Abstract
Short sub-100 ms visual feedback latencies are common in many types of human-computer interactions yet are known to markedly reduce performance in a wide variety of motor tasks from simple pointing to operating surgical robotics. It remains unclear, however, whether these latencies impair not only skilled motor performance but also the implicit sensorimotor learning that underlies its acquisition. Inspired by neurophysiological findings showing that cerebellar LTD and cortical LTP would both be disrupted by sub-100 ms latencies, we hypothesized that implicit sensorimotor learning may be particularly sensitive to these short latencies. Remarkably, we find that improving latency by just 60 ms, from 85 to 25 ms in continuous-feedback experiments, increases implicit learning by 50% and proportionally decreases explicit learning. This resulted in a dramatic reorganization of sensorimotor memory from a 45/55 to a 70/30 implicit/explicit ratio. This 70/30 ratio is more than double that observed in any previous study examining the effect of latency on sensorimotor learning, including a recent study which provided time-advanced visual feedback, suggesting that the low-latency continuous visual feedback we provided is critical for efficiently driving implicit learning. We go on to show that implicit sensorimotor learning is considerably more sensitive to latencies in the sub-100 ms range than to higher latencies, in line with the latency-specific neural plasticity that has been observed. This suggests a clear benefit for latency reduction in computer-based training that involves implicit sensorimotor learning and that across-study differences in computer-based experiments that have examined implicit sensorimotor learning might be explained by differences in unmeasured feedback latencies.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98652-2.
Subject terms: Learning and memory, Motor control, Sensorimotor processing
Introduction
Visual feedback latencies are an inherent component of human-computer interactions that rely on continuous task feedback, from everyday computer mouse use1 to sophisticated virtual and augmented reality systems used for skill training in tasks like the operation of surgical robotic systems2,3, rehabilitation4, and flight simulators5. While these latencies due to delayed system response times are commonly short (< 100 ms) – a level at which they are often not perceived6– research has shown that even sub-100 ms feedback latencies can markedly reduce motor performance in tasks such as reaching, tracking, steering, and collaborative control7–12.
These latencies are common in experiments that measure sensorimotor learning. Reliable reports range from a relatively low 36 ms13 to 60–80 ms14–16 to 145 ms17. However, latencies are unfortunately seldom measured, and values near or above 100 ms are likely not uncommon based on personal communications with colleagues. Latency values above 100 ms are not surprising given that many experimental setups use video projectors for visual feedback, and older projectors commonly inject display latencies on the order of 100 ms18, that would add to latencies from sensor input, computer processing, and graphics output to the display device especially when common double-buffering schemes are used for graphics. The existence of these latencies raises the questions of whether experimental setups may often impair the very sensorimotor learning processes they try to measure, and whether between-setup differences in latency complicate the comparison of findings across different labs.
While the effect of visual feedback latency on sensorimotor learning has been the subject of multiple studies13–15,17,19–31, only a fraction13–15,17,19 reliably measured the baseline latency upon which additional experimentally-imposed delays were administered. Therefore, remarkably, both the actual latency of the experimental conditions and of the reference conditions to which they were compared were unknown in most studies of visual feedback latency to date. This is especially problematic for studies examining short sub-100 ms delays20,22,23, but might not be a critical concern for studies that tested the effects of long experimentally-imposed delays ≥ 1000 ms27–31 or even those with delays ≥ 200 ms21,24–26, which are likely, in retrospect, to be well above the baseline latency, although we cannot be certain. Two long-delay studies dissected learning into implicit and explicit contributions and consistently found both decreased implicit and increased explicit learning following added delays of 200 ms or greater13,15. But with no data at shorter delays, we cannot know whether these findings might apply to the sub-100 ms latency range common in human-computer interactions including experiments for studying sensorimotor learning. This is especially the case because the few studies that examined small delays that might correspond to sub-100 ms latencies (1) reported conflicting results, (2) failed, in all but one case, to measure actual latencies, so that the studied latencies were not known, and (3) did not dissect learning into implicit and explicit contributions19,20,22,23. Two, including the only one to measure latencies19, reported that sensorimotor learning was not affected by added delays of up to 60 ms19,20 whereas the other two, one of which was based only on data from a single animal, reported reduced learning for 50 ms of added delay without measuring the actual latency22,23. It is therefore unclear how sub-100 ms latencies, typical of human-computer interactions and motor learning experiments, might affect sensorimotor learning. If these small latencies can have large effects on sensorimotor learning, then experiment-to-experiment variability in latency within the sub-100 ms range or just above it might explain the wide discordance in the amount of implicit or explicit motor adaptation observed. For example, even for studies using the same error-clamp paradigm to isolate implicit adaptation, the capacity for implicit visuomotor adaptation has been reported to be as low as 12° and as high as 25°, a greater than 2-fold difference32–34.
Moreover, evidence from neurophysiology suggests that latencies as small as 20 ms can disrupt the neural plasticity that may underlie implicit sensorimotor learning. Both the spike timing-dependent plasticity (STDP) that mediates LTP in cortical neurons and the neural plasticity that mediates LTD in cerebellar Purkinje cells are governed by precisely-timed coincident input, with plasticity windows on the order of 20 ms35–40. This leads to two possibilities regarding the effect of short latencies upon implicit sensorimotor learning. If sensorimotor learning relies on neurons tuned to a specific physiological latency associated with sensory input, then it should be exquisitely sensitive to short latencies. Alternatively, if sensorimotor learning could instead rely on one of several subpopulations of neurons tuned to a broad range of different preferred latencies, then it could be robust against changes in latencies provided they are within the distribution of these preferred latencies – which, for cerebellar Purkinje cells, is up to 150 ms wide40. Previous work cannot distinguish between these two possibilities, as results are only consistent for delays ≥200 ms13–15,21,24–31 corresponding to latencies well above 200 ms, which lie beyond both the narrow tuning of individual cells and the width of the distribution of preferred latencies, thus predicting impaired learning in both cases.
To disambiguate between these two possibilities, we examined visuomotor learning with a short, 85 ms visual feedback latency, comparing it against the 25 ms optimized latency of our setup and a larger 300 ms latency, as a long-latency reference. Using an aim report paradigm15,41, we dissected the learning measured on every training trial into implicit and explicit components, and found that implicit learning increased by a remarkable 50% when latency improved by just 60 ms, from 85 to 25 ms, whereas, explicit learning, by contrast, decreased. This dramatically altered the balance between implicit and explicit adaptation from a 45/55 to a 70/30 implicit/explicit ratio, double that observed in any previous study examining the effect of latency on sensorimotor learning13,15,17. Surprisingly, we also found that the sensitivity of implicit learning to sub-100 ms latencies was 5–10 fold greater than for higher latencies. These findings support the idea that implicit sensorimotor adaptation relies on neuronal populations narrowly tuned around a specific latency. The exquisite sensitivity of sensorimotor learning to sub-100 ms latencies that we uncover may explain across-study differences in the amount of implicit adaptation that can be attained.
