Abstract
Tuber magnatum Picco (the Italian white truffle) is the most valuable and widely appreciated truffle. It is an ectomycorrhizal fungus known to associate with many broadleaf tree species. However, its mycorrhizae are rarely observed in the field, suggesting possible alternative symbiotic strategies, such as endophytism with non-ectomycorrhizal plants. In order to test potential endophytic interactions of T. magnatum with wild plants, a combination of polymerase chain reaction (PCR) and Fluorescence In Situ Hybridization (FISH) approaches were used. Specific FISH probes for T. magnatum were designed, tested in vitro on hyphae and/or ectomycorrhizae, and selected for their specificity. These probes were then used on a wide variety root samples of wild plants collected from three T. magnatum production areas in Italy and previously tested for the presence of T. magnatum mycelium using PCR-specific primers. Molecular analyses detected the presence of T. magnatum in 21 of 100 plant samples analyzed. FISH analysis confirmed the extracellular presence of active T. magnatum hyphae inside the root system of Carex pendula Huds plant. This study provides the first evidence of T. magnatum acting as an endophyte in an herbaceous plant. The newly designed, highly specific T. magnatum FISH probes can be used for further investigations to confirm the endophytic tendencies of T. magnatum and to understand their influence on the life cycle and biology of this fungus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00248-025-02542-z.
Keywords: FISH, Endophytism, Non-ectomycorrhizal host plants, Truffle ecology
Introduction
Among many fungi fruiting hypogeously, true truffles refer to all fungi belonging to the genus Tuber within the family Pezizaceae, phylum Ascomycota [1, 2]. Approximately 180–220 species have been estimated in the genus [3], but only a few produce ascomata with significant gastronomic and economic value: the European species Tuber magnatum Picco (the Italian white truffle), Tuber melanosporum Vittad. (the Perigord black truffle), Tuber aestivum Vittad. (the Summer truffle), and Tuber borchii Vittad. (the Bianchetto truffle) are the most sought-after, with prices ranging from €30 to €6,000 per kilogram, depending on the species, size of the ascomata, season, ripeness, and availability. Among these, T. magnatum stands out for its intense aroma and unique flavor, making it the most prized truffle. Consequently, retail prices for T. magnatum in Italy are higher than for any other truffle, placing it among the world’s most expensive foods [4].
Tuber magnatum is an ectomycorrhizal fungus known to associate with many broadleaf tree species, particularly Populus spp., Quercus spp., and Tilia spp. [5]. In Italy, numerous cultivation attempts using inoculated plants from nurseries were undertaken from the late 1990 s, yielding variable and sporadic results [4, 6]. Notably, all these plantations were established in areas where T. magnatum naturally occurred. Considering this, it cannot be ruled out that production in the early T. magnatum plantations was a spontaneous event resulting from secondary local inoculation, rather than a direct result from planting inoculated plants. Only recently, a T. magnatum orchard established in France outside its natural range in 2015 [7] yielded the first ascoma production [8]. This undoubtedly confirmed the possibility of cultivating this Tuber species by planting trees that had been previously inoculated in greenhouses.
Interestingly, T. magnatum mycorrhizae can be obtained under greenhouse conditions [9–11], but they are rarely found in the field, even in productive places [12, 13]. Despite the rarity of its mycorrhizae, T. magnatum mycelium is consistently present and extensively colonizes the soil in the productive areas [14, 15]. The ectomycorrhizal habit of T. magnatum is also suggested by its limited capacity for saprotrophic metabolism of its genome and the challenges in isolating its mycelium in vitro [4, 16], characteristics common among many mycorrhizal fungi [17–19].
Compared to mycorrhizal fungi, endophytes produce no clear external morphological features, are often less abundant in plant tissues, and do not strongly contribute to host nutrition [20]. Recently, other Tuber species such as T. melanosporum, T. aestivum, and T. borchii have been shown to develop as endophyte within non-ectomycorrhizal plants including orchids [21–27]. Their role in Tuber biology is still not clear, but some authors speculated that orchids and other herbaceous species could be involved in Tuber spp. life cycle acting like reservoir of the paternal genotype [28, 29] although only maternal genotype was found in herbaceous roots to date [26]. Taschen et al. [30] documented that T. melanosporum developed more efficiently (with higher mycelial abundance) in rhizotrons containing non-ectomycorrhizal plants, with negative effects on the growth of these plants. Other authors found opposite results with a decrease of T. melanosporum mycelium and ectomycorrhizae in a dual cultivation of truffle mycorrhized plants with herbaceous or aromatic plants [31, 32]. In the field, T. melanosporum mycelium was not statistically affected by the presence of aromatic plants [33]. Our current knowledge of the interaction between truffles and these non-ectomycorrhizal plants is limited, and it is not known whether or not such endophytism is important in the life cycle of truffles. It cannot be excluded a sort of nutritional exchange between these plants and the Tuber mycelium, including carbon extraction from Tuber spp. by orchids [34] or providing of N to the plant [26]. However, it is a challenging task to distinguish the carbon flux originating from ectomycorrhizal plants, which is typically large and consistent [35–37], from that of non-ectomycorrhizal endophyted hosts where it varies widely among species [38], as both types of hosts are simultaneously colonized by truffle mycelium.
Most of these studies relied on polymerase chain reaction (PCR) or next-generation sequencing (NGS) methods to identify Tuber endophytisms. Notably, T. melanosporum endophytism was confirmed in one study through Fluorescence In Situ Hybridization (FISH) targeting cytoplasmic ribosomes, which demonstrated endophytism within the roots and excluded simple rhizoplane colonization [26]. FISH is a valuable tool for investigating the spatial distribution and interactions of microorganisms within complex environmental samples. By utilizing nucleic acid probes specific to ribosomal RNA, FISH enables direct visualization of living microorganisms, including fungi, within their natural habitats, providing critical insights into their ecological roles and interactions [39–43]. Recent advancements, such as confocal laser scanning microscopy (CLSM) and multicolor FISH using extended fluorophore sets, have enhanced its ability to detect multiple microbial taxa simultaneously [44–47]. Applied to fungal communities, these methodologies can provide detailed insights into their spatial distribution in natural matrices beyond molecular evidence. Furthermore, optimized protocols for sample fixation and hybridization have improved the resolution and accuracy of bacterial and fungal detection in highly complex matrices like soils and rhizospheres [42, 48–51]. In fungal ecology, FISH is particularly valuable for elucidating the structure and function of ectomycorrhizal fungi. However, its application to label fungal hyphae directly has been limited. Instead, most studies so far have focused on fungal interactions with bacteria, providing insights into belowground ecosystem dynamics [52–54].
This study aims to investigate the potential interaction of T. magnatum mycelium with wild plants, mainly herbaceous, collected in the field, combining PCR and FISH approaches. Specifically, the objectives were (1) to collect ectomycorrhizal and non-ectomycorrhizal plants from T. magnatum production areas; (2) to detect molecularly the presence of T. magnatum mycelium in the soil and within the roots of these plants, using species-specific primers; (3) to design and select specific FISH probes targeting T. magnatum mycelium and ectomycorrhizae; and (4) to use the selected probes to confirm the presence of T. magnatum inside roots resulted positive through species-specific PCRs.
Material and Methods
Sites of Study
Three natural fruiting sites (Supplementary Table S1) were selected to investigate the potential endophytic behavior of T. magnatum with ectomycorrhizal and non-ectomycorrhizal host plants that share its habitat. The first site was a rural area near Città della Pieve (subsequently named CDP, Perugia, Umbria, Italy), at an elevation of approximately 430–455 m a.s.l. This hilly region is characterized by alternating scrubland and cultivated fields. The putative host plants in this area include Corylus avellana L., Ostrya carpinifolia Scop., Populus nigra L., Populus alba L., Quercus cerris L., Quercus pubescens Willd., Quercus robur L., and Salix spp. The second site, the Panfilia Forest (subsequently named PF), is an ancient lowland forest (30 m a.s.l) located between the provinces of Bologna and Ferrara, near Sant’Agostino (Ferrara, Emilia Romagna, Italy). The predominant putative host plants in this area include C. avellana, P. alba, Q. robur, Salix spp., and Tilia cordata Mill. The third site is an Apennine mountainous area near Montefalcone nel Sannio (subsequently named MNS, Campobasso, Molise, Italy) at 345 m a.s.l. This site consists of a mesophilic deciduous forest, where possible symbiotic partners include C. avellana, O. carpinifolia, P. alba, Q. cerris, Q. pubescens, and Q. robur. Additional characteristics of the sites (soil, average precipitation and temperatures) are reported in Supplementary Table S1.
Ascomata, Soil, and Wild Plants Sampling
In January 2022, the study sites were visited with trained dogs in order to detect the T. magnatum production patches. The truffle hunters indicated T. magnatum fruiting points (FPs) based on previous harvests or picking up new ascomata for each site and their positions were georeferenced (GPS coordinates of each ascoma collection point were recorded but not showed at the request of truffle hunters). Six, three, and one FPs were respectively found for CDP, PF and MNS.
