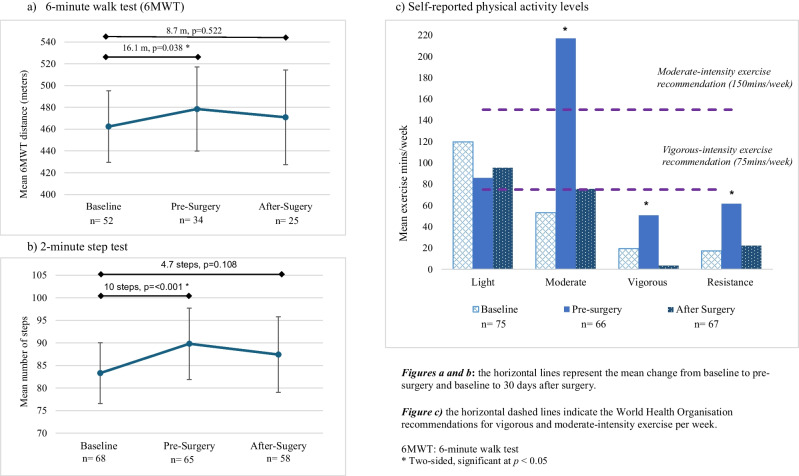
Functional outcomes are presented in Table 4, Fig. 2 and Supplementary Table 3. Before-to-after intervention assessments showed positive changes in functional capacity using the 6MWT (MD, +16.1 m, p=0.038) and 2-min step test (MD, +10 steps, p ≤ 0.001). Some improvement was maintained at 30 days after surgery, although it was no longer statistically significant. However, the 6MWT could not be completed on 110 (50%) occasions. Reasons included COVID-19 restrictions and the move to telehealth (n=86), adverse weather conditions (outdoor track) (n=13), pre-existing medical conditions contraindicating participation (n=6), inappropriate footwear (n=3) or participant declined (n=2). There were no significant changes in other functional measures.
Table 4.
Functional, nutritional and psychological well-being outcome measures
| Baseline, n =77 | Pre-surgery, n =72 | 30 days after surgery, n =72 | Change from baseline to pre-surgery | Change from baseline to after surgery | |||
|---|---|---|---|---|---|---|---|
| Mean change a (95% CI) | p-value | Mean change a (95% CI) | p-value | ||||
| Functional assessment | |||||||
| 6-minute walk test (6MWT) (metres), mean (SD) | 462.4d (118.30) | 471.7d (110.70) | 482.0d (105.34) | 16.1 (0.9, 31.4) | 0.038* | 8.7 (−17.8, 35.2) | 0.522 |
| Missing | 25 | 38 | 47 | ||||
| 2-minute step test (steps), mean (SD) | 83.3 (26.43) | 89.8 (30.77) | 87.4 (32.61) | 10.0 (6.0, 14.0) | <0.001* | 4.7 (−1.0, 10.4) | 0.108 |
| Missing | 9 | 7 | 14 | ||||
| Handgrip strength—right (kg), mean (SD) | 32.3 (12.04) | 32.7d (12.49) | 32.9d (12.72) | 1.0 (0.0, 1.9) | 0.058 | −0.8 (−1.8, 0.6) | 0.092 |
| Missing | 17 | 29 | 41 | ||||
| Handgrip strength—left (kg), mean (SD) | 30.5 (11.03) | 30.7d (16.35) | 31.2d (11.03) | 0.4 (−0.5, 1.3) | 0.409 | −1.3 (−2.3, −0.4) | 0.004* |
| Missing | 17 | 29 | 41 | ||||
| Nutritional assessment | |||||||
| Weight (kg), mean (SD) | 71.7 (18.42) | 72.2 (18.46) | 68.9 (17.65) | 0.5 (0.2, 0.8) | 0.002* | −2.4 (−3.1, −1.8) | <0.001* |
| Missing | 0 | 3 | 6 | ||||
| Body Mass Index (BMI) (kg/m2), mean (SD) | 24.8 (5.45) | 25.5 (5.16) | 24.5 (4.66) | 0.4 (−0.6, 1.4) | 0.448 | −0.5 (−1.4, 0.4) | 0.265 |
| Missing | 0 | 3 | 6 | ||||
| Nutrition status | |||||||
| Malnourished (PG-SGA B or C), n (%) | 15 (19.5) | 7 (10) | 25 (38.2) | 0.57e (0.36, 0.89) | 0.015* | 1.98e (1.23, 3.17) | 0.005* |
| PG-SGA score, mean (SD) | 6.4 (3.75) | 4.2 (1.46) | 6.2 (4.26) | −1.7 (−2.2, −1.1) | <0.001* | 1.8 (0.6, 2.9) | 0.003* |
| Missing | 0 | 3 | 6 | ||||
| Psychological well-being | |||||||
| Distress thermometer | 7.3 (2.9) | 8.1 (2.5) | 8.4 (2.5) | 0.7 (0.1, 1.2) | 0.016 | 1.1 (0.3, 1.8) | 0.006 |