Results
We examined the effect of short sub-100 ms visual feedback latencies on sensorimotor learning by measuring the learning curves for both implicit and explicit components of the adaptive response following exposure to a 30-degree visuomotor rotation (VMR) at different visual feedback latencies. Secondarily, we examined subsequent extended learning following a block of no-feedback movements and measured the generalization of adaptive responses across different movement directions to provide additional insight into implicit and explicit learning. We studied three latency conditions: 25 ms, 85 ms, and 300 ms. 25 ms, measured with high-speed video, was the lowest visual feedback latency we could achieve following optimization of the experimental setup (see Methods) and thus served as our low-latency reference. To this reference, we added 60 ms (a short additional latency) and 275 ms (a longer additional latency similar to values tested in previous work13,14,21,24,25, used as a long-latency reference) in software to implement the 85 and 300 ms latency conditions, respectively (Fig. 1c,d), in order to investigate how sensorimotor learning is altered by sub-100 ms vs. longer latencies. In brief, participants (n = 42) performed point-to-point reaching movements on a digitizing tablet, while receiving visual feedback in the form of an onscreen cursor that continuously tracked hand motion. The cursor was displayed on a computer monitor that was updated at 120 Hz and positioned horizontally above the tablet (Fig. 1a). After a short task familiarization period where visual feedback was presented at a 25 ms latency, the latency was set with equal probability to 25 ms, 85 ms, or 300 ms for a 100-trial baseline period and subsequent 120-trial VMR training period, where participants moved in a single target direction while a fixed clockwise (CW) or counterclockwise. (CCW) 30° VMR (counterbalanced – see Methods) was applied. They then performed a generalization block of 114 trials across 19 different directions where visual feedback was withheld, followed by 60 extended training trials where visual feedback was provided with the same latency and the same VMR as in the 120-trial training period. This extended training block was followed by a second 114-trial no-feedback generalization block, and finally by a zero-rotation washout block of 25 trials where the latency was maintained, but the VMR was removed, to complete the superset. Participants then performed another superset of baseline, training, generalization, and washout blocks, but with a different visual feedback latency, a different target direction, and an oppositely signed VMR during training. The combination of the VMR inversion and the ±120° target direction change successfully minimized carryover effects from the previous superset. In particular, amplitude of the difference between the baseline movement biases in the pre-training period at the beginning of supersets preceded by CW vs. CCW VMR training was < 0.2° for the overall movement direction and also for implicit and explicit contributions to it, and not significantly different from zero (t < 0.4 and p > 0.7 in all 3 cases). Moreover, visual latency, target directions, VMR directions, and test order were independently balanced across participants within each experiment, so that any systematic effect of target direction, VMR direction or test order would be independent of the latency condition (see Methods). Experiments 1a (n = 24) and 1b (n = 18) were identical except that the target directions differed and participants were studied at two vs. three latencies, respectively, i.e. each participant performed two vs. three supersets of baseline/training/washout.
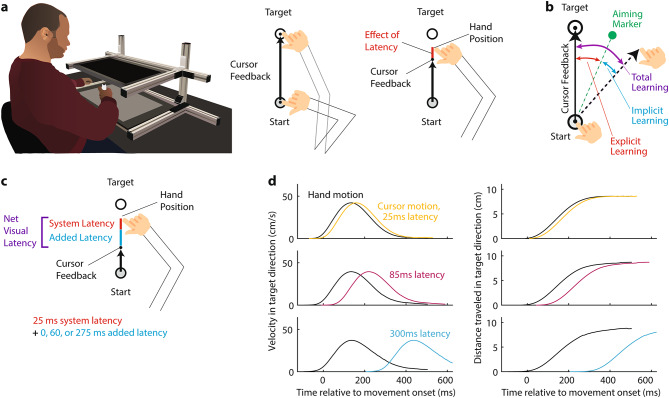
Fig. 1.
Visual latency in a reaching task. (a) Participants made point-to-point reaching movements on a digitizing tablet while a cursor provided visual feedback on a horizontal screen positioned above the hand. Any latency in the visual display will make the cursor lag behind true hand motion. (b) Visuomotor rotation (VMR) with aiming paradigm. Users indicated their aim strategy before each movement by positioning an on-screen marker (green), allowing us to dissect total learning into explicit strategy vs. implicit components (the angle between the aiming marker and target in red vs. the angle between the hand motion and aiming marker in blue). (c)The overall visual latency in our experiments consisted of a base system latency that was optimized down to a 25 ms value that combined with experimentally-imposed delays of 0, 60 or 275 ms to yield latencies of 25, 85 or 300 ms. (d) Comparison the hand-cursor discrepancy induced by a 25 vs. 85 vs. 300 ms latency is in terms of velocity in target direction (left column) and distance traveled in target direction (right column). The black curves indicate the average hand velocity and distance profiles for each latency condition. The colored curves indicate the corresponding velocity and distance profiles for cursor motion. Note that because movement durations were rapid with durations of about only 300 ms, the shifts produced by these latencies led to large changes in the velocity and position profiles between cursor motion and hand motion.
Implicit sensorimotor adaptation increases and explicit strategy decreases when latency is reduced
Inspection of the learning curves for overall adaptation (shades of purple in Fig. 2a) reveals them to be remarkably similar in both shape and amplitude across all three tested latency conditions. Correspondingly, when we quantified overall sensorimotor adaptation by computing its asymptotic level in the training block (operationally defined as the average adaptation over the last 20 trials of the 120-trial training period, excluding trials following rest breaks, see the grey “Late learning” regions indicated in Fig. 2a–c), we found essentially identical overall adaptation levels for all three latency conditions (25 ms: 29.5 ± 0.4°, 85 ms: 28.8 ± 0.4°, 300 ms: 29.0 ± 0.5° [mean ± SEM], see Fig. 2d). Correspondingly, an ANOVA examining the effects of latency, test order, and VMR direction revealed no main effect of latency nor interactions between latency and the other variables, indicating that latency had little effect on overall adaptation. It also found no effect of test order or VMR direction or an interaction between the two (See Supplementary Table S1). However, decomposing adaptation into implicit and explicit components using aim reports41,42 (Fig. 1b, see Methods) revealed large opposing effects for implicit vs. explicit sensorimotor adaptation. An ANOVA on implicit adaptation revealed no main effects of test order or VMR direction, or interaction between them, but a clear main effect of latency (F(2,87) = 13.0, p = 0.000012), with no interactions between latency and either test order or VMR direction. Likewise, an ANOVA on explicit adaptation revealed no main effects of test order or VMR direction, or interaction between them, but a clear effect of latency (F(2,87) = 12.4, p = 0.000018), also with no interactions between latency and either test order or VMR direction. When we looked at implicit adaptation across the three latency conditions, we found it to be weakest in the 300 ms condition, but it grew stronger as the latency of visual feedback decreased to 85 and then 25 ms (shades of blue in Fig. 2b). In contrast, explicit adaptation was strongest in the 300 ms condition but grew weaker as the latency of visual feedback decreased (shades of red in Fig. 2c). Implicit adaptation was a remarkable 50% higher when latency was 25 ms compared to 85 ms (20.4 ± 1.3° vs. 13.8 ± 1.4°, respectively, t(66) = 3.4, p = 0.00049) and twice as high compared to the 300 ms condition (20.4 ± 1.3° vs. 10.8 ± 1.6°, t(65) = 4.7, p = 7.2 × 10− 6). In contrast, explicit adaptation was 40% lower when latency was 25 ms compared to 85 ms (9.1 ± 1.3° vs. 15.0 ± 1.5°, respectively, t(66) = 3.0 p = 0.0022) and 50% lower compared to the 300 ms condition (9.1 ± 1.3° vs. 18.4 ± 1.4°, t(65) = 4.9, p = 3.2 × 10− 6).
Fig. 2.
Small reductions in latency improve implicit and decrease explicit learning. (a–c) Learning curves for (a) overall, (b) implicit, and (c) explicit adaptation for the three latency conditions studied shows increasing implicit and decreasing explicit learning as latency is reduced, with overall learning largely unaffected by latency. The gray rectangle indicates the late learning period analyzed in panel d. Vertical dashed lines indicate trials following 60s breaks. These trials were excluded from the main analysis and hence not shown here, but are examined in Figure S3 in the supplementary materials. (d) Late learning vs. latency. While overall learning is largely unaffected by latency, implicit learning is increased by 50% when latency is reduced from 85 to 25 ms and doubled when latency is reduced from 300 to 25 ms. In contrast, explicit learning is reduced by 40% when latency is reduced from 85 to 25 ms and by 50% from 300 to 25 ms. (e) Sensitivity of implicit learning to changes in latency. This sensitivity, the rate at which implicit learning increases per unit of latency decrease, is nominally 5-fold higher across the sub-100 ms latency interval between 25 and 85 ms compared to the interval between 85 and 300 ms. Shading and error bars indicate ± SEM; note that error bars for overall learning in panel (d) are shown in white for visibility, as they would be occluded by the circle symbols otherwise.
