Abstract
To compare postoperative changes in peripapillary retinal nerve fiber layer (pRNFL) thickness between macula-off and macula-on rhegmatogenous retinal detachment (RRD). Patients with RRD who had undergone a single, uncomplicated vitrectomy and been followed for ≥ 3 years postoperatively were included. Based on preoperative status, patients were categorized into a macula-on group (Group 1) and a macula-off group (Group 2). The baseline was established after complete gas dissipation from the vitreous cavity, followed by three additional examinations at 1-year intervals. In total, 62 eyes were analyzed: 30 in Group 1 and 32 in Group 2. Global pRNFL thicknesses in Group 1 were 100.0 ± 19.5, 99.4 ± 19.6, 98.4 ± 19.4, and 97.0 ± 20.3 μm at baseline, 1 year, 2 years, and 3 years, respectively (P = 0.001). In Group 2, the corresponding values were 99.6 ± 15.0, 96.2 ± 16.4, 95.4 ± 16.3, and 94.1 ± 17.6 μm (P < 0.001). Sectoral analysis showed statistically significant changes in the inferotemporal (P < 0.001) and inferonasal (P = 0.003) sectors in Group 2. The reduction rates of global pRNFL thickness were − 0.89 μm/y in Group 1 and − 1.81 μm/y in Group 2; these rates significantly differed between the groups (P = 0.026). Among RRD patients, pRNFL thickness gradually declined over time, with a more pronounced reduction in the macula-off group. A substantial decrease in inferior pRNFL thickness was observed in macula-off patients.
Keywords: Rhegmatogenous retinal detachment, Retinal nerve fiber layer, Axial length
Subject terms: Diseases, Eye diseases, Retinal diseases
Introduction
Rhegmatogenous retinal detachment (RRD) occurs when liquefied vitreous humor enters the subretinal space through retinal breaks, causing the separation of the retinal pigment epithelium layer from the sensory retinal layer. It is more prevalent in men than in women and is most commonly observed in individuals aged 20–60 years, although it can occur at any age1,2 Risk factors for RRD include myopia, lattice degeneration, inflammation, retinal injury, atopic dermatitis, and posterior vitreous detachment3,4 Surgical intervention is often required to treat RRD; it may involve procedures such as scleral buckling and vitrectomy, performed either alone or in combination depending on the specific case. These surgeries aim to achieve retinal reattachment by closing retinal breaks and relieving traction5.
Surgical treatment for RRD is typically highly successful in reattaching the retina6 However, visual prognosis after reattachment may be affected by several factors; one of the most critical is the macular detachment status prior to surgery7 Retinal reattachment can lead to microstructural changes in the retina, including alterations in the external limiting membrane, ellipsoid zone, and interdigitation zone at the macula, which may impact visual outcomes. These changes are often influenced by macular involvement during detachment8,9 Furthermore, RRD may induce changes in peripapillary retinal nerve fiber layer (pRNFL) thickness, even after successful reattachment10 It is hypothesized that more severe pRNFL damage progression occurs in macula-off RRD, where detachment ends to be more extensive in the posterior pole area. However, no studies have specifically evaluated this aspect.
The purpose of this study was to compare postoperative changes in pRNFL thickness between macula-off and macula-on RRD.