For each FP, nine equidistant soil cores were collected with 1.6 cm diameter disposable polyvinyl tubes along two diagonal lines in a 2 m2 area centered in FPs. The upper layer of organic matter was removed, and the soil was sampled down to a depth of 20 cm. The soil was maintained at 4 °C and extracted from the tubes within 24 h. Organic debris was removed, then the soil was sieved using 2-mm mesh and lyophilized. Successively, the soil was ground and stirred in a mortar. All the tools were washed and then sterilized with 5% bleach solution. The nine soil samples of each FP were mixed and the total DNA was extracted from three samples of 0.5 g taken from the composite sample according to Iotti et al. [55]. Then, the DNA was purified using the Nucleospin Plant II kit (Macherey–Nagel, Germany) and preserved at − 80 °C pending molecular analysis.
The mycelial presence in each selected FP was verified by amplification of the T. magnatum DNA in the soil with a nested PCR approach. The fungal DNA was first amplified with the fungal universal primers ITS1f/ITS4 [56, 57] (Supplementary Table S2), then the amplified fragments were subjected to a second PCR round by using the specific primers TmagI-TmagII [58] (Supplementary Table S2).
Since the Panfilia Forest is renowned as one of the most productive T. magnatum forests in northern Italy [59, 60], it was selected as representative fruiting site to investigate the most suitable season to detect root colonization by T. magnatum in ectomycorrhizal and non-ectomycorrhizal plants. Thus, in winter, spring, summer, and autumn 2022, for each FP where T. magnatum DNA was detected in soil samples, plant samples were collected from the nodes of a 1 × 1 m grid with an approximately 20-cm pitch placed on FPs (Fig. S1). The 10 plants closest to the FP were selected for each FP. The collected plants were identified, and the epigeal part was separated and preserved in the herbarium “Centro di Micologia” of Bologna (CMI-UNIBO). The roots were washed under tap water and analyzed on the same day. The same sampling procedure was applied in the other two fruiting sites in spring 2023, which was inferred as the most suitable season from the analysis of the PF root samples.
PCR Detection of T. magnatum in Plant Roots and RNA Fixation Process
Three thin and healthy roots were selected from each plant sample and cut into three 1-cm fragments. The proximal and distal fragments were used for DNA extraction. They were superficially sterilized by immersion in 70% ethanol solution for 5 min, in 0.9% sodium hypochlorite solution for 15 s and then rinsed three times in sterile water following the protocol of Cao et al. [61]. This protocol was previously applied to detect the endophytic root colonization of T. aestivum and T. melanosporum in non-ectomycorrhizal plants [26]. To check for the presence of T. magnatum DNA inside the roots, total DNA extraction from proximal and distal fragments were completed as previously reported by Graziosi et al. [27] and nested PCRs were performed using specific primers for T. magnatum (TmagI-TmagII, [58]).
The 1 cm central fragment was put into a 4% paraformaldehyde in 1 × Phosphate Saline Buffer (PBS 1 ×), added with 0.1% of Tween20. All the solutions were prepared with Ultrapure Water, Milli-Q®. The fixing solution was stored on ice before sample immersion. Three successive vacuums were then applied. The last vacuum was maintained for 1 h at room temperature. Then the root pieces were rinsed three times in PBS 1 × for 10 min and immersed consecutively in ethanol 10%/PBS 1 × solution for 30 min, in ethanol 30%/PBS 1 × solution for 30 min and, at the end, in ethanol 50%/PBS1 × solution for 30 min. These last immersion steps were conducted at room temperature. The samples were preserved at − 20 °C pending FISH experiments.
Design of rRNA Specific Probes for T. magnatum Involved into FISH and Microscopy Observations
The first step was the in silico design of new probes based on the alignment of SSU ribosomal DNA (rDNA) sequence of T. magnatum and other Tuber spp., alongside sequences from various species in the Pezizales and other orders obtained from genomic data published in GenBank (NCBI, [62]) and Mycocosm (The Fungal Genomics Resource-JGI, [63]) databases (Supplementary Table S3). SSU rDNA sequences were aligned using the Multalin algorithm [64] (Fig. S2) to find variable domains for developing T. magnatum species-specific or Tuber genus-specific probes.
Four regions exhibiting polymorphisms were retained as a basis for designing probes targeting Tuber spp. or T. magnatum, including closely related species in the Tuberaceae family. Probes were evaluated for their accessibility [65] and in silico specificity by using iterative BLAST searches with low stringency algorithm parameters in the GenBank and SILVA databases [66]. Finally, the number of mismatches of the designed probes, the in silico accessibility, and specificity were taken into account to choose four probes named T.mag185, T.mag645, T.mag1313, and T.mag1647, each presenting a different degree of specificity to T. magnatum (Supplementary Table S4). All FISH-probes were commercially synthesized by Biomers.net (Ulm/Donau, Germany) including 5′-end labeled with Atto fluorochromes and stored in sterile DNA-grade water at − 20 °C.
To highlight genetic diversity between SSU sequences of T. magnatum and other closely related fungal species, a phylogeny was inferred by the Maximum Likelihood (ML) option implemented in MEGA11 software [67]. ML analyses were performed with 1000 throughout bootstrap replicates (100 runs), applying the models of nucleotide substitution K2 + G + I (Fig. S3).
Collection and Fixation of Mycelial Samples and Ectomycorrhizae
To evaluate the in vitro sensitivity and specificity of the FISH probes and to establish confocal acquisition parameters, strains of several fungal species were selected based on their genetic closeness to T. magnatum by assessing the similarity of their SSU rDNA sequences. Closely related Tuber spp. and outgroup species in the order Pezizales included in the analysis were: T. aestivum (strain 5230), T. borchii (strain IT1) Tuber brumale Vittad. (strain 5256), T. melanosporum (strain TME2, [68]), and outgroup species belonging to Pezizales order, Morchella eximia Boud. (strain MD14) and Dissingia leucomelaena (Pers.) K. Hansen & X.H. Wang (strain DILE1). Several of these fungal species were chosen also because they often share the same habitat of the white truffle, occasionally colonizing the roots of the same host tree [60].
All selected pure cultures were cultivated on M9 Minimal Medium (M9 MM, [69]) at 22.5 °C in the dark. Each pure culture, excepted T. magnatum, was previously isolated from ascomata by excising and aseptically transferring 1–2-mm fragments of fungal tissue from the inner part of the ascoma onto M9 MM. Tuber magnatum sample (herbarium number 5377) was taken from the gleba tissue of a young ascoma provided by Truffleland s.r.l (Sant’Anatolia di Narco, Perugia, Umbria, Italy). Only for M. eximia, a diluted spore suspension in sterile water was employed, added to M9 MM with antibiotics (200 μg/mL of streptomycin, ampicillin, and chloramphenicol). The ascomata were then dried and deposited in the herbarium of the “Centro di Micologia” of Bologna (CMI-UNIBO). Once fungal elements were discernible to the naked eye on the Petri dishes, agar plugs containing hyphae were harvested and fixed as previously reported and finally stored at − 20 °C in 50% ethanol-PBS 1 × until use. The same process was applied to T. magnatum gleba tissue pieces.
Moreover, Tuber spp. ectomycorrhizae from inoculated seedlings were included in this preliminary phase to evaluate the efficiency of the FISH probes on fungal hyphae interacting with plants in semi-natural conditions. Young T. magnatum, T. aestivum, and T. melanosporum ectomycorrhizae were individually sampled from Q. pubescens inoculated plants produced by Robin Pépinières (St Laurent du Cros, France). Each T. magnatum ectomycorrhiza was cut in half with a razor blade, and one half was immediately fixed in a 4% paraformaldehyde solution. After 4 h of fixation on ice, the ectomycorrhizae were washed three times in PBS 1 × solution, partially dehydrated in an ethanol series and then stored at − 20 °C in 50% ethanol/PBS 1 × until use. The other half was used for DNA isolation using Extract-N-Amp Tissue DNA extraction (Sigma, Merck KGaA, Germany). DNA extraction was performed according to manufacturer instructions. For T. magnatum ectomycorrhizae, the internal transcribed spacer (ITS) was amplified using universal fungal primers ITS1 F and ITS2 (Supplementary Table S2) [57], and sequenced by Eurofins (Ebersberg, Germany). BLASTn against the NCBI-nr database was used to confirm the species identity. The sequences were deposited in the GenBank under the following accession numbers: PQ818236 to PQ818245.