| Missing | 2 | 5 | 5 | ||||
| HADS—anxiety, mean (SD)b | 5.8 (3.76) | 4.7 (4.23) | 4.5 (3.49) | −1.1 (−1.8, −0.3) | 0.004* | −1.3 (−2.2, −0.4) | 0.004* |
| Anxiety >4, n (%) | 47 (62.6) | 32 (47.8) | 28 (41.8) | 1.3 (1.0, 1.8) | 0.053e | 1.5 (1.1, 2.1) | 0.010e* |
| HADS- depression, mean (SD)b | 4.1 (4.0) | 3.5 (3.61) | 4.4 (4.39) | −0.5 (−1.2, 0.2) | 0.174 | 0.4 (−0.8, 1.5) | 0.528 |
| Depression >4, n (%) | 26 (34.7) | 24 (35.8) | 25 (37.3) | 1.0 (0.6, 1.5) | 0.885e | 0.9 (0.6, 1.4) | 0.742e |
| Missing | 2 | 5 | 5 | ||||
| Quality of life: EORTC QLQ-C30, global health, mean (SD)c | 65.2 (21.54) | 71.8 (19.19) | 64.2 (22.14) | 6.2 (1.8,10.7) | 0.006* | −1.7 (−0.8, 4.5) | 0.583 |
| Missing | 2 | 5 | 5 | ||||
SD standard deviation, 6MWT 6-minute walk test, kg kilograms, PG-SGA Patient-Generated Subjective Global Assessment, HADS Hospital Anxiety and Depression Scale, EORTC QLQ-C30 European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire
aDerived from the imputation of missing data and adjustment for confounders, including age, gender, and surgeon. A Gaussian distribution estimated mean differences for continuous outcomes, and a Poisson distribution estimated relative risks for dichotomous outcomes
bScores range from 0–21, with higher scores indicating greater levels of anxiety or depression. A score >8 indicates high symptoms of anxiety or depression
cEORTC QLQ-C30—Global health: A higher score indicates a better quality of life and functioning
dIndicates >30% of participants missing
eRelative risks
*Two-sided, significant at p < 0.05
Fig. 2.
Functional capacity and exercise behaviours
At baseline, 15 participants (19.5%) were malnourished (PG-SGA B/C) (Table 4 and Supplementary Table 3). UGI participants had higher incidence of malnutrition compared to colorectal participants (colorectal, 17.5% vs. UGI, 28.6%, p=0.455) (Supplementary Table 3). There were no significant differences in characteristics and surgical outcomes between well-nourished and malnourished participants. PG-SGA scores, an indication of nutritional-related risk, improved from baseline to pre-surgery with prehabilitation (baseline, 6.4 vs. pre-surgery, 4.2, MD −1.7 (−2.2, −1.1), p ≤ 0.001). No significant changes were seen in body composition measures (Supplementary Table 3).
Patient-reported outcomes are outlined in Table 4 and Supplementary Table 3. Global health-related QOL, emotional, fatigue, dyspnoea subscales and anxiety symptoms significantly improved from baseline to pre-surgery. Of these, only the improvements in anxiety symptoms were maintained 30 days after surgery.