Methods
Patients
This retrospective, longitudinal, observational study adhered to the tenets of the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board/Ethics Committee of Konyang University Hospital, Daejeon, Republic of Korea (No. 2024-10-022). Patients who visited the retinal clinic between March 2015 and May 2024 were screened for inclusion. The Institutional Review Board/Ethics Committee of Konyang University Hospital waived the requirement for informed consent due to the retrospective study design. Patients with RRD who underwent a single, uncomplicated vitrectomy and had a minimum follow-up period of 3 years postoperatively were included. The surgeries were performed by two surgeons (Y.H.L. and M.W.L.). All patients underwent 25-gauge pars plana vitrectomy, and phacoemulsification with posterior chamber lens implantation was performed in patients with cataracts. The preexisting peripheral retinal breaks were used to drain the subretinal fluid after filling with perfluorocarbon liquid. Subsequently, fluid-air exchange was performed and endo photocoagulation was applied around the breaks in the peripheral retina. After PFCL removal, gas endo tamponade (C3F8 or SF6) was applied, and patients were advised to maintain a prone position for two weeks following the surgery. Patients who received silicone oil injections, underwent internal limiting membrane peeling or retinotomy during vitrectomy, which could affect pRNFL thickness measurements, or had concurrent epiretinal membrane or vascular diseases such as retinal vein occlusion were excluded. Based on preoperative status, patients were categorized into a macula-on group (Group 1) and a macula-off group (Group 2). The baseline was established after complete gas dissipation from the vitreous cavity, followed by three additional examinations at 1-year intervals. We excluded patients with a history of ocular diseases other than RRD, pathologic myopia with diffuse chorioretinal atrophy, and intraocular pressure > 21 mmHg after gas dissipation. During the follow-up period, we excluded not only patients with RRD-related complications such as epiretinal membrane but also those with retinal abnormalities that were less directly related to RRD, including diabetic retinopathy, retinal vein occlusion, or age-related macular degeneration.
pRNFL thickness measurements
A skilled examiner measured pRNFL thickness using spectral-domain optical coherence tomography (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Scans were obtained in a circular scanning pattern with a 3.5 mm diameter around the optic nerve head, capturing pRNFL thickness in both global and sectoral areas, including the temporal, superotemporal, superonasal, nasal, inferonasal, and inferotemporal sectors. Scans with a quality score below 15, as well as images showing decentration, misalignment, or severe segmentation errors, were excluded from the analysis.
Statistical analysis
Baseline demographic characteristics and ocular parameters were compared between groups using independent t-tests, while categorical variables were analyzed with Chi-squared tests. Linear mixed models were utilized to assess changes in pRNFL thickness over time within each group, including the rate of thickness reduction, with a random intercept at the eye level. Generalized linear mixed models were used to identify factors associated with changes in pRNFL thickness over time. Statistical analyses were performed with SPSS software (version 18.0; IBM Corp., Armonk, NY, USA).
Results
Demographic characteristics
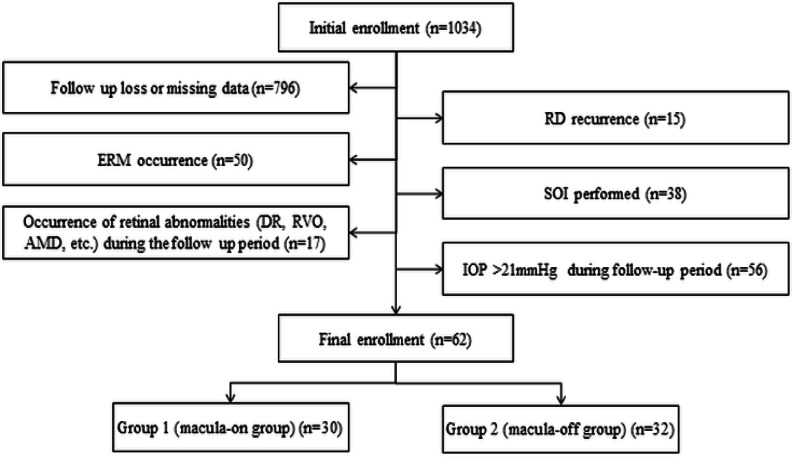
In total, 62 eyes were included in the study; 30 in Group 1 and 32 in Group 2 (Fig. 1). The mean ages were 48.6 ± 14.8 years in Group 1 and 48.6 ± 13.9 years in Group 2 (P = 0.999) (Table 1). The mean duration from the diagnosis of RRD to surgery was 3.3 ± 1.5 days in Group 1 and 3.1 ± 1.4 days in Group 2 (P = 0.821). The mean times from surgery to baseline were 9.7 ± 11.7 months in Group 1 and 7.5 ± 5.4 months in Group 2 (P = 0.344). The mean best-corrected visual acuity significantly differed between the groups: 0.11 ± 0.22 in Group 1 and 0.38 ± 0.35 in Group 2 (P = 0.001). The mean axial lengths were 25.8 ± 2.1 mm in Group 1 and 25.7 ± 1.8 mm in Group 2 (P = 0.298). Other factors, including sex, spherical equivalent, intraocular pressure, symptom duration, and location of retinal elevation, Showed no significant differences between the groups.