A subsequent 60-trial extended training block within each superset, with a target direction, VMR, and latency that were all identical to the 120-trial training block, was separated from that training block by 114 no-feedback trials in different target directions (these trials were used to measure directional generalization - see Methods and the generalization analysis section later in the Results). This extended training block provides information about the effects of extended learning, as 50% more training was added to the initial training block. Analysis of sensorimotor learning in this block mirrors the findings from the first training block presented above. As before, we averaged data from the final 20 trials of the block to assess the asymptotic adaptation levels for overall, implicit, and explicit adaptation. An ANOVA on overall adaptation again revealed no main effect of latency nor interactions between latency and the other either test order or VMR direction. Moreover, there was no effect of test order or VMR direction or an interaction between the two (See Supplementary Table S1). In contrast, an ANOVA on implicit adaptation revealed no main effects of test order or VMR direction, or interaction between them, but a clear effect of latency (F(2,87) = 8.6, p = 0.00038), with no interactions between latency and either test order or VMR direction. And, an ANOVA on explicit adaptation revealed no main effects of test order or VMR direction, or interaction between them, but a clear effect of latency (F(2,87) = 8.2, p = 0.00055), with no interactions between latency and either test order or VMR direction. In line with the training block data above, we again observed little difference in overall adaptation between latency conditions (Fig. 3a,d), but found pronounced increases in implicit adaptation (Fig. 3b,d) and decreases in explicit adaptation (Fig. 3c,d) as latency was reduced. Implicit adaptation was in this case 60% higher when latency was 25 ms compared to 85 ms (19.9 ± 1.3° vs. 12.4 ± 1.7°, respectively, t(66) = 3.5, p = 0.00036) and again nearly double compared to the 300 ms condition (19.9 ± 1.3° vs. 11.2 ± 1.7°, t(65) = 4.0, p = 7.2 × 10− 5). In contrast, explicit adaptation was again 40% lower when latency was 25 ms compared to 85 ms (9.8 ± 1.4° vs. 16.6 ± 1.7°, respectively, t(66) = 3.1, p = 0.0015) and 50% lower compared to the 300 ms condition (9.8 ± 1.4° vs. 18.5 ± 1.4°, t(65) = 4.3, p = 2.7 × 10− 5). Together these findings provide evidence for a dramatic increase in implicit sensorimotor learning alongside complementary decreases in explicit strategy arising from a small 60 ms reduction in the latency of visual feedback in the sub-100 ms range.
Fig. 3.
Extended learning data. (a–d) Same as Fig. 2a–d but for the 60-trial extended learning period. (e) Sensitivity of late implicit learning to changes in latency. The left pair of bars compares sensitivities across the 25–85 ms interval vs. 85–300 ms intervals for the extended learning data. The right pair shows this comparison for the combined learning and extended learning data. In both cases, the sub-100 ms interval displays markedly higher sensitivity of implicit learning to changes in latency than the longer latency interval. As in Fig. 2, post-break trials are not shown here, but are examined in Figure S3 in the supplementary materials. Shading and error bars indicate ± SEM.
The sensitivity of implicit sensorimotor adaptation to latency markedly increases at low visual feedback latencies
These findings reveal that short, sub-100 ms visual feedback latencies can not only markedly reduce implicit adaptation, but also have a disproportionately larger effect than the higher latencies previously studied. In particular, training period data show that the 60 ms decrease in latency from 85 to 25 ms results in roughly double the improvement in implicit sensorimotor adaptation than the 3-fold greater 215 ms decrease in latency from 300 to 85 ms (6.6 ± 1.9° vs. 3.1 ± 2.2°). This results in a sensitivity of adaptation per unit latency reduction that is nominally 7-fold greater for the 25–85 ms interval compared to the 85–300 ms interval (1.09 ± 0.32° vs. 0.14 ± 0.10° of adaptation per 10 ms of added latency, respectively (Fig. 2e)). Data from the 60-trial extended training block that followed the first generalization block, mirror the training block findings. In this case, they show that the 60 ms decrease in latency from 85 ms to 25 ms results in triple the improvement in implicit sensorimotor adaptation than the 215 ms decrease in latency from 300 ms to 85 ms (7.5 ± 2.1° vs. 1.2 ± 2.4°). This results in a sensitivity that is nominally 20-fold greater over the 25–85 ms interval compared to the 85–300 ms interval (1.26 ± 0.35° vs. 0.06 ± 0.11° of adaptation per 10 ms of added latency, respectively (Fig. 3e)). Combining these training block and extended trainiing block data to obtain the most accurate comparison of the sensitivity between adaptation and latency, reveals a sensitivity of 1.18 ± 0.32°/10 ms on the 25–85 ms interval compared to 0.10 ± 0.10°/10 ms on the 85–300 ms interval (F(1,98) = 7.5, p = 0.0074, Fig. 3e). The marked contrast between these intervals underscores the exceptional sensitivity of implicit adaptation to short visual feedback latencies. Our results demonstrate that, as latency is reduced, this sensitivity increases in a rapidly accelerating non-linear trajectory. If this trajectory were to be maintained for latency reductions beyond 25 ms, substantial further improvement in implicit learning might be attained for latency improvements exceeding what we were able to achieve (see Methods).
Effects of latency on movement speed
Within the same analysis window, an ANOVA found no effect of latency on peak movement speed across the three latency conditions (25 ms condition: 51.4 ± 1.4 cm/s [mean ± SEM]; 85 ms condition: 48.6 ± 1.9 cm/s; 300 ms condition: 47.5 ± 2.8 cm/s, F(2,98) = 0.9, p = 0.40). A similar analysis found an effect of latency on the time it took participants to reach peak speed (25 ms condition: 145 ± 4 ms; 85 ms condition: 156 ± 6 ms; 300 ms condition: 168 ± 7 ms, F(2,98) = 3.9, p = 0.024). Post-hoc 2-tailed comparisons found significant differences between the 25 and the 300 ms conditions (p = 0.0051) but not between the 25 and 85 ms conditions (p = 0.14) or the 85 ms and 300 ms conditions (p = 0.20). These findings are illustrated in Fig. S1.
Visual feedback latencies affect how both implicit and explicit adaptation generalize to different movement directions
We proceeded to investigate how visual feedback latencies might affect the generalization of adaptation to different movement directions, as this may provide evidence about its underlying neural representation43–46. During generalization blocks performed after both the training and extended training periods, participants reached to targets in 19 different movement directions in the absence of visual feedback (−135° to 135° relative to training, spaced 15° apart in a pseudorandom order, Fig. 4a). The overall across-direction generalization patterns that we observed (shades of purple in Fig. 4b) have shapes that resemble the combination of a hump-shaped component and a fixed vertical offset that correspond, respectively, to locally-generalizing and globally-generalizing contributions46,47. We thus characterized the across-direction generalization patterns as the sum of a gaussian-shaped local component and a flat-shaped global component (i.e. a fixed offset; see Eq. 1), by regressing each generalization pattern onto these two components. The two-parameter fits associated with these regressions explain 89%, 83%, and 73% of the variance in the participant-averaged data for the overall generalization pattern for the 25 ms, 85 ms, and 300 ms latencies, suggesting that they capture the shape of these patterns well.
Fig. 4.
Latency reductions improve locally-generalizing implicit learning and decrease globally-generalizing explicit learning. (a) Generalization of VMR learning was measured with no-feedback movements across 19 different test directions that were centered on the trained target direction in 15° steps. (b–d) Effects of different latencies on the shape of (b) overall, (c) implicit, and (d) explicit generalization patterns. Thick lines indicate Gaussian fits (Eq. 1) used to dissect adaptation into its locally-generalizing and globally-generalizing components. Implicit learning primarily generalizes locally and is stronger at lower latencies, whereas explicit learning primarily generalizes globally and is stronger at high latencies. (e–g) Local and global generalization components extracted using Eq. 1. Decreased latencies display higher locally-generalizing and lower globally-generalizing overall learning, driven by increases in the primarily locally-generalizing implicit learning and decreases in the primarily globally-generalizing explicit learning. Error bars indicate ± SEM.
When we dissected the overall generalization pattern (Fig. 4b) into separate patterns for implicit and explicit generalization (Fig. 4c–d), we found that whereas the overall pattern was comprised of sizeable contributions from both local and global components (Fig. 4e), implicit generalization was dominated by its local component, whereas explicit generalization was dominated by its global component (Fig. 4f–g). Implicit generalization displayed local components of 5.5–8° vs. global components of 1.4–2.1° for the 25 ms, 85 ms, and 300 ms latencies, in stark contrast to local components of < 1° vs. global components of 7.5–18.5° for explicit generalization. Moreover, we found that, like implicit adaptation during the training and extended training periods, the local component of implicit generalization grew as latency decreased (5.6 ± 0.9° vs. 7.7 ± 0.7° for 85 ms vs. 25 ms, t(66) = 1.7, p = 0.043; and 5.5 ± 1.0° vs. 7.7 ± 0.7° for 300 ms vs. 25 ms, t(66) = 1.7, p = 0.043). And like explicit adaptation during the training and extended training periods, the global component of explicit generalization contracted as latency decreased (16.9 ± 1.9° vs. 7.9 ± 2.1° for 85 ms vs. 25 ms, t(30) = 3.2, p = 0.0015; and 18.3 ± 1.7° vs. 7.9 ± 2.1° for 300 ms vs. 25 ms, t(30) = 3.9, p = 0.00026).