Length of hospital stay was 2.1 days shorter for prehabilitation participants compared to historical controls (prehabilitation, 6.9 days; SD, 5.8, vs. historical control, 8.8 days; SD, 7.3, p=0.010) (Table 5). Colorectal participants had a shorter length of stay than UGI participants (5.8 vs. 11.9 days, p=0.062). Thirty (42%) participants experienced at least one complication (Table 5). No differences were seen in the number and severity of complications, hospital readmission rates, unplanned intensive care unit admissions or discharge destination.
Table 5.
Postoperative outcomes—Prehab-GI intervention vs. historical control groups
| Operative outcomes | Prehab-GI, n =72 | Historical control group (2019), n = 123 | Adjusted estimates a (95% CI) | p -value |
|---|---|---|---|---|
| Complications, n (%) b | ||||
| Total (at least 1 complication) | 30 (41.7) | 52 (42.3) | RR, 1.0 (0.7, 1.4) | 0.933 |
| Severe, Clavien-Dindo grade ≥3 | 6 (8.3) | 14 (11.4) | RR, 0.6 (0.2, 1.4) | 0.226 |
| Length of hospital stay (days), mean (SD) b | 6.9 (5.6) | 8.8 (7.3) | MD, −2.1 (−3.6, −0.5) | 0.010* |
| Unplanned ICU admissions, n (%) | 4 (5.6) | 8 (6.6) | RR, 0.8 (0.7, 2.7) | 0.791 |
| Hospital readmission, n (%) b | 6 (8.3) | 9 (7.3) | RR, 1.1 (0.4, 3.1) | 0.707 |
| Discharge destination, n (%) b | ||||
| Home | 71 (98.6) | 98 (79.7) | 0.0002* | |
| Rehab | 1 (1.4) | 25 (20.3) | ||
SD standard deviation, ICU intensive care unit, MD mean difference, RR relative risk
aAdjustment for confounders, including age, gender and surgeon. Continuous outcomes were assessed using linear regression (MD) and dichotomous variables using logistic regression (RR or χ2)
bMeasured at 30 days
*Two-sided, significant at p < 0.05
Adoption
Fourteen surgeons were invited to participate: eight colorectal and six UGI. All invited colorectal surgeons participated. Among the UGI surgeons, two did not operate at the hospital during the study period, and one did not refer to the study. Of the referrals, 70 (91%) came directly from surgeons, while 7 (9%) were identified through the preadmission clinic. Referrals fluctuated throughout the study period due to COVID-19 disruptions including cancellation of elective surgeries. (Supplementary Figure 1). Following completion of the study, 10 of the 11 (91%) participating surgeons incorporated prehabilitation into their standard care. An additional surgeon who was not involved in the study adopted the program. All surveyed clinicians reported the program helped patients prepare for surgery and would highly recommend it.
Clinician 7: “A great initiative, a no-brainer. The benefits go way beyond shortening hospital stay”.
Clinician 6: “What a great program. Patients love it and do so well”.
Implementation
All participants (n=77) completed their baseline assessment. Five withdrew because of psychosocial stressors (n=3), change in cognition (n=1) and cancellation of surgery due to disease progression (n=1). Completion rates for pre-surgery (96%, 69/72) and 30 days after surgery (92%, 66/72) assessments were high. However, some participants could not complete their assessments due to prehabilitation staff unavailability (n=4), COVID-related restrictions (n=3) or participant decline (n=2). Overall, 35% (74/212) of assessments were conducted via telehealth.
Most participants attended one supervised exercise session per week, with two participants attending the second optional exercise session. Approximately one-third (32%) of the supervised exercise sessions were delivered through videoconferencing. No serious adverse events were reported.