Fig. 1.
Flow diagram showing number of subjects included in the study population. RD retinal detachment, ERM epiretinal membrane, SOI silicone oil injection, DR diabetic retinopathy, RVO retinal vein occlusion, AMD age-related macular degeneration, IOP intraocular pressure.
Table 1.
Baseline demographic characteristics.
| Group 1 (n = 30) | Group 2 (n = 32) | P-value | |
|---|---|---|---|
| Age (years, mean ± SD) | 48.6 ± 14.8 | 48.6 ± 13.9 | 0.999 |
| Sex (male, %) | 11 (36.7) | 16 (50.0) | 0.343 |
| Laterality (right, %) | 15 (50.0) | 12 (37.5) | 0.264 |
| Diabetes (n, %) | 0 (0.0) | 3 (9.4) | 0.096 |
| Hypertension (n, %) | 3 (10.0) | 7 (21.9) | 0.247 |
| Symptom duration (days, mean ± SD) | 8.6 ± 9.8 | 10.2 ± 11.5 | 0.571 |
| Location of retinal elevation (n, %) | 0.351 | ||
| Superior | 16 (53.3) | 12 (37.5) | |
| Inferior | 4 (13.3) | 6 (18.8) | |
| Both | 10 (33.3) | 14 (43.8) | |
| Lens status (pseudophakic, %) | 16 (53.3) | 18 (56.3) | 0.933 |
| SE (diopters, mean ± SD) | -3.28 ± 3.74 | -2.99 ± 2.58 | 0.244 |
| IOP (mmHg, mean ± SD) | 14.2 ± 3.6 | 13.8 ± 3.1 | 0.407 |
| Axial length (mm, mean ± SD) | 25.8 ± 2.1 | 25.7 ± 1.8 | 0.298 |
| BCVA (logMAR, mean ± SD) | 0.11 ± 0.22 | 0.38 ± 0.35 | 0.003 |
SE spherical equivalent, IOP intraocular pressure, BCVA best-corrected visual acuity. Values in boldface (P < 0.050) are statistically significant.
pRNFL thickness at each visit
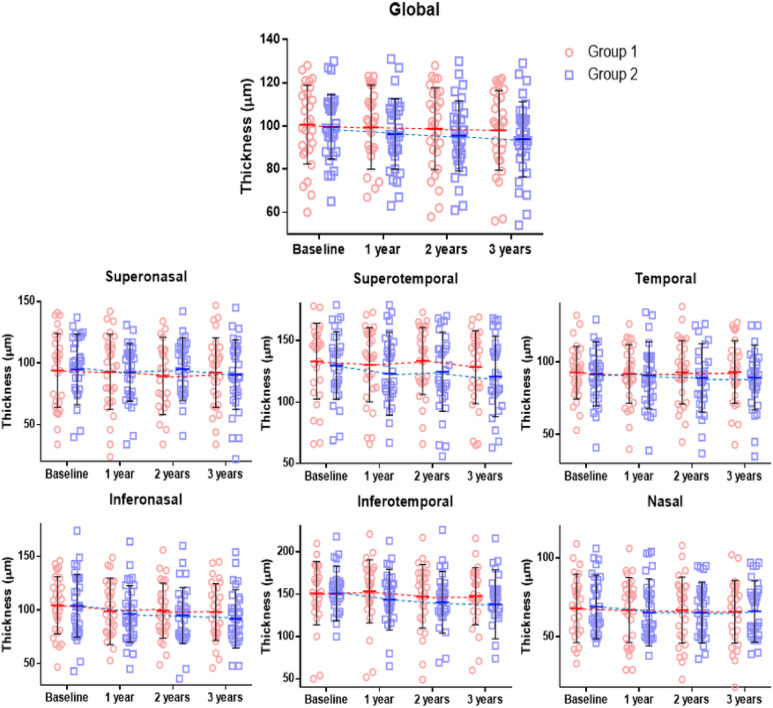
In Group 1, global pRNFL thicknesses were 100.0 ± 19.5, 99.4 ± 19.6, 98.4 ± 19.4, and 97.0 ± 20.3 μm at baseline, 1 year, 2 years, and 3 years, respectively (P = 0.001), which showed a significant reduction over time (Table 2). In Group 2, global pRNFL thicknesses were 99.6 ± 15.0, 96.2 ± 16.4, 95.4 ± 16.3, and 94.1 ± 17.6 μm at baseline, 1 year, 2 years, and 3 years, respectively (P < 0.001), which also showed a significant reduction over time. Although most sectors in both groups showed a trend of decreasing thickness, statistically significant reductions were observed only in the inferotemporal (P < 0.001) and inferonasal (P = 0.003) sectors in Group 2 (Fig. 2).