Together, our findings demonstrate that even short sub-100 ms latencies can dramatically alter the internal composition of sensorimotor learning by decreasing implicit adaptation and increasing explicit strategy. Moreover, we find that implicit sensorimotor learning is far more sensitive to sub-100 ms latencies than larger latencies, indicating that latency reduction within the sub-100 ms range can substantially improve implicit learning.
Discussion
Here we investigated the effects of short, sub-100 ms visual feedback latencies on implicit and explicit sensorimotor adaptation. Specifically, we compared the learning and generalization of a visuomotor rotation when visual feedback was presented with a short, optimized 25 ms latency vs. an intermediate sub-100 ms latency (85 ms) and a longer 300 ms latency that provided a reference condition comparable to the long latencies examined in previous studies that dissected implicit and explicit sensorimotor learning. We found that, reducing latency by just 60 ms during training, from 85 to 25 ms, led to a dramatic 50% increase in implicit adaptation and a complementary 40% decrease in explicit adaptation. This resulted in a reorganization of sensorimotor memory from a 45/55 to a 70/30 implicit/explicit learning ratio. Remarkably, the sensitivity of implicit adaptation to changes in latency was about 7-fold greater for short latencies (reducing latency from 85 to 25 ms) compared to longer latencies (reducing latency from 300 to 85 ms). This highlights the outsized importance of sub-100 ms latencies in driving increased implicit sensorimotor adaptation relative to more commonly studied longer latencies. These effects were consistent when separately examined in the subsequent extended learning period, whereby reducing latency from 85 to 25 ms led to a 60% increase in implicit adaptation, with the corresponding sensitivity to latency about 20-fold greater for short vs. long latencies. Secondarily, we examined the directional generalization of learned adaptation under each latency condition and found implicit sensorimotor learning to be dominated by locally-generalizing learning, with explicit learning instead dominated by globally-generalizing learning. This indicates that our dissection of overall adaptation identifies implicit and explicit components with very different underlying representations.
The most important findings are related to the surprisingly large impact that we uncover for short sub-100 ms latencies on implicit learning, especially when compared to previous work at higher latencies. Whereas previous studies had shown that latency increases can impair implicit and promote explicit learning at high latencies13,15, we find not only that this effect extends down to sub-100 ms latencies in the range relevant for common human computer interactions, but also that the strength of this effect grows dramatically in that range. Specifically, we demonstrate an accelerating reorganization of sensorimotor memory from a 40/60 implicit/explicit learning ratio at 300 ms of latency to 45/55 at 85 ms to a 70/30 ratio at 25 ms of latency. Critically, this 70/30 ratio is more than double any previously reported in studying the effect of feedback latency13,15,17. This underscores the potential of low latency feedback to unleash implicit learning, but also suggests that commonly-used setups, even when the latencies that characterize them are relatively modest, on the order of 85 ms, can unwittingly decrease implicit learning and increase explicit learning. Finally, we find that the sensitivity of implicit learning to latency differences (i.e. the increase in implicit learning per unit latency reduction) is many-fold greater in the range of 25–85 ms than in the range of 85–300 ms which is, in turn, many-fold greater than what has previously been observed at longer latencies13,15. This indicates that even modest improvements in latency can drive meaningful improvements in implicit learning in the sub-100 ms range. Together these observations underline the need to widely measure, report, and ultimately minimize these latencies.
Previous work on the effect of feedback latencies on motor adaptation
Most studies examining the effect of visual feedback latency on sensorimotor learning have studied large latencies above 200 ms13–15,21,24–31 and often beyond 1000 ms15,27–31. In fact, we were able to identify only four studies that examined the effects of latency on sensorimotor learning that used delays of 100 ms or less and thus could have potentially examined sub-100 ms latencies (and we could find no additional studies with delays below 200 ms). Three of these four, however, neglected to measure the any of the actual latencies in their experimental conditions, and so the delayed condition, the baseline condition, or both could have had latencies above 100 ms, rather than below it20,22,23. The fourth study19 measured a latency of 36 ms at baseline, so that the 60 ms delay they studied corresponded to a 96 ms latency. However, despite a close correspondence to the 25 ms and 85 ms latencies studied here, they found no effect of delay. But because they did not dissect adaptation into implicit and explicit components, the only available measure was overall learning where we also find no effect of delay. Consequently, the details of this result are in line with what we find, despite a robust effect in our data when implicit and explicit adaptation are dissected out, because the 60 ms delay we levied had opposing effects on implicit and explicit contributions that largely cancelled out when overall learning was measured. It should be noted, however, that this study attempted to isolate implicit learning by requiring short 200 ms reaction times, which can reduce explicit strategy48–51, but the effect of this manipulation may have been tempered by the endpoint-only task feedback employed, which has the opposing effect of increasing explicit strategy41,52,53.
For the three studies that examined sub-100 ms delays but did not measure actual latencies, it would be useful if we could make accurate post-hoc estimates of the baseline latencies. The first of these studies21 used the analog signals from x and y potentiometer-based sensors fed directly into an oscilloscope for visual feedback. Because oscilloscopes from the era of this study had CRT displays, the latency would likely be limited by phosphor persistence and thus be rather small, likely below 10 ms, although without the make and model number for the oscilloscope used or direct information about its phosphor screen coating, we cannot be certain as phosphor chemistry can vary and greater persistence is possible. And the potentiometer-based position sensor input should have had little delay unless the output signal was intentionally smoothed by adding capacitance, which was not indicated, suggesting that the baseline visual display latency could have been rather small, perhaps as low as 10 ms, but again, we cannot be certain.
The other two studies22,23 were more likely to have been plagued by higher baseline latencies. In both of these studies, a signal from a screen-mounted touch sensor was fed into a computer to detect movement endpoint so that LCD shutter glasses could be triggered to open and allow for direct endpoint feedback of the hand touching the screen where the target was displayed. The shutter glasses were reported to be able to open in just 1 ms, suggesting they added little to the baseline latency. However, information was provided neither about the latency of the touch sensor nor the time needed to read-in the sensor output from it nor to process the data to determine movement completion. Contemporary devices that rely on touch sensors, such as smartphones and tablets, often suffer from touch-to-display latencies of up to 100–200 ms54–56, suggesting that the 3-decade-old the Kitazawa et al. setup – with the first study published in 1995, when touch sensors were a newer technology – may have suffered from latencies on the high end of that range or higher. Likewise, it’s difficult to be certain about the latency associated with reading-in the sensor data to the in-the-loop computer, processing it, and sending out the shutter glass command signal, but even in our highly-optimized setup, that used a modern, much faster PC, the computer in-the-loop latency likely doubled the sensor latency (see Methods).
These three studies where latency was not measured yielded conflicting results. Held et al., 199120, like Tanaka et al., 201119 which measured latency, found no effect of latency for imposed delays up to 60 ms. However, as with the Tanaka study19 the Held et al. study did not dissect learning into implicit and explicit components, and so it may also have been that a latency-driven decrease in implicit learning could have been offset by an increase in explicit learning, especially as no specific efforts to abate an explicit contribution were reported. At odds with these negative results, two studies by Kitazawa et al.22,23 reported significant effects for 50 ms delays. One of these, however, was based on data from a single monkey23. The other was based on a more reasonable sample size of 21 in humans22; however, like the Tanaka et al. and Held et al., studies, implicit and explicit learning were not dissected out of the measured adaptation. It is thus unclear to us why the results would be positive in this case, especially as our post-hoc analysis of the probable baseline latency suggests that it was likely higher than that in both the Tanaka and Held et al. studies, and this increased baseline latency should blunt the sensitivity of the adaptive response to visual feedback delays. In sum, previous work employing short 50–60 ms added delays provides conflicting results about the effect of sub-100 ms latencies on sensorimotor learning, likely due to a combination of unmeasured latencies for the experimental conditions and varying contributions from implicit and explicit learning that were not dissected out.