Adherence to the study was high: 85% (61/72) of participants attended the available supervised exercise sessions, 90% (65/72) consumed the prescribed high-protein supplements, and 93% (67/72) received the provided nurse calls. Reasons for nonadherence included time constraints with medical appointments (n=5 exercise), exercise contraindications (n=1), forgetfulness (n=5; 3 nutrition, 2 exercise), participant declined (n=5; 3 exercise, 2 nursing), supplement tolerance (n=4) and interpreter unavailability (n=3 nursing). On 22 occasions, the supervised exercise sessions or nursing calls could not be delivered due to staff unavailability (e.g., illness/leave, COVID-19 disruptions). Cancer type, age, gender, language, employment status and surgeon did not affect adherence. Nearly all (96%) surveyed participants were strongly satisfied or satisfied with the program and would recommend it to others.
P12: “Very organised, it kept me motivated before surgery. I really enjoyed the Zoom sessions”.
P34: “I feel stronger and happier”
P70: “The support was great! It got me ready for my surgery.”
P22: “A fantastic program; the staff were exceptional.”
Maintenance
After completing the study, some modifications were made to the prehabilitation program to ensure its sustainability. Complementary nutritional supplements were no longer provided, and patients had to purchase their own. The exercise type, intensity and frequency were modified, with a change in delivery from exercise physiologists to physiotherapists due to staff availability.
In 2022, 49/117 (42%) eligible patients participated in the prehabilitation program: 34 (69%) colorectal and 15 (31%) UGI cancer. Of these, 39/49 (80%) received the exercise intervention, 44/49 (90%) received nursing support, and all 49 (100%) received the nutrition intervention.
Discussion
National and international guidelines recommend prehabilitation as an essential part of cancer and surgical pathways [14, 27]. Despite its benefits, prehabilitation is not routinely practised. This pragmatic study demonstrates that prehabilitation can be effectively integrated into routine clinical care for patients undergoing gastrointestinal cancer surgery, using telehealth services to combat pandemic-related challenges.
Using the RE-AIM framework [11], we found that our multimodal prehabilitation program was highly acceptable and had good fidelity. Almost all eligible patients referred participated, consistent with similar prehabilitation programs [28]. However, despite our efforts to promote the program across a multidisciplinary setting, the most common reason for ineligibility was insufficient time before surgery (<14 days) (n=64). Hospital administrative processes and the cancellation of elective surgeries in the public health system due to COVID-19 disruptions further impacted patient recruitment. According to current cancer treatment guidelines, patients should receive primary definitive treatment, including surgery, within 31 days, with delays potentially impacting survival outcomes [29, 30]. Given this narrow window, integrating a digital dashboard with automated risk stratification in the preoperative workflow could help identify suitable patients earlier, improve recruitment and maximise the prehabilitation duration while reducing the reliance on individual clinicians for screening [31].
Despite the challenges brought about by COVID-19, we achieved high completion and adherence rates, exceeding the average reported in the literature [28]. This success can be attributed to the program’s design, individualised intervention, coordination, supervision and involvement of experienced gastrointestinal surgical and oncology multidisciplinary staff, including a dietitian, exercise physiologists and nurses. The use of telehealth in response to the COVID-19 pandemic also played a significant role ensuring continuity of care. Many participants felt a sense of control over their health and viewed the program as a positive distraction while waiting for surgery. Research suggests that peer support and supervised exercise are associated with higher adherence rates [32]. Although our program mainly relied on group-based supervised exercise sessions, it is essential for future programs to consider local resources and patient needs when designing a prehabilitation program.
In line with the literature [8, 33], we observed improvements in functional capacity, exercise behaviours and nutritional status. While improvements diminished 30 days after surgery, functional outcomes and exercise behaviours remained higher than baseline. However, the results must be interpreted cautiously as some outcomes were omitted due to COVID-19 disruptions and the transition to telehealth. Surgical nutrition and oncology exercise guidelines recommend completing a pre-intervention assessment and monitoring to ensure safety and provide guidance [14, 34]. Although virtual assessments were quickly implemented, at the time of the study, there was limited evidence supporting the use of validated-telehealth functional outcome measures, including the 6MWT. Since then, several outcome measures have emerged and recommended in a telehealth setting [35].