Table 2.
Peripapillary retinal nerve fiber layer thickness in each group at each visit.
| Group 1 | Group 2 | P-value* | |
|---|---|---|---|
| Global | |||
| Baseline | 100.0 ± 19.5 | 99.6 ± 15.0 | 0.137 |
| First year | 99.4 ± 19.6 | 96.2 ± 16.4 | |
| Second year | 98.4 ± 19.4 | 95.4 ± 16.3 | |
| Third year | 97.0 ± 20.3 | 94.1 ± 17.6 | |
| P-value† | 0.001 | < 0.001 | |
| Sector | |||
| Temporal | |||
| Baseline | 92.5 ± 17.9 | 91.8 ± 22.0 | 0.595 |
| First year | 91.7 ± 20.2 | 90.8 ± 23.1 | |
| Second year | 92.4 ± 21.8 | 89.1 ± 23.5 | |
| Third year | 92.7 ± 21.6 | 89.2 ± 22.1 | |
| P-value† | 0.356 | 0.300 | |
| Superotemporal | |||
| Baseline | 133.4 ± 30.4 | 125.8 ± 35.5 | 0.854 |
| First year | 130.3 ± 30.2 | 123.2 ± 33.7 | |
| Second year | 129.9 ± 26.7 | 121.7 ± 32.1 | |
| Third year | 127.9 ± 30.9 | 120.5 ± 32.8 | |
| P-value† | 0.109 | 0.082 | |
| Superonasal | |||
| Baseline | 93.4 ± 31.8 | 94.9 ± 28.6 | 0.261 |
| First year | 93.0 ± 30.6 | 92.6 ± 23.3 | |
| Second year | 92.7 ± 31.3 | 92.2 ± 25.6 | |
| Third year | 91.6 ± 30.4 | 91.4 ± 28.3 | |
| P-value† | 0.276 | 0.174 | |
| Nasal | |||
| Baseline | 67.1 ± 23.1 | 68.9 ± 20.1 | 0.470 |
| First year | 66.9 ± 20.5 | 65.4 ± 21.2 | |
| Second year | 66.7 ± 21.1 | 65.2 ± 19.2 | |
| Third year | 64.8 ± 21.8 | 64.9 ± 19.2 | |
| P-value† | 0.172 | 0.077 | |
| Inferonasal | |||
| Baseline | 103.3 ± 28.4 | 104.0 ± 29.3 | 0.933 |
| First year | 98.8 ± 31.0 | 96.4 ± 26.4 | |
| Second year | 99.0 ± 26.1 | 94.9 ± 26.1 | |
| Third year | 97.1 ± 27.6 | 91.1 ± 28.1 | |
| P-value† | 0.215 | 0.003 | |
| Inferotemporal | |||
| Baseline | 149.7 ± 38.8 | 150.8 ± 32.1 | 0.254 |
| First year | 148.2 ± 37.3 | 143.6 ± 35.8 | |
| Second year | 146.7 ± 38.1 | 140.3 ± 36.4 | |
| Third year | 145.6 ± 36.5 | 135.3 ± 42.3 | |
| P-value† | 0.059 | < 0.001 | |
Values in boldface (P < 0.050) are statistically significant. *Independent t-test for baseline values. †Linear mixed model.