Test of Newly Designed Probe In Vitro Specificity
The FISH experiment was conducted similarly to Laurent-Webb et al. [43]. Rapidly, samples of both ascomata and mycelium were gradually dehydrated with an ethanol series (50%, 70%, 96%, 100%) in ultra-pure water and then rehydrated with an ethanol series (70%, 50%, 30%, 10%) in PBS-T (1 × PBS with 0.1% Tween 20), for 15 min each, ending with three 10-min PBS-T baths. All steps occurred at room temperature.
Samples were treated with digestion solution (25,000 U/mL Lyticase, 12.5 U/mL Chitinase in PBS 1 ×) for 15 min at 30 °C to weaken fungal cell walls. A pre-hybridization buffer (30% formamide, 5 × SSC, 0.1% Tween 20, 100 μg/mL tRNA, 0.2 × Denhardt’s solution) was then added, followed by a 30-min incubation at 46 °C. The buffer was replaced with a hybridization solution containing probes (0.35 pmol/µL of hybridization solution each), incubated for 2 h at 46 °C. After a 10-min wash (0.2 M Tris HCl pH 8, 0.005 M EDTA pH 8, 0.7 M NaCl, 0.1% SDS) and PBS 1 × rinse, samples were mounted in Slowfade Diamond Antifadent (Molecular Probes, Eugene, OR, USA, Thermofisher) solution containing 0.1% SR2200, a cell wall dye (Renaissance Chemicals, North Duffield, UK) and visualized with a confocal microscope (Zeiss LSM980, Carl Zeiss, Oberkochen, Germany) coupled to ZEN Blue 3.3 software (Carl Zeiss, Oberkochen, Germany). The acquisitions were performed in confocal mode. Fungal samples were co-hybridized with the universal eukaryote probe Euk516 and the four designed probes individually or with an anti-sense, non-binding probe (AS-Euk516 or AS-Rus899), each tagged with distinct fluorescent dyes (Supplementary Table S4), or undertaken the hybridization process without any probe addition as a control. The probe AS-Rus899 is specific to Russula spp. and Lactarius spp. Atto565 and Atto633 probes were excited at 561 nm and 639 nm, respectively, and detected with bandpass filters (570–630 nm, 640–720 nm). The cell wall dye SR2200 was excited at 405 nm and detected with a 420–477 nm filter.
In these co-hybridization experiments, probe sensitivity and specificity to bind to the 18S RNA target were evaluated in comparison to the positive signal obtained with the eukaryotic probe Euk516 [70]. Fluorescence intensity signal obtained for each sense probe was also compared with negative hybridization controls, i.e., FISH experiments realized with AS-Euk516, AS-Rus899 probes, without probes or FISH realized with sense probes on other fungal species than T. magnatum. These initial steps were used to calibrate laser power and photodetector gain parameters while minimizing tissue autofluorescence. Moreover, FISH experiments were conducted to verify the specific binding of T. magnatum FISH probes outside of in vitro conditions, on fungus-plant tissue of ectomycorrhizae. The hybridization protocol was the same as that applied to ectomycorrhizal and non-ectomycorrhizal plant root samples, as reported in the paragraph: “Hybridization and observation of T. magnatum hyphae in roots of wild plants.” Along with T. magnatum, T. aestivum and T. melanosporum ectomycorrhizae were added as negative controls. For each T. magnatum specific probe, three sets of independent FISH experiments were performed and each time the signal originated from eight Z stacks were compared for both every fungal species and FISH experiment. The hybridization signals were obtained and analyzed by using the same parameters (laser power and gain of detector) and were also used to image the control samples: samples without any probe or samples hybridized with the anti-sense probes.
Hybridization and Observation of T.magnatum Hyphae in Roots of Wild Plants
The T. magnatum specific T.mag1647 FISH probe was selected and used for FISH experiments applied on the roots from ectomycorrhizal and non-ectomycorrhizal host plants. FISH experiments on root samples of wild plants follow the same protocol, adapted from Laurent-Webb et al. [43]. Root fragments positive for T. magnatum through species-specific PCR were selected (TmagI-TmagII, [58], Supplementary Table S2). One PCR-negative root fragment for each of the PCR positive species was added to the experiment as control. Co-hybridization FISH experiments were done by using two probes targeting 18S rRNA simultaneously: the universal eukaryote probe Euk516 coupled with Atto-565 dye and newly designed T.mag1647 specific probe (Supplementary Table S4) coupled with Atto-633 dye. These fluorochromes were carefully chosen to reduce autofluorescence background of plant/fungal tissues [43].
Root samples were dehydrated using an ethanol series in ultrapure sterile water (50%, 70%, 96%, 100%) for 20 min per step at room temperature. They were immersed in pure methanol for 10 min at room temperature, refreshed, and stored at − 20 °C overnight. After two 5-min methanol washes at room temperature, the samples were rehydrated through a methanol series (70%, 50%, 30%, 10%) prepared in PBS-T, with each step lasting 20 min at room temperature. Samples were treated with a mix of plant and fungi cell-wall digestion cocktails [43] at 35 °C for 15 min to weaken cell walls, and rinsed three times in PBS-T at room temperature. They were then treated with 0.08 µg/µL of Proteinase K solution for 15 min at 37 °C. The reaction was stopped with 0.2% glycine prepared in PBS-T for 2 min, followed by a 30-min incubation in a post-fixative solution (4% paraformaldehyde in PBS-T). After three rinses in PBS-T, samples were prehybridized at 46 °C for 30 min, and then incubated with a hybridization buffer containing probes (0.35 pmol/µL) for 2 h at 46 °C. After a stringent 10-min wash at 46 °C in a buffer (0.2 M Tris–HCl pH 8, 0.005 M EDTA pH 8, 0.7 M NaCl, 0.1% SDS), samples were mounted in an antifade solution containing 0.1% SR2200 to visualize plant and fungi cell walls. Hybridized samples were stored at 4 °C until microscope observation.
Root samples were observed using a Zeiss LSM 980 confocal microscope equipped with EC-Plan Neofluar 10 ×/0.3 dry, W Plan-Apochromat 20 ×/1.0 water, and Plan-Apochromat 63 ×/1.4 oil objectives. Imaging employed the Airyscan 2 module in multiplex 4Y mode, with Atto565 and Atto633 probes excited at 561 nm and 639 nm, respectively, and detected with bandpass filters (570‒630 nm, 640‒720 nm). The cell wall dye SR2200 was excited at 405 nm and detected with a 420–477-nm filter. Fields of view were imaged with optical sections (Z-step: 0.15‒1 μm). Image analysis was conducted using ZEN Blue 3.5, ZEN 2.1 LITE (Zeiss), Vision4D 3.0.1 (Arivis AG, Germany), and FIJI-ImageJ softwares. The hybridization signals were obtained by using parameters (laser power and gain of detector) optimized during FISH experiments on pure fungi and were also used to image the control samples: samples without any probe or samples hybridized with the anti-sense probes.
Results
Endophytic Colonization Verification via PCR
The mycelium of T. magnatum was successfully detected in the soils of all FPs by PCR with T. magnatum species-specific primers. A total of 100 plant samples (60 from CDP, 30 from PF, and 10 from MNS; Table 1, Supplementary Table S5) were collected in the spring of 2022 and 2023 at fruiting points from the three fruiting sites. Most of the collected plants were non-ectomycorrhizal (97), while only three seedlings of ectomycorrhizal species were sampled (C. avellana, P. alba, and T. cordata).
Table 1.
Results of species-specific PCRs for Tuber magnatum Picco on plant samples collected in this study in spring 2022 and 2023
| Fruiting sites | Fruiting points (FPs) | Analyzed plant samples (analyzed species) | Positive plant samples | Percentage positive plant samples | Positive speciesa |
|---|---|---|---|---|---|
| Città della Pieve (PG) | 6 | 60 (23) | 15 | 25 | Arum italicum {2}, Sison amomum, Taraxacum dissectum, Galium verrucosum, Hedera helix {3}, Primula vulgaris {3}, Ranunculus bulbosus, Urtica dioica {2}, Viola odorata |
| Panfilia Forest (FE) | 3 | 30 (10) | 5 | 17 | Acer campestre, Carex pendula {4} |
| Montefalcone nel Sannio (CB) | 1 | 10 (6) | 1 | 10 | Ajuga reptans |
| Total | 7 | 100 | 21 |
aWithin brackets, numbers of plant samples with more than one positive sample
Among non-ectomycorrhizal plants, Asteraceae showed the highest species richness (Fig. S4), with seven taxa, followed by Apiaceae, Geraniaceae, Ranunculaceae, and Rosaceae with two species each. All other families exhibited just one species. In terms of samples, the families Arialaceae, Cyperaceae, and Asteraceae were the most abundant with 15, 12 and 12 plant samples, respectively. Primulaceae, Ranunculaceae, Araceae, Rosaceae, Geraniaceae, Violaceae, Urticaceae, Apiaceae, Plantaginaceae, and Lamiaceae showed a decreasing number of plant samples, from nine to two, while the other families accounted for just one plant sample.