Compared to historical data, prehabilitation patients had shorter hospital stays. However, the impact of other confounding factors, such as COVID-19 [36], surgical complexity and a patient’s self-motivation to participate, cannot be excluded. As the pandemic evolved, many services were redirected, reducing non-urgent diagnostic and surgical procedures to prioritise emergency and high-priority elective cases. Private hospital facilities were used, enabling public patients to receive treatment earlier. Additionally, extra efforts were made to discharge patients as soon as they were medically cleared to reduce the risk of hospital-acquired COVID-19 exposure [37], likely impacting the length of stay.
Having accessible and experienced prehabilitation team members with strong relationships with surgeons or anaesthetists facilitated the adoption of prehabilitation. Almost all invited surgeons adopted the program, making up 91% of the referrals. However, the long-term success of prehabilitation relies on continued engagement and dedicated resources. Unfortunately, there was no ongoing dedicated funding for our prehabilitation service, resulting in several modifications after the study’s maintenance phase. While a formal cost-effectiveness evaluation is underway, early economic evaluations suggest that prehabilitation can save up to £3000 (AUD 5200) per person [38] due to reduced complications, shorter hospital stays, decreased intensive care needs and fewer services needed upon discharge. These potential cost savings could be used to establish, maintain and expand prehabilitation programs.
As the demand for supporting vulnerable surgical patients increases, telehealth services are becoming a viable option, especially for the elderly or people living in rural or remote areas. While they cannot fully replace essential in-person visits, they can help reduce health inequities and improve access to services, regardless of geographical location [39]. A systematic review found that the travel distance to the exercise facility often influenced participation and adherence [40]. Interestingly, approximately 30% of our program was conducted via videoconference, with good patient acceptance. With the increasing demand for home-based or virtual prehabilitation programs, further research is needed to evaluate the safety, feasibility and effectiveness of web-based platforms, apps and wearables.
We acknowledge that our study has several limitations. Due to COVID-19 pandemic disruptions, a large amount of data for several key outcomes, including the 6MWT, is missing. To try to address this, we used the GEE method. Although we did not have a prospective control arm, we attempted to mitigate this limitation by using a historical control arm. Nevertheless, one strength of the study is that the program was implemented in a real-world clinical setting and included patients from diverse backgrounds. In response to the COVID-19 pandemic challenges, we quickly adapted the program to include a telehealth component. This adaption ensured the ongoing delivery during a time of great uncertainty, and helped overcome additional logistical barriers surgical patients may face, particularly those with work or family commitments or living far from the hospital. While the Maintenance phase of the RE-AIM framework was not extensively explored, key barriers included resources and fragmented screening/referral pathways during the transition to standard care. Further exploration of the barriers and facilitators to maintaining the program is needed from the perspectives of patients, clinicians, and institution.
Conclusion
The healthcare system is complex, and new concepts must be tailored to local settings. We showed that a multimodal prehabilitation program can be successfully implemented in face-to-face and telehealth settings, with a trend towards improving functional and surgical outcomes. While the current program has not expanded, it has provided valuable insights for the development and implementation of other prehabilitation programs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Colorectal and Upper Gastrointestinal (UGI) surgeons and surgical departments, Concord Cancer Centre and medical oncologists, and the Nutrition and Dietetics Department at Concord Hospital for their unwavering support and contributions. They also thank Prof. Pierre Chapuis and Gael Sinclair for maintaining and providing access to the Colorectal Bowel Cancer Database. Lastly, a heartfelt thank you is extended to all participating patients.
Author contribution
Conceptualisation, design and methodology: JV, KLR, MS. Data collection/curation: KLR, JT, CS, SG, SK, YL, CW, KYCC. Project administration: KLR, JV, MS. Formal analysis and interpretation of results: SE, JV, KLR, SYT. Writing- original draft: KLR, JV, SYT, SE. Writing – review and editing: KLR, JV, SYT, SE, MS, JT, CS, SG, SK, YL, CW, KYCC, GB, PL. Supervision: JV, SYT.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Fresenius Kabi supplied the high-protein oral nutritional supplements and funded a 1-year part-time study dietitian/coordinator position (KLR). Concord Cancer Centre provided funding through a seed research grant to support the part-time exercise physiologist position. Neither Fresenius Kabi nor Concord Cancer Centre was involved in the study design, data collection, results analysis, interpretation or dissemination.