Fig. 2.
Scatter plots and line graphs showing means and standard deviations of peripapillary retinal nerve fiber layer thickness at each visit.
Additionally, we performed the analysis after excluding patients with an axial length greater than 26.5 mm in each group, resulting in 22 in Group 1 and 25 in Group 2. The global pRNFL thicknesses were 102.4 ± 18.3, 101.7 ± 20.0, 101.3 ± 19.2, and 100.0 ± 18.9 μm in Group 1, and 101.7 ± 15.8, 99.6 ± 16.1, 98.2 ± 16.5, and 97.5 ± 15.7 μm in Group 2, respectively. Both groups showed a significant decrease in RNFL thickness over time (Group 1, P = 0.049; Group 2, P < 0.001), but the rate of decrease in Group 2 was significantly greater (P = 0.041).
Reduction rate and factors associated with changes in pRNFL thickness
The rates of reduction in global pRNFL thickness were − 0.89 μm/y in Group 1 and − 1.81 μm/y in Group 2, indicating a significant difference between the groups (P = 0.026) (Table 3). In terms of sectoral thickness, Group 2 exhibited a greater reduction tendency compared with Group 1, although a statistically significant difference between the groups was identified only in the inferotemporal sector (P < 0.001).
Table 3.
Rates of change in peripapillary retinal nerve fiber layer thickness, calculated using linear mixed models.
| Group 1 | Group 2 | *P-value | |
|---|---|---|---|
| Global | -0.89 (-1.43 to -0.35) | -1.81 (-2.40 to -1.21) | 0.026 |
| Sector | |||
| Temporal | 0.55 (-0.65 to 1.75) | -0.76 (-2.23 to 0.71) | 0.169 |
| Superotemporal | -1.12 (-2.49 to 0.25) | -1.33 (-2.83 to 0.17) | 0.836 |
| Superonasal | -0.85 (-2.39 to 0.69) | -1.20 (-2.96 to 0.56) | 0.569 |
| Nasal | -0.62 (-1.53 to 0.29) | -0.91 (-1.92 to 0.10) | 0.714 |
| Inferonasal | -1.36 (-3.53 to 0.82) | -3.20 (-5.23 to -1.17) | 0.108 |
| Inferotemporal | -1.63 (-3.32 to 0.06) | -3.64 (-5.58 to -1.72) | < 0.001 |
Values in boldface (P < 0.050) are statistically significant. *Interaction between group and duration in linear mixed models.
In univariate analysis, significant factors associated with changes in pRNFL thickness included axial length (estimate = -3.45, P = 0.003), final best-corrected visual acuity (estimate = -20.72, P = 0.030), and time (estimate = -1.37, P < 0.001), with time indicating whether the change in pRNFL thickness is significant with each passing year (Table 4). Multivariate analysis identified axial length (estimate = -3.38, P = 0.003) and time (estimate = -1.18, P < 0.001) as significant factors. In the group-based analysis, axial length was not significantly associated with changes in pRNFL thickness in Group 1 (estimate = -2.56, P = 0.201), whereas a significant association was found in Group 2 (estimate = -5.24, P < 0.001).
Table 4.
Linear mixed-effect model determination of factors associated with changes in peripapillary retinal nerve fiber layer thickness.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| Age | 0.17 (-0.14 to 0.48) | 0.281 | ||
| Sex | -4.42 (-13.28 to 4.44) | 0.323 | ||
| Symptom duration | 0.31 (-0.11 to 0.72) | 0.143 | ||
| Baseline BCVA | -4.55 (-18.34 to 9.25) | 0.512 | ||
| IOP | 0.20 (-1.13 to 1.53) | 0.760 | ||
| AXL | -3.45 (-5.65 to -1.26) | 0.003 | -3.38 (-5.57 to -1.19) | 0.003 |
| Final BCVA | -20.72 (-39.36 to -2.07) | 0.030 | -11.88 (-29.25 to 5.49) | 0.175 |
| Time | -1.37 (-1.78 to -0.96) | < 0.001 | -1.18 (-1.72 to -0.65) | < 0.001 |
BCVA best-corrected visual acuity, IOP intraocular pressure, AXL axial length. Values in boldface (P < 0.050) are statistically significant.