The mycelium of T. magnatum was detected inside plant samples only in spring, while no sample from the other seasons (47% of the total plant samples) showed positive results. In detail, it was found in 21 out of the 100 examined plant samples, considering as positive the samples where at least one root gave specific amplicons (Table 1). All the positive samples were from non-ectomycorrhizal plants whereas the ectomycorrhizal plants gave no positive results. The highest number of positive plant samples were found in CDP with 15 samples (25% of total plant samples) followed by PF with five samples (17%) and MS with just one sample (10%).
The plant families with the highest number of positive samples were Cyperaceae (4), Araliaceae (3), Primulaceae (3), Araceae (2), and Urticaceae (2). In contrast, Apiaceae, Asteraceae, Lamiaceae, Ranunculaceae, Rubiaceae, Sapindaceae, and Violaceae yielded only one positive sample each. All other families provided no positive samples. See Supplementary Table S5 for a detailed report.
Among the 39 examined plant species, 12 showed positive results (Table 1, Fig. 1). In details, Acer campestre L. and Galium verrucosum Huds. were represented by just one collected plant sample resulted positive by species-specific PCR (100%). Other species had just one positive plant sample, but more than one plant was collected: Aiuga reptans L. (2 plant samples in total, 50% positive plant samples), Ranunculus bulbosus L. (4, 25%), Sison amomum L. (2, 50%), Taraxacum dissectum (Ledeb.) Ledeb. (2, 50%), and Viola odorata L. (3, 33%). Two or more positive plant samples were detected in four species: Arum italicum Mill., (6 plant samples in total, 33% positive plant samples) Carex pendula Huds. (12, 33%), Hedera helix L. (7, 43%), P. vulgaris Huds. (3, 33%), and Urtica dioica L. (2, 67%) (Fig. 1).
Fig. 1.
Percentual distribution of positive and negative plant samples collected in Spring 2022 and 2023 after PCR with Tuber magnatum Picco specific primers
Selection of Probes by In Vitro Pre-tests
The strategy of targeting 18S RNA for probes design is based on its high abundance in cells, which greatly enhances its detection. The design of the FISH probes was carefully tailored to achieve either a broad or narrow spectrum of specificity, to ensure the target recognition. The SSU sequences of Tuber species, including T. magnatum, exhibit an exceptionally high degree of identity, not only among themselves but also with closely related fungi such as Choiromyces spp. This high sequence conservation posed a significant challenge for probe design, as it left very few regions with polymorphisms suitable for differentiation. Consequently, the available regions for probe targeting were limited, influencing the specificity range of the probes. The designed probes demonstrated a specificity spectrum that was inherently constrained, being more or less selective for T. magnatum. In our design and based on in silico analysis, two probes were expected to be highly specific to T. magnatum (T.mag185 and T.mag1647), while probe T.mag1313 would include a subset of other Tuber species (such as T. magnatum, T. borchii, and T. puberulum) and probe T.mag645 should recognize almost all Tuber spp. as well as some Choiromyces spp. (Fig. S2).
During our pre-test of FISH probes, all the four newly designed probes (Supplementary Table S4) showed a positive binding with T. magnatum ascocarp (Fig. 2, Fig. S5). The correct hybridization signal of these probes or the general Euk516 probe resulted in clearly colored patches inside the cytoplasm (Fig. 2). Two non-sense probes were used in our study: AS-Euk516 and AS-Rus899, which are inverted and complemented probes of the generalist Euk516 probe or of a Russula-specific probe [43], respectively. None of these anti-sense probes showed any hybridization signal. Thus, it did not highlight the hyphal cytoplasm (Fig. 2; Fig. S5), indicating the absence of non-specific hybridization and excluding also a random binding of the fluorochrome. Moreover, in the hybridization experiments conducted without probe addition, no fluorescence was detected at the laser power and detector gain settings used to image the sense probes.
Fig. 2.
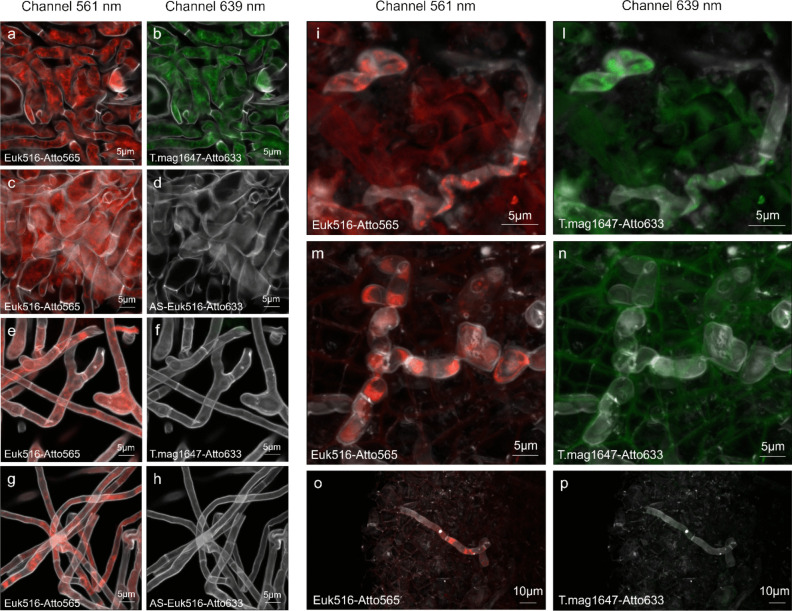
Evaluation of the Fluorescence In Situ Hybridization (FISH) probe T.mag1647, targeting the 18S ribosomal RNA of Tuber magnatum Picco, was conducted using either ascomata or pure cultures. In the figure is showed the comparison between the ascoma of T. magnatum (a–d, left panel), a pure culture of Tuber melanosporum Vittad. (e–h, left panel), and ectomycorrhizae of T. magnatum (i, l), Tuber aestivum Vittad. (m, n), and T. melanosporum (o, p) (right panel). The co-hybridizations were performed on T. magnatum ascoma (herbarium number 5377), T. aestivum (strain 5230), and T. melanosporum (strain TME2). The mycorrhizae were provided by Robin Pépinières nurseries (St. Laurent du Cros, France). Co-hybridization experiments were performed with a generalist probe targeting eukaryotic cells, Euk516 (in red), as a positive control. The positive control showed high signal intensity in the cytoplasm of fungal cells (a, c, e, g, i, m, and o), in combination with the T.mag1647-specific probe (b, d, f, h, l, n, and p). An anti-sense probe, AS-Euk516, was used as a negative control to verify the absence of non-specific binding of the probe or random fluorochrome binding. For each co-detection, 2D images from individual channels are presented. Fluorochrome Atto565 and Atto633 were excited at 561 nm and 639 nm, respectively, and detected with bandpass filters (570‒630 nm, 640‒720 nm). The cell wall dye SR2200 was excited at 405 nm and detected with a 420–477-nm filter
Images collected during the three sets of independent FISH experiments on pure cultures were compared and analyzed (Fig. S5) and images with higher magnification and better resolution are presented with the specific probe T.mag1647 on T. magnatum and T. melanosporum (Fig. 2).
The hybridization efficiency of each FISH probe is shown in Fig. S5. In particular, the probe T.mag185 (Fig. S5IV) demonstrated a high specificity for T. magnatum, and exclusively labeled the target species. This is evidenced by the overlapping signals of the Euk516 probe and the T.mag185 probe when applied to T. magnatum gleba tissue (Fig. S5IV-A1) and the absence of random fluorochrome binding with an anti-sense probe (Fig. S5IV-F6). The SSU alignment supported this result, as it revealed at least one single-nucleotide polymorphism (SNP) in all other sequences used for the probe design. However, the probe signal intensity appeared weaker compared to other probes with a similar level of specificity.
In contrast, the probes T.mag645 (Fig. S5III) and T.mag1313 (Fig. S5II) produced more intense signals. Nevertheless, they also recognized other Tuber spp. Specifically, the T.mag645 probe detected T. borchii, T. brumale, and T. aestivum (Fig. S5III-B2, C3, D4), as well as species of the Tuber and Choiromyces (not tested experimentally) genera, displaying an extended hybridization spectrum (Fig. S2). This probe shows no complementarity and hybridization with more distant species (Fig. S2, Fig. S5).
The T.mag1313 probe was much more specific than T.mag645 and showed a positive binding on T. magnatum and T. borchii only (Fig. S5II-B2). However, a very weak signal was also observed on T. aestivum (Fig. S5II-D4). For T. magnatum, the signal was also detected but less pronounced than with the other probes, indicating that 18S region has a lower hybridization efficiency. As with the T.mag645 probe, the SSU alignment explained these results since this probe precisely matched the T. borchii sequence and exhibited only a single SNP with T. aestivum. This likely also explained the very faint signal observed (Fig. S2).