Data availability
Available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Ethics approval was obtained from the Sydney Local Health District Human Ethics Review Committee (HREC: CH62/6/2019-114). Permission was granted to obtain the data for the historical control group from the Bowel Cancer Database (HREC: 16/CRGH/263). A waiver of consent was obtained, as participants had consented to the use of their de-identified data in other research studies. The study was conducted in accordance with the principles of the Declaration of Helsinki (2013).
Consent to participate
Prehab-GI participants received a Participant Information Sheet outlining the study details. Written consent was obtained prior to the commencement of the study.
Consent for publication
All PREHAB-GI participants received a Participant Information Sheet explaining publication and confidentiality information. Written consent was obtained.
Competing interests
KLR and the Concord Repatriation General Hospital Nutrition and Dietetics department disclose that funding was received for a part-time wage for one year from Fresenius Kabi to support the study.
Footnotes
Trial registration: Australian and New Zealand Clinical Trials Registry (ACTR:12620000409976. Date: 20/12/2020).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benitez Majano S, Di Girolamo C, Rachet B, Maringe C, Guren MG, Glimelius B et al (2019) Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. The Lancet Oncology. 20(1):74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Service NCRaA. National cancer registration and analysis service—chemotherapy, radiotherapy and surgical tumor resections in England. [Available from: https://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/main_cancer_treatments. Accessed 18 Feb 2024
- 3.Govaert JA, van Dijk WA, Fiocco M, Scheffer AC, Gietelink L, Wouters MW et al (2016) Nationwide outcomes measurement in colorectal cancer surgery: improving quality and reducing costs. J Am Coll Surg. 222(1):19-29.e2 [DOI] [PubMed] [Google Scholar]
- 4.Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J et al (2016) The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 160(5):1189–201 [DOI] [PubMed] [Google Scholar]
- 5.Lawrence VA, Hazuda HP, Cornell JE, Pederson T, Bradshaw PT, Mulrow CD et al (2004) Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 199(5):762–72 [DOI] [PubMed] [Google Scholar]
- 6.Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ et al (2010) Randomized clinical trial of prehabilitation in colorectal surgery. British Journal of Surgery. 97(8):1187–97 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS et al (2013) Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surgical Endoscopy. 27(4):1072–82 [DOI] [PubMed] [Google Scholar]
- 8.Lambert JE, Hayes LD, Keegan TJ, Subar DA, Gaffney CJ (2021) The impact of prehabilitation on patient outcomes in hepatobiliary, colorectal, and upper gastrointestinal cancer surgery: a PRISMA-accordant meta-analysis. Annals of Surgery. 274(1):70–7 [DOI] [PubMed] [Google Scholar]
- 9.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383-94 [DOI] [PubMed]
- 10.Morris ZS, Wooding S, Grant J (2011) The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 104(12):510–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC et al (2019) RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 7:64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raso KL, Suen M, Turner J, Khatri S, Lin Y, Wildbore C et al (2023) Prehabilitation before gastrointestinal cancer surgery: protocol for an implementation study. JMIR Res Protoc. 12:e41101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health and Medical Research Council (2013) Australian dietary guidelines. Canberra: national health and medical research council. www.nhmrc.gov.au/guidelines-publications/n55. Accessed 18 Feb 2024
- 14.Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S et al (2021) ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 40(7):4745–61 [DOI] [PubMed] [Google Scholar]
- 15.Prado CM, Purcell SA, Laviano A (2020) Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 11(2):366–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riba MB, Donovan KA, Andersen B, Braun I, Breitbart WS, Brewer BW et al (2019) Distress management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 17(10):1229-49 [DOI] [PMC free article] [PubMed]