Notably, we found two previous studies that dissected out the effects of latency on implicit and explicit sensorimotor learning13,15. These studies, however, examined only much larger latencies, above 200 ms in both cases, and thus did not provide information on the sub-100 ms latencies prevalent in human-computer interactions including experiments for studying sensorimotor learning when latency is not isolated as a variable of interest. The Brudner et al. study15 measured a baseline latency of 70 ms, and examined an additional delay of 1000 ms corresponding to latencies of 70 and of 1070 ms for the two conditions they compared. Although they found clear evidence for decreased implicit learning in the higher-latency condition, the baseline condition was already dominated by explicit learning, as 70–75% of overall adaptation was explicit compared to only 25–30% for implicit (34° of explicit vs. 12° of implicit for the 45° VMR). Thus their 70 ms baseline condition displayed far less implicit learning than we observed in our 25 ms baseline condition (25–30% vs. 65–70% of overall adaptation, corresponding to 12° vs. 20° of implicit learning, despite the larger 45° perturbation). Their result also displayed less implicit learning than our 85 ms or 300 ms latency conditions, corresponding to 60 and 275 ms delays in our study. The surprisingly low level of implicit learning observed in the Brudner et al. baseline condition might be explained by two factors in their experimental design known to reduce implicit and promote explicit contributions to sensorimotor learning: the use of larger amplitude perturbations57 and endpoint-only feedback41,52,53,58. They also studied an even longer 5000 ms delay with similar results. We also note that the larger amplitude perturbation they used (45° instead of 30°), might also affect the ratio of implicit to explicit learning as implicit learning capacity may be limited32,57. The Schween et al. study13 was similar to Brudner et al. but examined delays of 200 and 1500 ms rather than 1000 and 5000 ms, and, like the current study, employed a 30° perturbation. They reported a baseline latency of 27 ms; however, this estimate was not based on direct comparison of visual feedback and live hand motion as in the current study, Brudner et al.15 or Tanaka et al.15,19. Instead, they measured the partial latency from a statement in their code calling for the sensor reading associated with movement termination and the reading of a photodiode signal that confirmed the display of endpoint feedback. This measurement is useful but excludes the latency between actual arm motion and the time at which sensor feedback of this motion is made ready to be read-in by the computer, i.e. the sensor input latency, which constitutes nearly half of the 25 ms baseline latency in our setup. Like Brudner et al., the results from Schween et al., showed significant reductions in implicit learning for the high latencies they studied, with corresponding increases in explicit learning. At their baseline latency, they found a level of implicit learning (40–45% of the total learning, corresponding to 11°) that was already dominated by explicit learning and far smaller than the 65–70% level, corresponding to 20°, we observed at 25 ms. This lower-than-expected level is consistent with the combination of a baseline latency that was likely higher than the reported 27 ms value and the use of an endpoint-only rather than continuous feedback task. Grossly, however, both the Brudner and Schween studies are consistent with our current results in that they reported a balance between implicit and explicit learning that shifts towards being more explicit-dominated as latency increases, suggesting that reductions in latency promote implicit learning, whereas increases promote explicit learning. The current study adds the critical information that this balance is shifted even for small latency increases in the sub-100 ms range, relevant to human-computer interactions and experiments studying sensorimotor learning, and that the sensitivity of this shift to changes in latency is, in fact, far greater in this sub-100 ms range than at the longer latencies at which it had previously been identified.
A recent study tried to assess the effects of reducing latency below the base setup latency. For each movement, Wang et al.17 estimated heading direction shortly after movement onset and projected this direction out to an endpoint distance where feedback was provided. This could be viewed as reducing the feedback latency at this endpoint by the movement time for each trial. The study consistently found that adaptation was higher for their advanced feedback condition compared to their standard base latency endpoint feedback condition, but not higher than when continuous visual feedback was provided, as in the current study. For their VMR condition, the only condition where implicit and explicit learning were measured and the one which is most comparable to the current and to previous studies, they found that implicit accounted for only about 25% of the total learning for their standard feedback condition and about 35% with advanced feedback. However, this 35% of the total learning in the advanced feedback condition is far smaller than the 65–70% observed for implicit learning for low latency condition in the current study, with the absolute implicit adaptation level (12–13°) also smaller than what we observe (20°), despite a larger 45° VMR perturbation. Even in their error-clamp condition, which employed a large 15° error signal to provide a large, continuous drive for adaptation that dwarfed the modest 0.5–2° errors that drove adaptation after the first 3–5 trials in the initial training period in the current study (see the overall learning plots in Figs. 2a and 3a), Wang et al. observed asymptotic learning that either merely matched implicit learning for our minimum-delay condition (20°, from their online experiments) or fell short (15°, from laboratory-controlled experiments). These observations suggest that it is the reduction of latency from positive baseline levels to near-zero values, rather than the creation of “negative” latency with advanced feedback, which is likely responsible for the enhanced learning observed by Wang et al.17 We do note however, that complicating comparisons with our work and other studies where latencies were precisely controlled, the effective latency reduction for the advanced feedback condition varied widely with the movement time for each trial. This resulted in a wide range even for the mean latency reductions for different individuals, with the reported values ranging between 0 and 300 ms.
Effects of temporal latency on neural plasticity
Evidence from neurophysiology suggests that short, sub-100 ms latencies can strongly disrupt the neural plasticity that underlies learning. Spike-timing dependent plasticity in cortical synapses relies on a precise temporal coupling between synaptic input and cell firing that dictates a switch between maximally positive and maximally negative plasticity in just 10–20 ms35–39. In contrast, it had long been thought that plasticity in the cerebellum did not require such precise temporal coincidence. Plasticity in cerebellar Purkinje cells – widely associated with error-driven sensorimotor learning59–62 – is governed by paired activity of climbing fibers carrying error signals and parallel fibers carrying contextual sensorimotor information63–65, with the coincidence of this timing believed to be tolerant of a broad 150 ms range of climbing fiber-parallel fiber latencies. This belief was based on population data from the cerebellar vermis66,67. However, more recent work that examined neural responses at different latencies within individual vermal neurons found that, instead, each neuron exhibits much tighter tuning - with a tolerance of just 20–30 ms or less – around its preferred latency40. The previously reported broad tuning would thus be due to different cells in the population displaying different preferred latencies ranging from 0 to 150 ms around which the tight 20–30 ms plasticity window operates. The narrow tuning of these plasticity windows around specific preferred latencies – where plasticity is reduced by latencies both above and below the preferred one – suggests that negative latencies should impair learning like positive latencies do, with the ideal feedback latency being zero. This observation lines up with our assessment of the enhanced learning observed by Wang et al. as likely due to reducing latency from positive baseline levels to near-zero values rather than the creation of negative latency. Together, the current evidence suggests that the neural plasticity underlying learning, in both cortex and cerebellum, requires tight temporal coupling of neural activity that could be disrupted by latencies as small as 50–100 ms, in line with the high sensitivity of implicit sensorimotor learning to latency that we demonstrate here. The requirement for tight temporal coupling for neural plasticity to proceed can be viewed as a neurophysiological mechanism for aligning neural plasticity with agency, promoting plasticity underlying implicit sensorimotor learning when agency is high and restricting it when agency is low68,69.
Implications for sensorimotor learning studies
Our finding that even short latencies can dramatically reduce implicit adaptation may explain wide across-study differences in the amount of implicit adaptation, which persist even when studies with closely aligned experiment design are compared. For example, three studies using the same type and magnitude of visuomotor perturbation – a 15° clamped cursor error – for comparable durations of training and multi-target training schedules, reported implicit adaptation levels ranging from 12° to about 25°32–34. Our findings suggest that previous reports of low asymptotic implicit adaptation may be, at least in part, due to high visual feedback latencies in the experiment setup. Unfortunately, we cannot know the exact contribution of different setup latencies to the different levels of implicit adaptation reported, as these were not assessed in the first place. This highlights the need to widely measure and report these “baseline” latencies so that latency effects can be meaningfully accounted for when interpreting study findings and the need to minimize these latencies to abate setup-specific learning impairments that can occur even for sub-100 ms latencies. Our experience indicates that low-latency performance can be attained by optimizing software, such as by using direct rather than buffered graphics updates, in combination with modern low-input-lag computer gaming displays. Taking latency into consideration becomes even more important with the emergence of sensorimotor learning studies that are conducted online rather than in the lab17,70. With each participant using their own device, it is challenging to measure latency and, crucially, inter-individual differences in device latency could inject substantial inter-individual variability into implicit and explicit sensorimotor learning measurements due to differences in participants’ devices rather than to their learning abilities, as well as reduce group-average implicit and increase group-average explicit learning to an extent that depends the on severity and frequency of high-latency visual feedback experienced by remote participants.