Finally, among the four probes studied, T.mag1647 (Fig. 2, Fig. S5) appears particularly promising for our study, providing a clear hybridization signal, a high degree of specificity for T. magnatum without highlighting other Tuber spp. or species outside of the Tuber genus (Fig. 2, Fig. S5I-A1, B2, C3, D4, E5). During the FISH experiments, this probe showed a better hybridization signal than T.mag185. The T.mag1647 SSU sequence confirmed this specificity, with at least three SNPs differentiating it from other SSU fungal sequences. None of the probes gave positive signal in the hyphae of M. eximia or D. leucomelaena.
Therefore, we decided to test the T.mag 1647 probe on ectomycorrhizae to further demonstrate its specificity and validate hybridizations on plants. For all the examined ectomycorrhizae of T. magnatum, T. melanosporum, and Tuber aestivum, the binding with the generalist eukaryotic probe Euk516 occurred rarely, indicating that many hyphae of the mantle were inactive. The high specificity of the T.mag1647 probe was also confirmed in T. magnatum active hyphae on the ectomycorrhizal mantle (Fig. 2).
FISH Evidence of Endophytic Colonization
After observing all the PCR positive plant samples through FISH and confocal laser scanning microscopy, we successfully observed generic fungal hyphae in all the root samples highlighted by the universal Euk516 probe (Fig. 3a, b). In fact, it was possible to recognize hyphae of endomycorrhizal fungi (Glomeromycota) inside the roots, along with their characteristic arbuscules. However, the latter hyphae were not highlighted by the T.mag1647 T. magnatum-specific probes, supporting the specificity of T.mag1647.
Fig. 3.

Observation of plant roots by confocal microscopy after Fluorescence In Situ Hybridization (FISH) with the Tuber magnatum Picco specific probe T.mag1647 (b, d; green) and the eukaryotic Euk516 probe (a, c; red) as a positive control. a, b Endomycorrhizal fungi found into a Carex pendula Huds. root sample with no colocalization of Euk516 and T.mag1647 probes. c, d Colocalization of Euk516 and T.mag1647 probes into a C. pendula roots. The arrows indicate hyphae bonded just with the Euk516 probe. Fluorochrome Atto565 and Atto633 were excited at 561 nm and 639 nm, respectively
In most of the roots examined by FISH with the Euk516 probe, many hyphae were not active (no cytoplasmic labeling). Hyphae specifically marked by the T. magnatum-specific probe T.mag1647 were observed in only positive fragments from C. pendula (Fig. 3c, d). The root showed in Fig. 3c, d is colonized by two distinct species of endophytic fungus, one of which is T. magnatum (perfect colocalization of Euk516 and T.mag1647 probes fluorescence), the other showing only fluorescence from the Euk516 probe (white arrows). Nevertheless, unvital hyphae characterized by suspected Tuber-like morphology, as the hyphal thick diameter, septa presence, and absence of clamp-junctions [4, 68], were observed in the inner tissues of PCR-positive plant roots. The overlapping signal between the Euk516 and T.mag1647 probes on the same hyphal region together with their contemporary absence in other sections confirmed the correct binding of the probe. No T. magnatum hyphae were observed in fragments negative for T. magnatum specific PCR (n = 12 observed in total). Tuber magnatum hyphae were localized within the roots, probably in the apoplast between cells that were lined by the hyphae and appeared intact.
Discussion
In this study, specific FISH-probes designed for T. magnatum firstly highlighted the presence of this truffle species as an endophyte inside the root of a non-ectomycorrhizal plant raising new questions about its biology. The seasonal sampling of plants conducted in PF has provided key insights into the distribution and dynamics of T. magnatum mycelium. Detection of T. magnatum by PCR in roots was confined to spring samples from a limited number (21%) of non-ectomycorrhizal plants, mainly herbaceous with the exception of A. campestre. No positive PCR was obtained from ectomycorrhizal plant root samples. However, we cannot exclude possible endophytic interactions with ectomycorrhizal plants, considering the limited number of samples examined. The PCR detection of T. magnatum only in plant samples collected in spring aligns with previous observations that T. magnatum reaches the peak of abundance and the wider distribution of soil mycelium in this season [15]. This further supports that T. magnatum mycelium is more active during spring, when the soil water potential is high [71] and allows colonization of the soil and the roots of non-ectomycorrhizal plants. In contrast, other truffle species like T. aestivum and T. melanosporum were detected inside non-ectomycorrhizal roots in autumn and summer, respectively [25, 26]. This may be related to the different periods of maximum mycelial growth in the soil and, consequently, to ascoma production. Nevertheless, the growth of T. melanosporum mycelium can vary significantly depending on the climatic conditions of different years; Queralt et al. [72] observed that the mycelium of T. melanosporum began growing in the summer, reached its peak development in the winter, and then declined sharply.
The highly specific FISH-probes designed for T. magnatum demonstrated a significant affinity since they successfully labeled the hyphae of T. magnatum gleba tissue inside the examined ascoma. Although microscopy and FISH of thick plant tissues are challenging [73], T. magnatum specific probes were also able to identify hyphae in the ectomycorrhizal mantle of this species. Interestingly, just a few hyphae appeared viable, similar to findings in T. aestivum and T. melanosporum ectomycorrhizae, indicating a selective viability among mantle hyphae and/or a possible rapid vitality loss along mantle formation. This characteristic was also observed in other ectomycorrhizal species, where hyphae of the outer layers of the mantle were mostly inactive [42, 74]. Similarly, the endophytic hyphae of T. magnatum colonizing roots during spring might rapidly lose their vitality inside the plant tissue. Alternatively, endophytic hyphae have low metabolic activity inside host tissues and reach undetectable levels, which could explain both the restricted season during which they can be found inside the roots and the discrepancy between the number of PCR-positive results and FISH observations. In fact, FISH applied to herbaceous plants yielded only one T. magnatum positive detection on C. pendula roots, whereas 21 positive plant samples were found using species-specific PCRs. Of course, difficulty of visualization (including the fact that PCR with T. magnatum specific primers were carried out on a different, although close, piece of root) can also contribute to this discrepancy. Moreover, contamination of the rhizoplane by T. magnatum mycelium cannot be entirely ruled out; however, the external mycelium of T. magnatum has never been observed on either PCR-positive or PCR-negative root fragments when using the FISH technique. This further supports evidence of a loss of hyphal vitality within the PCR-positive roots although it also warrants further exploration.
It is notable that the only positive plant species, C. pendula, is one of the most prevalent herbaceous plants in the PF [75]. Other species of the same genus, Carex muricata L. and Carex flacca Schreb., were found to be associated with T. aestivum and T. melanosporum, respectively [25, 76]. Based on molecular methods, these truffles share some endophytic partners of the same species or genus with T. magnatum. For instance, H. helix appears capable of hosting T. aestivum, T. melanosporum, and T. magnatum [25, 26]. Similarly, the genus Viola is shared among these three truffle species. However, most of the T. magnatum endophytic host plant species found in this study are not shared with the other two truffles in the current (limited) knowledge [25, 26, 76]. Only T. aestivum and T. magnatum share the genus Taraxacum as a non-ectomycorrhizal host.
Without excluding the possible influence of annual weather variations and specific fungal ecological characteristics, T. magnatum appears to be more restrictive as an endophyte within non-ectomycorrhizal host plant roots, interacting with a reduced number of individuals and plant species (21% of the collected plant samples and 31% of the examined species). Other Tuber species, such as T. melanosporum and T. aestivum, were more frequent and widespread within non-ectomycorrhizal plant species [25, 26, 76]. For instance, T. aestivum was positively detected in 54% of collected plant samples and 80% of examined plant species [26]. Regarding T. melanosporum, Schneider-Maunoury et al. [25] reported a very high percentage of non-ectomycorrhizal plant colonization, with 79% of collected plant samples and 90% of plant species. In a subsequent study, T. melanosporum was detected in 53% of the collected plant samples and 90% of the examined plant species by PCR, and its presence was subsequently confirmed by FISH in the four most colonized species [26]. The endophytic ability of T. magnatum further supports the idea that such a trait is widespread among Tuber spp., as well as other ectomycorrhizal fungi [21–27, 43, 77], and is also congruent to the hypothesis that endophytism may have predated the emergence of ectomycorrhizal abilities in fungi (the Waiting Room Hypothesis, where endophytism is a waiting room to closer symbiosis [26, 78]).
A possible explanation for the reduced endophytic abundance of T. magnatum compared to other truffle species may lie in the differences in mycelial biomass and in the vertical soil distribution of its mycelium. The mycelial biomass density of T. aestivum and T. melanosporum in soil appears to be up to three orders of magnitude higher than that of T. magnatum [15, 24, 72]. In particular, T. aestivum seems to produce relatively dense soil mycelium compared to other ectomycorrhizal fungi [15, 24]. Furthermore, the density of T. aestivum mycelium on the root surface of ectomycorrhizal plants appears to be very high and may be comparable to that found in radical portions provided with ectomycorrhizae. This type of association between T. aestivum mycelium and root surfaces other than ectomycorrhizae could explain its tendency to also interact with the roots of non-ectomycorrhizal plant species.