Discussion
In vitrectomized eyes, changes in oxygen distribution within the vitreous cavity can impact the extracellular matrix of the trabecular meshwork, potentially reducing aqueous outflow and increasing the risk of glaucoma11,12 Additionally, RRD may contribute to inner retinal damage through anterograde degeneration13 High myopia, a common and significant risk factor for RRD, can also influence changes in pRNFL thickness14 Thus, it is important to carefully analyze pRNFL thickness in patients who have undergone vitrectomy for RRD. Although the extent of retinal detachment may lead to varying degrees of pRNFL damage, no previous studies have provided a longitudinal analysis of this phenomenon. In this study, we performed a longitudinal and comparative analysis of changes in pRNFL thickness over time between macula-on and macula-off RRD cases. Our findings demonstrated a significant decrease in pRNFL thickness over time in both groups; a more rapid reduction was evident in patients with macula-off RRD patients. Notably, the inferior sectors exhibited the greatest thickness reduction in patients with macula-off RRD. Such reduction in pRNFL thickness among patients with RRD showed a significant association with axial length.
Both groups in this study showed a significant reduction in pRNFL thickness over time. This reduction may be associated with long axial lengths in both cohorts. Lee et al.14 reported that highly myopic eyes experienced a significantly greater decrease in pRNFL thickness over 2 years relative to normal eyes, with reduction rates of -0.95 μm /y in individuals aged 30 to 39 years, -1.70 μm/y in those aged 40 to 49 years, and − 1.69 μm/y in those aged 50 to 59 years. They explained this reduction to globe elongation, which leads to mechanical stretching and thinning of the retina. The patients in our study had long axial lengths approaching high myopia, which may have contributed to the observed reduction in pRNFL thickness. Although direct comparison is challenging due to the use of different optical coherence tomography devices and the shorter axial lengths in our study, the reduction rate in Group 1, at -0.89 μm/y, appears to be slightly lower than that reported in the previous study on highly myopic patients. This finding suggests that the impact of RRD on changes in pRNFL thickness is smaller in Group 1.
The rate of pRNFL thickness reduction was significantly greater in Group 2 than in Group 1. This finding suggests that, because other factors (e.g., axial length) did not differ between the groups, macula-off RRD contributes to a more pronounced reduction in pRNFL thickness. Faude et al.13 reported that photoreceptor degeneration is accompanied by a substantial loss of ganglion cell axons and the degeneration of numerous ganglion cells in the inner layers of the detached retina. Consequently, pRNFL thickness may be influenced by anterograde degeneration of the second and third neurons in the area of the detached retina10 The faster rate of pRNFL reduction observed in Group 2, which has a relatively larger area of retinal detachment around the posterior pole compared with Group 1, is therefore expected. Additionally, the significant thickness reduction observed in Group 2 during the first year may be related to the resolution of postoperative edema and the decrease in Muller cell proliferation, which typically increases immediately after surgery. Further studies are needed to confirm this hypothesis.
Although most sectoral thicknesses tended to decrease over time, these changes were not statistically significant in either group, possibly due to the small sample size. However, the inferior sectors in Group 2 demonstrated a statistically significant and pronounced decrease over time. This observation may be related to the anatomical structure of the lamina cribrosa, through which the nerve fibers pass. Compared with other quadrants, the inferior portions of the lamina cribrosa contain larger pores and provide less connective tissue support for ganglion cell axons15 Larger laminar pores accommodate more nerve fiber bundles and require greater metabolic resources16 Consequently, this increased metabolic demand renders the inferior sector more susceptible to various types of damage17 Although fewer patients in this study had RRD in the inferior region compared with the superior region, the inferior sector exhibited a more pronounced reduction in thickness. This finding suggests that the anatomical structure of the lamina cribrosa plays a more central role in sectoral pRNFL thickness reduction than the location of the retinal detachment.