Materials and methods
Participants and consent statement
A total of 42 individuals took part in the study (average age: 22.0 ± 4.3 y.o., 17 male, 2 left-handed, 5 ambidextrous based on the Edinburgh Handedness questionnaire71; all participants used their right hand for the reaching task and operated the aim-report knob with their left, see details below). Participants provided informed consent in line with the Declaration of Helsinki, and the study protocol was approved by the Harvard University Committee on the Use of Human Subjects (IRB board).
Experiment setup and general task description
Participants sat on a chair and made 9-cm point-to-point reaching movements on a 200 Hz digitizing tablet (Intuos 3, Wacom Co., Japan) while grasping a lightweight plastic handle that contained a tablet stylus. Vision of the hand and arm was occluded. Instead, participants could receive continuously updated visual feedback in the form of a cursor (2.6 mm diameter) displayed on a screen mounted horizontally above the tablet (BenQ XL2411, refreshed at 120 Hz, Fig. 1a). The experiment room was dimly lit to improve screen contrast and facilitate attention to the task. To the left of the setup, we placed a knob which could be used to indicate the aiming target before each reaching movement. Participants were encouraged to move quickly to reach and stop at the target within 350 ms, in which case the experiment computer played a bell sound. The experiment was coded and executed using Matlab (Mathworks, Natick, MA) with the Psychtoolbox 3.1 extension for reading the digitizing tablet and for graphical output to display targets and to provide cursor feedback72.
Measurement and sources of system latency
To measure system latency, we simultaneously recorded physical motion of the handle and cursor motion using a high-speed (240 fps) camera positioned to see both in the same field of view. An LED was attached to the handle to facilitate tracking its position through the video. To obtain a measurement, we rapidly and irregularly moved the handle back and forth in the lateral direction for 5–10 s, while keeping it in the camera’s field of view. We processed the video to extract both handle and cursor motion in 2D, and used a form of cross-correlation (that was corrected for biased normalization present in standard cross-correlation that would skew latency estimates towards zero, especially for particularly short-duration video segments) to identify the latency between them. For our optimized setup, we found a latency of 24.8 ± 0.2 ms (mean ± SEM across four videos). Our latency assessment tool is available on Github (https://github.com/harvardmotorlab/Latency), along with a guide for optimizing latency for in similar experiment setups.
To estimate the effect of different setup components on the overall latency of our optimized system, we examined different hardware and software configurations. To assess the latency due to using the digitizing tablet as a source of position information, we replaced it with a low-latency computer mouse, which reduced latency by about 16 ms, i.e. 60–70% of the 25 ms overall latency. Despite the tablet being responsible for most of the overall latency, however, we did not substitute it with a mouse, as the tablet provided higher position precision and, importantly, provided absolute position measurements that would be reliable even if the pen were lifted and placed on a different location on the tablet. To assess the effect of the experiment environment, including execution time, we compared latency outside vs. within the experiment environment, using mouse input in both cases, finding that latency was merely 0.8 ms lower outside Matlab, i.e. about 3% of the 25 ms overall latency. Finally, our screen’s input lag was 4.4 ms73 or about 18% of the overall latency. A notable finding during the process of optimizing our setup was the importance of how the software handled screen updates. We coded our experiment to use asynchronous screen updates, as we found that synchronous screen updates added an additional ~ 17 ms (60–70% more) to the latency.
Implementation of increased latency conditions
For the 85 ms and 300 ms conditions, we imposed additional latencies of 60 ms and 275 ms respectively in addition to the base system latency. We validated these added latencies using the video method outlined above, obtaining 85.1 ± 1.2 and 299.6 ± 0.4 ms, respectively.
Experiment schedule
The experiment consisted of a familiarization block (17 movement trials to one of 19 different target directions with a latency of 25 ms and no visuomotor rotation (VMR)) followed by two (Experiment 1a) or three (Experiment 1b) supersets of experiment blocks in which sensorimotor learning was assessed. Within each superset, visual feedback, when provided, was delayed by a single latency (25 ms, 85 ms, or 300 ms) that was switched between supersets so that each participant in Experiment 1a experienced two latencies and each in Experiment 1b experienced three. The ordering of presentation of the three latencies was such that the probability of experiencing each latency in the first superset was 1/3 (as was the probability in the 2nd superset, and (in Experiment 1b) the probability in the 3rd one). Each superset began with 57 baseline trials with no VMR (3 pseudorandomly-ordered reaches each to targets in 19 movement directions, spaced at 15° apart and centered at the direction that would subsequently be used for VMR training). Participants then performed 16 additional no-VMR trials in the training direction followed by 120 training trials with a VMR of ± 30°. Both experiments were counterbalanced so that half of the supersets trained a + 30° counterclockwise VMR and half trained a −30° clockwise VMR, independently of the experienced latency. Additionally, half the participants began with a superset that trained a + 30° VMR and half that trained a −30° VMR. To reduce the possibility that learning from one superset might carry over to the next, the orientation of the VMR and the direction of the trained target location were always switched between supersets. This target direction was chosen from {−75°, or + 75°} in Experiment 1a and from {0°, −120°, or + 120°} in Experiment 1b, all equally likely, with 0° indicating the 12 o’clock direction.
The 120-trial single-direction training block was followed by a block testing for directional generalization of the trained adaptation. This 114-trial generalization block consisted of six reaches without visual feedback to each of the 19 different directions in the 114-trial pre-training baseline block – arrayed − 135° to 135° around the trained direction in increments of 15° (Fig. 4a). This was followed by a 60-trial extended training block, with the same VMR, target direction, and latency as in the training block, and then by a second 114-trial generalization block. Completing the superset, the second generalization block was followed by a 25-trial washout block, in which participants continued to make reaches to the same trained target location with the same visual feedback latency but with no VMR, i.e. the rotation turned off, to further reduce the chance of carryover of adaptation between supersets. Rest breaks, each at least one minute in duration, were given every 57 trials within the generalization blocks, and every 20–30 trials within the training and extended training blocks.
Decomposition of adaptation into implicit and explicit components using aim reports
We assessed implicit and explicit adaptation using aim reports immediately before each movement15,41,42. Prior to movement initiation, participants indicated the aim point location that they thought would result in on-target cursor motion by placing an aiming marker on the screen. The aiming marker’s distance from the start position was held constant and its direction was controlled using a rotating knob by the participant’s left hand. Participants “locked” their aiming selection by clicking on the knob before each movement. Once locked, the aiming marker’s location was fixed, and it remained visible until the end of the movement.
Data analysis
Measurement of reach and aim directions and dissection of adaptation into implicit and explicit components
Position data were recorded at 200 Hz and differentiated to estimate movement velocity. To estimate movement onset, we first found the moment of peak movement speed, and then went backwards until we found the moment velocity first exceeded a threshold of 6.35 cm/s. Movements were fast and so the time from this movement onset point to the peak speed point averaged 150–160 ms. We defined reach direction as the direction of a vector from the participant’s position at the movement onset point to the participant’s position at the peak speed point, and we defined relative target direction as the direction of a vector from the participant’s position at the movement onset point to the target. Relative reach direction was then defined as the difference between the reach direction and the relative target direction. We defined aim direction as the difference between the direction of a vector from the start position to the aiming point and the direction of a vector from the start position to the target.
For training and extended training, we defined explicit adaptation as the aim direction relative to the target, and implicit adaptation as the difference between the reach direction and the aim direction as illustrated in Fig. 1b41,42,57,74. For generalization, we used two different methods to dissect generalization patterns into their implicit and explicit components. In the first subtype (all generalization blocks in Experiment 1a and half the generalization blocks in Experiment 1b), we isolated implicit generalization by instructing participants to aim their hand through each probe target. In the second subtype (half the generalization blocks in Experiment 1b), we measured both implicit and explicit generalization by asking participants to report their aim prior to each probe in the same way as during training/extended training trials. To combine data from + 30° VMR (counterclockwise) and − 30° (clockwise) VMR supersets, we flipped clockwise data prior to further analysis. Trials immediately following rest breaks were not included in the main analysis, since these ~ 60 s breaks result in rapid decay of the temporally-volatile component of adaptation47,75,76, and the focus of this study was on examining the effect of visual latency on implicit vs. explicit adaptation. See Figure S3 in the supplementary materials for an analysis of these post-break trials.