Regarding the vertical distribution of the mycelium, as suggested by Ceruti [79] and Iotti et al. [71], the mycelium of T. magnatum may extend deeper into the soil than that of other ectomycorrhizal species [80–83], particularly under stress conditions such as very dry winters or summers [84]. During the sampling years, especially in 2022, winter seasons were exceptionally dry [85, 86], which may have caused the mycelium to develop at greater depths. The roots of non-ectomycorrhizal plant species usually grow in the upper soil layer, and T. magnatum mycelium might encounter difficulties in reaching them through dry soil layers.
In summary, our results seem to support the idea that T. magnatum mycelium exists within non-ectomycorrhizal plant roots, but it may be rare. Mycelial biomass within herbaceous roots is likely insufficient to play a major role in supporting the nutritional requirements of T. magnatum to colonize the soil and even less to fruit. While symbiotic Bradyrhizobia may contribute nitrogen to the T. magnatum mycelium and ascoma [16, 87], the nutritional role of non-ectomycorrhizal wild plants in carbon uptake remains unclear and requires further investigation. Nevertheless, the endophytic colonization of non-ectomycorrhizal plants by T. magnatum may be involved in other ecological mechanisms, such as protection of hyphae or habitat exploration. On the latter issue, Zampieri et al. [14] molecularly identified the T. magnatum mycelium in soil up to 100 m from the nearest productive tree. This considerable distance could have been traversed over a long period, with non-ectomycorrhizal plants serving as temporary refuges during the mycelial migration.
The highly specific FISH probes designed in this study open up new possibilities for further investigating the relationships between T. magnatum mycelium and plants, soil, and other organisms, emphasizing the role of vital hyphae while excluding those detected by relic T. magnatum DNA. Further investigations will be necessary to confirm the endophytic tendencies of T. magnatum and to understand the potential role this association plays in its life cycle and biology. It remains unclear whether the endophytism of T. magnatum is beneficial for the fungus, the host plant, or both. Assuming a beneficial interaction from endophytic colonization by T. magnatum in both ectomycorrhizal and non-ectomycorrhizal host plant species, this could support the introduction of these plants to enhance white truffle plantations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Truffleland s.r.l for the T. magnatum ascoma providing and for their help in localizing truffle grounds. We thank Mr. Matteo Rossi, Association “Il Tartufo aps”, Mr. Alessandro Giuggioli and “Quintosapore agricultural company” for their help in white truffle fruiting point localization and ascoma sampling. We would like to thank Bruno Robin for providing the mycorrhized T. magnatum plants. Image acquisition and/or image analysis were performed at the IBPS Imaging Facility (I2PS). The authors greatly acknowledge Chloé Chaumeton and France Lam of the IBPS Imaging Facility. The IBPS Imaging facility is supported by Region-Île-de-France, Sorbonne-University and CNRS. We also appreciate the highly valuable comments of the editor and reviewers, which helped to improve our manuscript substantially.
Author Contribution
SG, PR, AZ and MAS contributed to the study conception and design. Experiments and data collection were performed by SG, CB, ZC, AD, LD, MJ, CM, PR. Data analysis and preparation of the first draft were done by SG and PR. The manuscript was revised by MI, CM, MAS, AZ. All authors commented and approved the final manuscript.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. This work is funded by the European Union—NextGenerationEU under the National Recovery and Resilience Plan (PNRR)—Mission 4 Education and research—Component 2 From research to business—Investment 1.1 Notice Prin 2022—DD N. 104 del 2/2/2022, from title “Interactions of the white truffle Tuber magnatum with soil microbiome and plants,” proposal code K272X8 – CUP J53D23010090006. This study is part of the activities of the 37 th cycle of the Ph.D. course in “Health, Safety and Green System,” PON call “Research and Innovation 2014–2020.” The Institut Universitaire de France is supporting M.-A.S. The French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (ANR-11-LABX-0002–01, Lab of Excellence ARBRE) financed UMR IaM.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary material files. ITS partial sequences of T. magnatum ectomycorrhizae were deposited at NCBI (GenBank) with the following accession numbers: PQ818236 to PQ818245.
Declarations
Competing interests
The authors declare no competing interests.
Contributor Information
Simone Graziosi, Email: simone.graziosi5@unibo.it.
Philippe Rech, Email: philippe.rech@mnhn.fr.
References
- 1.Mello A, Murat C, Bonfante P (2006) Truffles: much more than a prized and local fungal delicacy. FEMS Microbiol Lett 260:1–8. 10.1111/j.1574-6968.2006.00252.x [DOI] [PubMed] [Google Scholar]
- 2.Selosse M-A (2020) Truffles. Curr Biol 30:R382–R383 [DOI] [PubMed] [Google Scholar]
- 3.Bonito G, Trappe JM, Rawlinson P, Vilgalys R (2010) Improved resolution of major clades within Tuber and taxonomy of species within the Tuber gibbosum complex. Mycologia 102:1042–1057. 10.3852/09-213 [DOI] [PubMed] [Google Scholar]
- 4.Graziosi S, Hall IR, Zambonelli A (2022) The mysteries of the white truffle: its biology, ecology and cultivation. Encyclopedia 2:1959–1971. 10.3390/encyclopedia2040135 [Google Scholar]
- 5.Hall IR, Brown GT, Zambonelli A (2007) Taming the truffle. The history lore and science of the ultimate mushroom. Timber, Portland, Oregon, USA
- 6.Donnini D, Bencivenga M, Baciarelli Falini L (2000) Risultati di esperienze pluriennali nella coltivazione di Tuber magnatum Pico in Umbria. Micol Ital 29:33–39 [Google Scholar]
- 7.Murat C (2015) Forty years of inoculating seedlings with truffle fungi: past and future perspectives. Mycorrhiza 25:77–81. 10.1007/s00572-014-0593-4 [DOI] [PubMed] [Google Scholar]
- 8.Bach C, Beacco P, Cammaletti P, Babel-Chen Z, Levesque E, Todesco F, Cotton C, Robin B, Murat C (2021) First production of Italian white truffle (Tuber magnatum Pico) ascocarps in an orchard outside its natural range distribution in France. Mycorrhiza 31:383–388 [DOI] [PubMed] [Google Scholar]
- 9.Mello A, Fontana A, Meotto F, Comandini O, Bonfante P (2001) Molecular and morphological characterization of Tuber magnatum mycorrhizas in a long-term survey. Microbiol Res 155:279–284. 10.1016/S0944-5013(01)80005-7 [DOI] [PubMed] [Google Scholar]
- 10.Rubini A, Paolocci F, Granetti B, Arcioni S (2001) Morphological characterization of molecular-typed Tuber magnatum ECMs. Mycorrhiza 11:179–185. 10.1007/s005720100116 [Google Scholar]
- 11.Pereira G, Palfner G, Suz LM, Sandoval P, Ramírez R, Chávez D, Atala C (2021) Success and failures in the inoculation of five introduced trees in Chile with Tuber magnatum Pico: first advances for the domestication of the white truffle in South America. Gayana Bot 78:19–28 [Google Scholar]
- 12.Murat C, Vizzini A, Bonfante P, Mello A (2005) Morphological and molecular typing of the below-ground fungal community in a natural Tuber magnatum truffle-ground. FEMS Microbiol Lett 245:307–313 [DOI] [PubMed] [Google Scholar]
- 13.Leonardi M, Iotti M, Oddis M, Lalli G, Pacioni G, Leonardi P, Maccherini S, Perini C, Salerni E, Zambonelli A (2013) Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 23:349–358. 10.1007/s00572-012-0474-7 [DOI] [PubMed] [Google Scholar]
- 14.Zampieri E, Murat C, Cagnasso M, Bonfante P, Mello A (2010) Soil analysis reveals the presence of an extended mycelial network in a Tuber magnatum truffle-ground. FEMS Microbiol Ecol 71:43–49 [DOI] [PubMed] [Google Scholar]
- 15.Iotti M, Leonardi M, Lancellotti E, Salerni E, Oddis M, Leonardi P, Perini C, Pacioni G, Zambonelli A (2014) Spatio-temporal dynamic of Tuber magnatum mycelium in natural truffle grounds. PLoS ONE 9:e115921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graziosi S, Puliga F, Iotti M, Amicucci A, Zambonelli A (2024) In vitro interactions between Bradyrhizobium spp. and Tuber magnatum mycelium. Environ Microbiol Rep 16:e13271. 10.1111/1758-2229.13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler A, Kuo A, Nagy LG et al (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47(4):410–415 [DOI] [PubMed] [Google Scholar]
- 18.Murat C, Payen T, Noel B et al (2018) Pezizomycetes genomes reveal the molecular basis of ectomycorrhizal truffle lifestyle. Nat Ecol Evol 2(12):1956–1965. 10.1038/s41559-018-0710-4 [DOI] [PubMed] [Google Scholar]
- 19.Martin FM, van Der Heijden MG (2024) The mycorrhizal symbiosis: research frontiers in genomics, ecology, and agricultural application. New Phytol 242(4):1486–1506. 10.1111/nph.19541 [DOI] [PubMed] [Google Scholar]
- 20.Wilson D (1995) Endophyte: the evolution of a term, and clarification of its use and definition. Oikos 73(2):274–276. 10.2307/3545919 [Google Scholar]