In patients with RRD, axial length was significantly associated with changes in pRNFL thickness, consistent with the previous study reporting thinner pRNFL in cases of high myopia14 As axial length increases, there is a tendency for continued elongation over time18 This stretching effect may lead to further thinning of the pRNFL. Notably, in the subgroup analysis, axial length was not significantly associated with changes in pRNFL thickness in Group 1, whereas a significant association was observed in Group 2. Changes in pRNFL thickness in macula-off RRD patients appear to be more sensitive to axial length compared to such changes in macula-on RRD patients. This sensitivity may be attributed to the larger area of retinal detachment at the posterior pole in macula-off patients, making the pRNFL more vulnerable to damage from the stretching effect. Further research is needed to elucidate the precise mechanisms involved.
Several studies have reported results different from ours19–21 Hwang et al.20 reported that no pRNFL thinning or thickening was observed at 3 years following surgery. This difference from our study can be due to the following reasons. First, the axial length of patients included in this study is longer compared to the previous study, which may have contributed to the more pronounced pRNFL thinning observed. Second, while the previous study reported no significant difference in pRNFL changes between the macula-off and macula-on groups, the sample size in that study was relatively small, with only 8 patients included in the macula-off group. Most importantly, the baseline measurement in the previous study was taken before surgery, whereas in this study, the baseline was defined as the point after the complete disappearance of intraocular gas. When examining only the postoperative period in the previous study, the trend of pRNFL thinning appears similar to that observed in our study. Thus, the results of the two studies are not contradictory. It is believed that the differences in the results of other studies, compared to ours, were also due to variations in inclusion criteria, such as differences in baseline time points or the inclusion of patients who received silicone oil injections19,21.
Our study had several limitations. First, its retrospective design inevitably introduced selection bias. Second, the number of patients was limited due to strict inclusion and exclusion criteria. Due to the limited number of cases, a small number of diabetic patients were included in Group 2. Although they did not progress to diabetic retinopathy during the follow-up period, their inclusion may have introduced some degree of bias. Third, although we enrolled patients without pRNFL defects or a history of intraocular pressure exceeding 21 mmHg, we could not completely rule out the possibility of including patients with preperimetric glaucoma at baseline. Fourth, while the axial lengths of the included patients were close to high myopia, reflecting actual clinical situations, this presents challenges in isolating the effects of RRD alone on changes in pRNFL thickness. Although similar results were observed in the analysis excluding high myopia patients with an axial length greater than 26.5 mm in this study, further research with a larger sample size is needed. Although the surgical procedures and methods were not different between the two surgeons, the inability to exclude bias due to inter-surgeon variability is another limitation of this study. The strength of our study lies in its longitudinal comparative analysis of changes in pRNFL thickness between macula-on and macula-off cases, a topic that has not been previously reported.
In conclusion, patients with RRD who underwent vitrectomy and experienced no additional complications showed a gradual decrease in pRNFL thickness over time; a more pronounced reduction was evident in those with macula-off RRD. Although more patients had superior area detachments relative to inferior detachments, the greatest reduction in inferior RNFL thickness was observed in macula-off patients. This finding is believed to result from the anatomical structure of the lamina cribrosa. Furthermore, changes in pRNFL thickness demonstrated a significant association with axial length; this association was stronger in macula-off patients. Physicians should consider these changes in pRNFL thickness when evaluating ophthalmic diseases where pRNFL measurements are important in patients who have undergone RRD surgery.
Acknowledgements
Contributions to Authors in each of these areas. Design and conduct of the study (M.W.L., J.T.K.); Collection of data (K.S.P., J.T.K., M.W.L.); Analysis and interpretation of data (M.W.L., .J.T.K.); Writing the article (M.W.L., J.T.K.); Critical revision of the article (M.W.L., Y.H.L, K.H.L.); Final approval of the article (J.T.K., K.S.P., K.H.L., Y.H.L., M.W.L.).