Dissection of adaptation into locally-generalizing and globally-generalizing components
We extracted locally-generalizing and globally-generalizing adaptation components by regressing the observed generalization pattern onto a local training-direction-centered gaussian and a constant offset46,47,77,78. In Eq. 1, the height of the gaussian  , corresponds to the strength of the locally-generalizing component that is centered on the corresponding training direction
, corresponds to the strength of the locally-generalizing component that is centered on the corresponding training direction 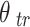 and the height of the constant offset
and the height of the constant offset  corresponds to the strength of the globally-generalizing component. Note that the width of the gaussian,
corresponds to the strength of the globally-generalizing component. Note that the width of the gaussian,  , was fixed to 30° in line with previous findings46.
, was fixed to 30° in line with previous findings46.
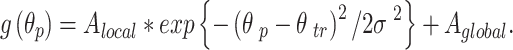 |
1 |
This curve was fit to each individual’s data, separately for the implicit and explicit generalization data and separately for each latency condition.
Baseline subtraction
To minimize the effects of any subject-specific biases and superset-to-superset carryover effects, we subtracted the average baseline from our data, separately for each individual and latency condition (i.e. within each superset), for both reaching and aiming directions (and thus for measurements of both implicit and explicit learning). For the training and extended training data, this baseline consisted of the last 15 trials before the onset of the ± 30° VMR training. For the generalization data, this baseline consisted of the last pre-VMR generalization block (57 trials, three to each of the 19 tested directions).
Data inclusion criteria
On a small fraction of trials (0.1%), a recording issue prevented us from accurately parsing the data so that we could not determine the reach angle. We also excluded about 0.8% of trials as outliers with reaching angles that were more than 3 times the across-subject interquartile range away from the across-subject median of the data (separately for each latency condition). In total, data exclusion amounted to 1.0 ± 0.1% of trials (mean ± SEM).
Statistical comparisons
Given that decreased implicit and increased explicit learning have been observed for larger (> 200 ms) latencies13,15, we hypothesized that the latency increases examined here would lead to decreased implicit and increased explicit learning and thus used the corresponding one-tailed t-tests to compare effects of latency.
Note that, a subset of participants (Experiment 1b) was tested under all three latency conditions, which would allow for paired comparisons; however, the remaining participants (Experiment 1a) were only tested under two out of the three latency conditions, meaning that isolating analysis to paired comparisons would exclude data. We thus used unpaired comparisons when analyzing the pooled data. We used F-tests to compare sensitivities of implicit learning to latency (Figs. 2e/3e).
Significance statement
This work demonstrates that implicit sensorimotor learning, a key process for motor skill acquisition, is strikingly sensitive to brief visual feedback latencies below 100 ms which are common in everyday human-computer interactions such as controlling a computer mouse. Specifically, we find that reducing latency just from 85 to 25 ms boosts implicit learning by 50% while proportionally decreasing explicit learning, dramatically reorganizing sensorimotor memory. This exquisite sensitivity to short latencies aligns with evidence that key neural plasticity mechanisms can be disrupted by brief latencies, and highlights the importance of minimizing latency for computer-based skill training that aims to build implicit sensorimotor learning with practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Support for this work was provided by the McKnight Scholar Award to MAS, a Sloan Research Fellowship to MAS, a grant from NIA (R01 AG041878) to MAS, and a grant from the NSF (2218427) to MAS, as well as a T32 Fellowship by the National Institute of Neurological Diseases and Stroke (T32NS100663) to AMH.
Author contributions
AMH and MAS designed the study. YD and TR collected data. AMH, TR and MAS analyzed data. AMH and MAS wrote the manuscript.
Data availability
Data and code for this paper’s analyses is maintained at https://github.com/AlkisMH/Latency.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alkis M. Hadjiosif, Email: alkis@seas.harvard.edu
Maurice A. Smith, Email: mas@seas.harvard.edu
References
- 1.Wimmer, R., Schmid, A. & Bockes, F. On the Latency of USB-Connected Input Devices. in Proceedings of the CHI Conference on Human Factors in Computing Systems 1–12 (Association for Computing Machinery, New York, NY, USA, 2019). (2019). 10.1145/3290605.3300650
- 2.Kumcu, A. et al. Effect of video lag on laparoscopic surgery: Correlation between performance and usability at low latencies. Int. J. Med. Robot. 13, e1758 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Anvari, M. et al. The impact of latency on surgical precision and task completion during robotic-assisted remote telepresence surgery. Comput. Aided Surg.10, 93–99 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Krakauer, J. W. et al. Comparing a novel neuroanimation experience to conventional therapy for high-dose intensive upper-limb training in subacute stroke: The SMARTS2 randomized trial. Neurorehabil Neural Repair.35, 393–405 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Oberhauser, M. & Dreyer, D. A virtual reality flight simulator for human factors engineering. Cogn. Technol. Work. 19, 263–277 (2017). [Google Scholar]
- 6.Hoover, A. E. & Harris, L. R. Detecting delay in visual feedback of an action as a monitor of self recognition. Exp. Brain Res.222, 389–397 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Pavlovych, A. & Stuerzlinger, W. The tradeoff between Spatial jitter and latency in pointing tasks. in 187–196 (2009).
- 8.MacKenzie, I. S. & Ware, C. Lag as a determinant of human performance in interactive systems. in 488–493 (1993).
- 9.Jay, C., Glencross, M. & Hubbold, R. Modeling the effects of delayed haptic and visual feedback in a collaborative virtual environment. ACM Trans. Comput. -Hum Interact. TOCHI. 14, 8–es (2007). [Google Scholar]
- 10.Friston, S., Karlström, P. & Steed, A. The effects of low latency on pointing and steering tasks. IEEE Trans. Vis. Comput. Graph. 22, 1605–1615 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Jota, R., Ng, A., Dietz, P. & Wigdor, D. How fast is fast enough? A study of the effects of latency in direct-touch pointing tasks. in 2291–2300 (2013).
- 12.Brenner, E. et al. How the timing of visual feedback influences goal-directed arm movements: Delays and presentation rates. Exp. Brain Res.241 (5), 1447–1457 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schween, R. & Hegele, M. Feedback delay attenuates implicit but facilitates explicit adjustments to a visuomotor rotation. Neurobiol. Learn. Mem.140, 124–133 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Honda, T., Hirashima, M. & Nozaki, D. Adaptation to visual feedback delay influences visuomotor learning. PLoS One. 7, e37900 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brudner, S. N., Kethidi, N., Graeupner, D., Ivry, R. B. & Taylor, J. A. Delayed feedback during sensorimotor learning selectively disrupts adaptation but not strategy use. J. Neurophysiol.115, 1499–1511 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Malla, C., López-Moliner, J. & Brenner, E. Dealing with delays does not transfer across sensorimotor tasks. J. Vis.14, 8–8 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Wang, T., Avraham, G., Tsay, J. S., Thummala, T. & Ivry, R. B. Advanced feedback enhances sensorimotor adaptation. Curr. Biol.34 (5), 1076–1085. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Projector & Central accessed 9/27/2023. www.projectorcentral.com.
- 19.Tanaka, H., Homma, K. & Imamizu, H. Physical delay but not subjective delay determines learning rate in Prism adaptation. Exp. Brain Res.208, 257–268 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Held, R. & Durlach, N. Telepresence, time delay and adaptation. Pict. Commun. Virtual Real. Environ. 232–246 (1991).
- 21.Held, R., Efstathiou, A. & Greene, M. Adaptation to displaced and delayed visual feedback from the hand. J. Exp. Psychol.72, 887–891 (1966). [Google Scholar]
- 22.Kitazawa, S., Kohno, T. & Uka, T. Effects of delayed visual information on the rate and amount of Prism adaptation in the human. J. Neurosci.15, 7644 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitazawa, S. & Yin, P. B. Prism adaptation with delayed visual error signals in the monkey. Exp. Brain Res.144, 258–261 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Farshchiansadegh, A., Ranganathan, R., Casadio, M. & Mussa-Ivaldi, F. A. Adaptation to visual feedback delay in a redundant motor task. J. Neurophysiol.113, 426–433 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Dix, A., Helmert, J. R. & Pannasch, S. Latency in cyber-physical Systems: the Role of Visual Feedback Delays on Manual Skill Learning, in 1138–1146 (Springer, 2022).