- 21.Ouanphanivanh N (2008) Could orchids indicate truffle habitats?: mycorrhizal association between orchids and truffles. Acta Biol Szeged 52:229–232. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2630. Accessed 11 Dec 2024
- 22.Illyés Z, Ouanphanivanh N, Rudnóy S, Orczán AK, Bratek Z (2010) The most recent results on orchid mycorrhizal fungi in Hungary. Acta Biol Hung 61:68–76 [DOI] [PubMed] [Google Scholar]
- 23.Teˇšitelová T, Teˇšitel J, Jersáková J, Rˇíhová G, Selosse M-A (2012) Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am J Bot 99(6):1020–1032. 10.3732/ajb.1100503 [DOI] [PubMed] [Google Scholar]
- 24.Gryndler M, Trilčová J, Hršelová H, Streiblová E, Gryndlerová H, Jansa J (2013) Tuber aestivum Vittad. mycelium quantified: advantages and limitations of a qPCR approach. Mycorrhiza 23:341–348. 10.1007/s00572-012-0475-6 [DOI] [PubMed] [Google Scholar]
- 25.Schneider-Maunoury L, Leclercq S, Clément C, Covès H, Lambourdiere J, Sauve M, Richard F, Selosse M-A, Taschen E (2018) Is Tuber melanosporum colonizing the roots of herbaceous, non-ectomycorrhizal plants? Fungal Ecol 31:59–68. 10.1016/j.funeco.2017.10.004 [Google Scholar]
- 26.Schneider-Maunoury L, Deveau A, Moreno M, Todesco F, Belmondo S, Murat C, Courty P-E, Jąkalski M, Selosse M-A (2020) Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, endophytically colonise roots of non-ectomycorrhizal plants in natural environments. New Phytol 225:2542–2556. 10.1111/nph.16321 [DOI] [PubMed] [Google Scholar]
- 27.Graziosi S, Leonardi P, Zambonelli A (2022) Symbiotic interactions between orchids and Tuber borchii. Ital J Mycol 51:58–65. 10.6092/issn.2531-7342/15348 [Google Scholar]
- 28.Qin J, Feng B (2022) Life cycle and phylogeography of true truffles. Genes 13(1):145. 10.3390/genes13010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galappaththi MCA, Dunstan WA, Hardy GES, McComb J, McHnery MP, Zambonelli A, Burgess TI (2024) Advances in molecular genetics have increased knowledge of Tuber species’ life cycle and population genetic structure, indicating ways to improve yield. Mycorrhiza 35:2. 10.1007/s00572-024-01177-1 [DOI] [PubMed] [Google Scholar]
- 30.Taschen E, Sauve M, Vincent B, Parladé J, Van Tuinen D, Aumeeruddy-Thomas Y, Assenat B, Selosse M-A, Richard F (2020) Insight into the truffle brûlé: tripartite interactions between the black truffle (Tuber melanosporum), holm oak (Quercus ilex) and arbuscular mycorrhizal plants. Plant Soil 446:577–594 [Google Scholar]
- 31.Mamoun M, Oliver JM (1997) Mycorrhizal inoculation of cloned hazels by Tuber melanosporum: effect of soil disinfestation and co-culture with Festuca ovina. Plant Soil 188(2):221–226. 10.1023/A:1004267405566 [Google Scholar]
- 32.Barou V, Rincón A, Calvet C, Camprubí A, Parladé J (2023) Aromatic plants and their associated arbuscular mycorrhizal fungi outcompete Tuber melanosporum in compatibility assays with truffle-oaks. Biology 12(4):628. 10.3390/biology12040628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Barreda S, Navarro-Rocha J, Gómez-Molina E, Barou V, Sanz MÁ, Sánchez S, Parladé J (2023) Intercropping of aromatic plants in truffle orchards: short-term effect on extraradical truffle mycelium and aromatic plant growth. Plant Soil 1–11. 10.1007/s11104-023-06106-3
- 34.Selosse M-A, Faccio A, Scappaticci G, Bonfante P (2004) Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb Ecol 47:416–426 [DOI] [PubMed] [Google Scholar]
- 35.Zeller B, Brechet C, Maurice J-P, Tacon F (2008) Saprotrophic versus symbiotic strategy during truffle ascocarp development under holm oak. A response based on 13C and 15N natural abundance. Ann For Sci 65:607–607. 10.1051/forest:2008037 [Google Scholar]
- 36.Le Tacon F, Zeller B, Plain C, Hossann C, Brechet C, Robin C (2013) Carbon transfer from the host to Tuber melanosporum mycorrhizas and ascocarps followed using a 13C pulse-labeling technique (Shaw P, ed). PLoS ONE 8:e64626. 10.1371/journal.pone.0064626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Tacon F, Zeller B, Plain C, Hossann C, Bréchet C, Martin F, Kohler A, Villerd J, Robin C (2015) Study of nitrogen and carbon transfer from soil organic matter to Tuber melanosporum mycorrhizas and ascocarps using 15 N and 13 C soil labelling and whole-genome oligoarrays. Plant Soil 395:351–373. 10.1007/s11104-015-2557-7 [Google Scholar]
- 38.Selosse M-A, Martos F (2014) Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends Plant Sci 19(11):683–685. 10.1016/j.tplants.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya D, Taga M (2001) Application of fibre-FISH (fluorescence in situ hybridization) to filamentous fungi: visualization of the rRNA gene cluster of the ascomycete Cochliobolus heterostrophus. Microbiology 147(5):1183–1187. 10.1099/00221287-147-5-1183 [DOI] [PubMed] [Google Scholar]
- 40.Baschien C, Manz W, Neu TR, Marvanová L, Szewzyk U (2008) In situ detection of freshwater fungi in an alpine stream by new taxon-specific fluorescence in situ hybridization probes. Appl Environ Microbiol 74(20):6427–6436. 10.1128/AEM.00815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jobard M, Rasconi S, Sime-Ngando T (2010) Fluorescence in situ hybridization of uncultured zoosporic fungi: testing with clone-FISH and application to freshwater samples using CARD-FISH. J Microbiol Methods 83:236–243. 10.1016/j.mimet.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 42.Vági P, Knapp DG, Kósa A, Seress D, Horváth ÁN, Kovács GM (2014) Simultaneous specific in planta visualization of root-colonizing fungi using fluorescence in situ hybridization (FISH). Mycorrhiza 24:259–266. 10.1007/s00572-013-0533-8 [DOI] [PubMed] [Google Scholar]
- 43.Laurent-Webb L, Rech P, Bourceret A, Chaumeton C, Deveau A, Genola L, Januario M, Petrolli R, Selosse M-A (2024) Endophytic and ectomycorrhizal, an overlooked dual ecological niche? Insights from natural environments and Russula species. bioRxiv 2024(0):24.576884. 10.1101/2024.01.24.576884
- 44.Thurnheer T, Gmür R, Guggenheim B (2004) Multiplex FISH analysis of a six-species bacterial biofilm. J Microbiol Methods 56:37–47. 10.1016/j.mimet.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 45.Behnam F, Vilcinskas A, Wagner M, Stoecker K (2012) A straightforward DOPE (double labeling of oligonucleotide probes)-FISH (fluorescence in situ hybridization) method for simultaneous multicolor detection of six microbial populations. Appl Environ Microbiol 78:5138–5142. 10.1128/AEM.00977-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schimak MP, Kleiner M, Wetzel S, Liebeke M, Dubilier N, Fuchs BM (2016) MiL-FISH: multilabeled oligonucleotides for fluorescence in situ hybridization improve visualization of bacterial cells. Appl Environ Microbiol 82:62–70. 10.1128/AEM.02776-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukumbuzya M, Schmid M, Pjevac P, Daims H (2019) A multicolor fluorescence in situ hybridization approach using an extended set of fluorophores to visualize microorganisms. Front microbiol 10:1383. 10.3389/fmicb.2019.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertaux J, Gloger U, Schmid M, Hartmann A, Scheu S (2007) Routine fluorescence in situ hybridization in soil. J Microbiol Methods 69:451–460. 10.1016/j.mimet.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 49.Rogers SW, Ong SK, Moorman TB (2007) Mineralization of PAHs in coal–tar impacted aquifer sediments and associated microbial community structure investigated with FISH. Chemosphere 69:1563–1573. 10.1016/j.chemosphere.2007.05.058 [DOI] [PubMed] [Google Scholar]
- 50.Naumann M, Schüßler A, Bonfante P (2010) The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes. ISME J 4:862–871. 10.1038/ismej.2010.21 [DOI] [PubMed] [Google Scholar]
- 51.Desirò A, Naumann M, Epis S, Novero M, Bandi C, Genre A, Bonfante P (2013) Mollicutes-related endobacteria thrive inside liverwort-associated arbuscular mycorrhizal fungi. Environ Microbiol 15:822–836. 10.1111/j.1462-2920.2012.02833.x [DOI] [PubMed] [Google Scholar]
- 52.Mogge B, Loferer C, Agerer R, Hutzler P, Hartmann A (2000) Bacterial community structure and colonization patterns of Fagus sylvatica L. ectomycorrhizospheres as determined by fluorescence in situ hybridization and confocal laser scanning microscopy. Mycorrhiza 9:271–278. 10.1007/PL00009991 [Google Scholar]
- 53.Bertaux J, Schmid M, Prevost-Boure NC, Churin JL, Hartmann A, Garbaye J, Frey-Klett P (2003) In situ identification of intracellular bacteria related to Paenibacillus spp. in the mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Appl Environ Microbiol 69(7):4243–4248. 10.1128/AEM.69.7.4243-4248.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertaux J, Schmid M, Hutzler P, Hartmann A, Garbaye J, Frey-Klett P (2005) Occurrence and distribution of endobacteria in the plant-associated mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Environ Microbiol 7:1786–1795. 10.1111/j.1462-2920.2005.00867.x [DOI] [PubMed] [Google Scholar]
- 55.Iotti M, Leonardi M, Oddis M, Salerni E, Baraldi E, Zambonelli A (2012) Development and validation of a real-time PCR assay for detection and quantification of Tuber magnatum in soil. BMC Microbiol 12:1–10. 10.1186/1471-2180-12-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY, USA, 18 pp 315–322.