Author contributions
Design and conduct of the study (M.W.L., J.T.K.); Collection of data (K.S.P., J.T.K., M.W.L.); Analysis and interpretation of data (M.W.L., .J.T.K.); Writing the article (M.W.L., J.T.K.); Critical revision of the article (M.W.L., Y.H.L, K.H.L.); Final approval of the article (J.T.K., K.S.P., K.H.L., Y.H.L., M.W.L.);
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laatikainen, L. & Tolppanen, E. M. Characteristics of rhegmatogenous retinal detachment. Acta Ophthalmol.63 (2), 146–154 (1985). [DOI] [PubMed] [Google Scholar]
- 2.Mitry, D. et al. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br. J. Ophthalmol.94 (6), 678–684 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Sung, J-Y. et al. Clinical characteristics and prognosis of total rhegmatogenous retinal detachment: a matched case-control study. BMC Ophthalmol.20, 1–6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazi, N. & Green, W. Pathology and pathogenesis of retinal detachment. Eye16 (4), 411–421 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Soni, C., Hainsworth, D. P. & Almony, A. Surgical management of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled trials. Ophthalmology120 (7), 1440–1447 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Heimann, H. et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology114 (12), 2142–2154 (2007). e4. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Costa, S. et al. Optical coherence tomography angiography based prognostic factors and visual outcomes in primary rhegmatogenous retinal detachment after Pars plana vitrectomy. Int. J. Retina Vitreous. 10 (1), 57 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sehemy, A. et al. Post-operative Photoreceptor Integrity and Anatomic Outcomes Based on Presenting Morphologic Stage of Rhegmatogenous Retinal Detachment. Retina 10.1097 (2022). [DOI] [PubMed]
- 9.Arend, N. et al. Morphological and clinical characterization of foveal Bulge sign three years after retinal detachment repair: A longitudinal prospective evaluation. Clin. Ophthalmol. 2024, 2261–2270 (2024). [DOI] [PMC free article] [PubMed]
- 10.Lee, Y-H. et al. Longitudinal changes in retinal nerve fiber layer thickness after vitrectomy for rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci.53 (9), 5471–5474 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Chang, S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am. J. Ophthalmol.141 (6), 1033–1043.e1 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Sahoo, N. K. et al. Retina and glaucoma: surgical complications. Int. J. Retina Vitreous. 4, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faude, F. et al. Experimental retinal detachment causes widespread and multilayered degeneration in rabbit retina. J. Neurocytol.30, 379–390 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Lee, M-W. et al. Longitudinal changes in peripapillary retinal nerve fiber layer thickness in high myopia: a prospective, observational study. Ophthalmology126 (4), 522–528 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Shin, J. W. et al. Clinical characteristics of choroidal microvasculature dropout in normal-tension glaucoma versus nonarteritic anterior ischemic optic neuropathy: an optical coherence tomography angiography study. Sci. Rep.11 (1), 21391 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas, J. B. et al. Human optic nerve fiber count and optic disc size. Investig. Ophthalmol. Vis. Sci.33 (6), 2012–2018 (1992). [PubMed] [Google Scholar]
- 17.Chung, H. S. et al. Regional differences in retinal vascular reactivity. Investig. Ophthalmol. Vis. Sci.40 (10), 2448–2453 (1999). [PubMed] [Google Scholar]
- 18.Lee, M. W., Lee, S-E., Lim, H-B. & Kim, J-Y. Longitudinal changes in axial length in high myopia: a 4-year prospective study. Br. J. Ophthalmol.104 (5), 600–603 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Lyssek-Boroń, A. et al. Assessment of vascular changes in patients after Pars plana vitrectomy surgery due to macula-off rhegmatogenous retinal detachment. J. Clin. Med.10 (21), 5054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang, Y. H., Byun, Z. Y. & Hwang, D. D. J. Changes in circumpapillary retinal nerve fiber layer thickness after vitrectomy for rhegmatogenous retinal detachment. Sci. Rep.12 (1), 9630 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurišić, D., Geber, M. Z., Ćavar, I. & Utrobičić, D. K. Retinal Layers Measurements Following Silicone Oil Tamponade for Retinal Detachment Surgeryv. 33 (Taylor & Francis, 2018). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.