- 17.Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240(2):205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 42(2):377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W (2013) Validity of the six-minute walk test in cancer patients. Int J Sports Med. 34(7):631–6 [DOI] [PubMed] [Google Scholar]
- 20.Jones CJ, Rikli RE, Beam WC (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research Quarterly for Exercise and Sport. 70(2):113–9 [DOI] [PubMed] [Google Scholar]
- 21.Ottery FD (2000) Patient-generated subjective global assessment. In: McCallum PD, Polisena CG (eds) The clinical guide to oncology nutrition. The American Dietetic Association, Chicago, pp 11–23 [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 85(5):365–76 [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 67(6):361–70 [DOI] [PubMed] [Google Scholar]
- 24.Panel PDPG (1999) NCCN practice guidelines for the management of psychosocial distress. National Comprehensive Cancer Network. Oncology (Williston Park) 13(5A):113–47 [PubMed] [Google Scholar]
- 25.Godin G (2011) The Godin-Shephard leisure time physical activity questionnaire. Health Fit J Can. 4:18–22 [Google Scholar]
- 26.Hardin JW, Hilbe JM (2002) Generalized estimating equations, 1st edn. Chapman and hall/CRC. 10.1201/9781420035285
- 27.Bloom E (2017) Prehabilitation evidence and insight review. London, UK: Macmillan cancer support. https://www.macmillan.org.uk/_images/prehabilitation-evidence-and-insight-review_tcm9-335025.pdf. Accessed 14 Apr 2024
- 28.McIsaac DI, Gill M, Boland L, Hutton B, Branje K, Shaw J et al (2022) Prehabilitation in adult patients undergoing surgery: an umbrella review of systematic reviews. British Journal of Anaesthesia. 128(2):244–57 [DOI] [PubMed] [Google Scholar]
- 29.Australian Institute of Health and Welfare (2014) Hospital performance: cancer surgery waiting times in public hospitals in 2012–13. Canberra: AIHW
- 30.Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E et al (2020) Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 371:m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahajan A, Esper S, Oo TH, McKibben J, Garver M, Artman J et al (2023) Development and validation of a machine learning model to identify patients before surgery at high risk for postoperative adverse events. JAMA Network Open 6(7):e2322285-e [DOI] [PMC free article] [PubMed]
- 32.Hageman D, Fokkenrood HJP, Gommans LNM, van den Houten MML, Teijink JAW (2018) Supervised exercise therapy versus home‐based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev (4) 10.1002/14651858.CD005263.pub4 [DOI] [PMC free article] [PubMed]
- 33.Suen M, Liew A, Turner JD, Khatri S, Lin Y, Raso KL et al (2021) Short-term multimodal prehabilitation improves functional capacity for colorectal cancer patients prior to surgery. Asia Pacific Journal of Clinical Oncology. 14:14 [DOI] [PubMed] [Google Scholar]
- 34.Stout NL, Santa Mina D, Lyons KD, Robb K, Silver JK (2021) A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin. 71(2):149–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyrusqué E, Granet J, Pageaux B, Buckinx F, Aubertin-Leheudre M (2022) Assessing physical performance in older adults during isolation or lockdown periods: web-based video conferencing as a solution. The Journal of nutrition, health and aging. 26(1):52–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MZ, Tay YK, Teoh WM, Kong JC, Carne P, D’Souza B et al (2022) Melbourne colorectal collaboration: a multicentre review of the impact of COVID-19 on colorectal cancer in Melbourne. Australia. ANZ J Surg. 92(5):1110–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feier CVI, Bardan R, Muntean C, Olariu A, Olariu S (2022) Impact of the COVID-19 pandemic on the elective surgery for colorectal cancer: lessons to be learned. Medicina. 58(10):1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risco R, González-Colom R, Montané-Muntané M, Cano I, Vela E, Sebio R et al (2023) Actionable factors fostering health value generation and scalability of prehabilitation: a prospective cohort study. Annals of Surgery. 278(2):e217–e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse CS, Krowski N, Rodriguez B, Tran L, Vela J, Brooks M (2017) Telehealth and patient satisfaction: a systematic review and narrative analysis. BMJ Open. 7(8):e016242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME (2018) Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho-Oncology. 27(3):713–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available from the corresponding author upon reasonable request.