- 57.Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. 10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- 58.Amicucci A, Zambonelli A, Giomaro G, Potenza L, Stocchi V (1998) Identification of ectomycorrhizal fungi of the genus Tuber by species-specific ITS primers. Mol Ecol 7:273–277 [Google Scholar]
- 59.Tonon G, Boldreghini P, Gioacchini P (2005) Seasonal changes in microbial nitrogen in an old broadleaf forest and in a neighbouring young plantation. Biol Fertil Soils 41:101–108. 10.1007/s00374-004-0813-8 [Google Scholar]
- 60.Leonardi P, Baroni R, Puliga F, Iotti M, Salerni E, Perini C, Zambonelli A (2021) Co-occurrence of true truffle mycelia in Tuber magnatum fruiting sites. Mycorrhiza 31:389–394. 10.1007/s00572-021-01030-9 [DOI] [PubMed] [Google Scholar]
- 61.Cao L, Qiu Z, You J, Tan H, Zhou S (2004) Isolation and characterization of endophytic Streptomyces strains from surface-sterilized tomato (Lycopersicon esculentum) roots. Lett Appl Microbiol 39:425–430. 10.1111/j.1472-765X.2004.01606.x [DOI] [PubMed] [Google Scholar]
- 62.NCBI (2023). https://www.ncbi.nlm.nih.gov (accessed 8 December 2023)
- 63.Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, Riley R, Salamov A, Zhao X, Korzeniewski F, Smirnova T, Nordberg H, Dubchak I, Shabalov I (2014) MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res 42:D699–D704. 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. 10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Metfies K, Medlin LK (2008) Feasibility of transferring fluorescent In situ hybridization probes to an 18S rRNA gene phylochip and mapping of signal intensities. Appl Environ Microbiol 74:2814–2821. 10.1128/AEM.02122-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glöckner FO, Yilmaz P, Quast C, Gerken J, Beccati A, Ciuprina A, Bruns G, Yarza P, Peplies J, Westram R, Ludwig W (2017) 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol 261:169–176. 10.1016/j.jbiotec.2017.06.1198 [DOI] [PubMed] [Google Scholar]
- 67.Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iotti M, Amicucci A, Stocchi V, Zambonelli A (2002) Morphological and molecular characterization of mycelia of some Tuber species in pure culture. New Phytol 155:499–505 [DOI] [PubMed] [Google Scholar]
- 69.Clements JM (1986) Molecular characterization of the 3-phosphoglycerate kinase gene from Aspergillus nidulans. University of Leicester, UK [Google Scholar]
- 70.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925. 10.1128/aem.56.6.1919-1925.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iotti M, Leonardi P, Vitali G, Zambonelli A (2018) Effect of summer soil moisture and temperature on the vertical distribution of Tuber magnatum mycelium in soil. Biol Fertil Soils 54:707–716. 10.1007/s00374-018-1296-3 [Google Scholar]
- 72.Queralt M, Parlade J, Pera J, de Miguel AM (2017) Seasonal dynamics of extraradical mycelium and mycorrhizas in a black truffle (Tuber melanosporum) plantation. Mycorrhiza 27:565–576. 10.1007/s00572-017-0780-1 [DOI] [PubMed] [Google Scholar]
- 73.Prieto P, Moore G, Shaw P (2007) Fluorescence in situ hybridization on vibratome sections of plant tissues. Nat Protoc 2:1831–1838. 10.1038/nprot.2007.265 [DOI] [PubMed] [Google Scholar]
- 74.Qian XM, Kottke I, Oberwinkler F (1998) Activity of different ectomycorrhizal types studied by vital fluorescence. Plant Soil 199:91–98. 10.1023/A:1004226220283 [Google Scholar]
- 75.Ferrari C, Speranza M (1984) Ecological inferences from phytosociological data in an alluvial forest on the Po plain (Northern Italy) Studia Geobotanica. Int J 4:41–47 (http://hdl.handle.net/10077/14993) [Google Scholar]
- 76.Gryndler M, Černá L, Bukovská P, Hršelová H, Jansa J (2014) Tuber aestivum association with non-host roots. Mycorrhiza 24:603–610. 10.1007/s00572-014-0580-9 [DOI] [PubMed] [Google Scholar]
- 77.Selosse M-A, Schneider-Maunoury L, Martos F (2018) Time to re-think fungal ecology? Fungal ecological niches are often prejudged. New Phytol 217:968–972 (https://www.jstor.org/stable/90018525) [DOI] [PubMed] [Google Scholar]
- 78.Selosse M-A, Dubois MP, Alvarez N (2009) Do Sebacinales commonly associate with plant roots as endophytes? Mycol Res 113:1062–1069. 10.1016/j.mycres.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 79.Ceruti A, Fontana A, Nosenzo C (2003) Le specie europee del genere Tuber: una revisione storica. Monografie 37. Museo Regionale di Scienze Naturali, Torino
- 80.Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620. 10.1111/j.1469-8137.2006.01936.x [DOI] [PubMed] [Google Scholar]
- 81.Jumpponen A, Jones K, Blair J (2010) Vertical distribution of fungal communities in tallgrass prairie soil. Mycologia 102:1027–1041. 10.3852/09-316 [DOI] [PubMed] [Google Scholar]
- 82.Baldrian P, Kolařík M, Štursová M, Kopecký J, Valášková V, Větrovský T, Žifčáková L, Šnajdr J, Rídl J, Vlček Č, Voříšková V (2012) Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J 6:248–258. 10.1038/ismej.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uroz S, Ioannidis P, Lengelle J, Cébron A, Morin E, Buée M, Martin F (2013) Functional assays and metagenomic analyses reveals differences between the microbial communities inhabiting the soil horizons of a Norway spruce plantation. PLoS One 8:e55929. 10.1371/journal.pone.0055929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Tacon F (2016) Influence of climate on natural distribution of Tuber species and truffle production. In: Zambonelli A, Iotti M, Murat C (eds) True truffles (Tuber spp.) in the world, soil biology, vol 47. Springer, Cham, pp 153–167. 10.1007/978-3-319-31436-5_10
- 85.ISPRA (2024). https://www.isprambiente.gov.it/it (accessed 11 December 2024)
- 86.ISTAT (2024). https://www.istat.it (accessed 11 December 2024)
- 87.Barbieri E, Ceccaroli P, Saltarelli R, Guidi C, Potenza L, Basaglia M, Fontana F, Baldan E, Casella S, Ryahi O, Zambonelli A, Stocchi V (2010) New evidence for nitrogen fixation within the Italian white truffle Tuber magnatum. Fungal Biol 114:936–942. 10.1016/j.funbio.2010.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary material files. ITS partial sequences of T. magnatum ectomycorrhizae were deposited at NCBI (GenBank) with the following accession numbers: PQ818236 to PQ818245.