- 26.Vassiliadis, P., Lete, A., Duque, J. & Derosiere, G. Reward timing matters in motor learning. Iscience25, 104290 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swinnen, S. P., Schmidt, R. A., Nicholson, D. E. & Shapiro, D. C. Information feedback for skill acquisition: Instantaneous knowledge of results degrades learning. J. Exp. Psychol. Learn. Mem. Cogn.16, 706 (1990). [Google Scholar]
- 28.Dyal, J. A., Wilson, W. J. & Berry, K. K. Acquisition and extinction of a simple motor skill as a function of delay of knowledge of results. Q. J. Exp. Psychol.17, 158–162 (1965). [Google Scholar]
- 29.Becker, P. W., Mussina, C. M. & Persons, R. W. Intertrial interval, delay of knowledge of results, and motor performance. Percept. Mot Skills. 17, 559–563 (1963). [DOI] [PubMed] [Google Scholar]
- 30.Boulter, L. R. Evaluation of mechanisms in delay of knowledge of results. Can. J. Psychol. Can. Psychol.18, 281 (1964). [DOI] [PubMed] [Google Scholar]
- 31.Greenspoon, J. & Foreman, S. Effect of delay of knowledge of results on learning a motor task. J. Exp. Psychol.51, 226 (1956). [DOI] [PubMed] [Google Scholar]
- 32.Morehead, J. R., Taylor, J. A., Parvin, D. E. & Ivry, R. B. Characteristics of implicit sensorimotor adaptation revealed by task-irrelevant clamped feedback. J. Cogn. Neurosci.29, 1061–1074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avraham, G., Morehead, J. R., Kim, H. E. & Ivry, R. B. Reexposure to a sensorimotor perturbation produces opposite effects on explicit and implicit learning processes. PLoS Biol.19, e3001147 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, H. E., Morehead, J. R., Parvin, D. E., Moazzezi, R. & Ivry, R. B. Invariant errors reveal limitations in motor correction rather than constraints on error sensitivity. Commun. Biol.1, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi, G. & Poo, M. Synaptic modifications in cultured hippocampal neurons: Dependence on Spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci.18, 10464–10472 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Froemke, R. C. & Dan, Y. Spike-timing-dependent synaptic modification induced by natural Spike trains. Nature416, 433–438 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Tzounopoulos, T., Kim, Y., Oertel, D. & Trussell, L. O. Cell-specific, spike timing–dependent plasticities in the dorsal cochlear nucleus. Nat. Neurosci.7, 719–725 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Dan, Y. & Poo, M. Spike timing-dependent plasticity of neural circuits. Neuron44, 23–30 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Zhang, L. I., Tao, H. W., Holt, C. E., Harris, W. A. & Poo, M. A critical window for cooperation and competition among developing retinotectal synapses. Nature395, 37–44 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Suvrathan, A., Payne, H. L. & Raymond, J. L. Timing rules for synaptic plasticity matched to behavioral function. Neuron92, 959–967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, J. A., Krakauer, J. W. & Ivry, R. B. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci.34, 3023–3032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto, Y. R., Wang, S. & Smith, M. A. Implicit adaptation compensates for erratic explicit strategy in human motor learning. Nat. Neurosci.23, 443–455 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Shadmehr, R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum. Mov. Sci.23, 543–568 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez Castro, L. N., Monsen, C. B. & Smith, M. A. The binding of learning to action in motor adaptation. PLOS Comput. Biol.7, e1002052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezazadeh, A. & Berniker, M. Force field generalization and the internal representation of motor learning. Plos One. 14, e0225002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brayanov, J. B., Press, D. Z. & Smith, M. A. Motor memory is encoded as a gain-field combination of intrinsic and extrinsic action representations. J. Neurosci.32, 14951–14965 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou, W., Fitzgerald, J., Colucci-Chang, K., Murthy, K. G. & Joiner, W. M. The Temporal stability of visuomotor adaptation generalization. J. Neurophysiol.118, 2435–2447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haith, A. M., Huberdeau, D. M. & Krakauer, J. W. The influence of movement preparation time on the expression of visuomotor learning and savings. J. Neurosci.35, 5109–5117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Ruiz, J., Wong, W., Armstrong, I. T. & Flanagan, J. R. Relation between reaction time and reach errors during visuomotor adaptation. Behav. Brain Res.219, 8–14 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Albert, S. T. et al. Competition between parallel sensorimotor learning systems. Elife11, e65361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leow, L. A., Gunn, R., Marinovic, W. & Carroll, T. J. Estimating the implicit component of visuomotor rotation learning by constraining movement Preparation time. J. Neurophysiol.118, 666–676 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schween, R., Taube, W., Gollhofer, A. & Leukel, C. Online and post-trial feedback differentially affect implicit adaptation to a visuomotor rotation. Exp. Brain Res.232, 3007–3013 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Hinder, M. R., Tresilian, J. R., Riek, S. & Carson, R. G. The contribution of visual feedback to visuomotor adaptation: How much and when? Brain Res.1197, 123–134 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Kaaresoja, T. & Brewster, S. Feedback is… late: measuring multimodal delays in mobile device touchscreen interaction. in 1–8 (2010).
- 55.Deber, J. et al. Hammer time! A low-cost, high precision, high accuracy tool to measure the latency of touchscreen devices. in 2857–2868 (2016).
- 56.Ng, A., Lepinski, J., Wigdor, D., Sanders, S. & Dietz, P. Designing for low-latency direct-touch input. in 453–464 (2012).
- 57.Bond, K. M. & Taylor, J. A. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J. Neurophysiol.113, 3836–3849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkley, V., Salomonczyk, D., Cressman, E. K. & Henriques, D. Y. Reach adaptation and proprioceptive recalibration following terminal visual feedback of the hand. Front. Hum. Neurosci.8, 705 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohmae, S. & Medina, J. F. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat. Neurosci.18, 1798–1803 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Najafi, F. & Medina, J. F. Beyond all-or-nothing climbing fibers: Graded representation of teaching signals in purkinje cells. Front. Neural Circuits. 7, 115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng, Y., Diedrichsen, J., Krakauer, J. W., Shadmehr, R. & Bastian, A. J. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol.98, 54–62 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Herzfeld, D. J., Kojima, Y., Soetedjo, R. & Shadmehr, R. Encoding of error and learning to correct that error by the purkinje cells of the cerebellum. Nat. Neurosci.21, 736–743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marr, D. & Thach, W. T. A theory of cerebellar cortex. In From the retina to the neocortex. Birkhäuser Boston. 11–50. 10.1007/978-1-4684-6775-8_3 (1991).
- 64.Albus, J. S. A theory of cerebellar function. Math. Biosci.10, 25–61 (1971). [Google Scholar]
- 65.Ito, M. & Kano, M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett.33, 253–258 (1982). [DOI] [PubMed] [Google Scholar]
- 66.Ekerot, C. F. & Kano, M. Stimulation parameters influencing climbing fibre induced long-term depression of parallel fibre synapses. Neurosci. Res.6, 264–268 (1989). [DOI] [PubMed] [Google Scholar]
- 67.Karachot, L., Kado, R. T. & Ito, M. Stimulus parameters for induction of long-term depression in in vitro rat purkinje cells. Neurosci. Res.21, 161–168 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Pitti, A., Mori, H., Kouzuma, S. & Kuniyoshi, Y. Contingency perception and agency measure in visuo-motor spiking neural networks. IEEE Trans. Auton. Ment Dev.1, 86–97 (2009). [Google Scholar]
- 69.Welniarz, Q., Worbe, Y. & Gallea, C. The forward model: A unifying theory for the role of the cerebellum in motor control and sense of agency. Front. Syst. Neurosci.15, 644059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsay, J. S., Lee, A., Ivry, R. B. & Avraham, G. Moving outside the lab: The viability of conducting sensorimotor learning studies online. ArXiv Prepr. arXiv:2107.13408 (2021).
- 71.Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]
- 72.Brainard, D. H. & Vision, S. The psychophysics toolbox. Spat. Vis.10, 433–436 (1997). [PubMed] [Google Scholar]
- 73.RTINGS.com. accessed 3/4/2024. https://www.rtings.com/monitor/reviews/benq/zowie-xl2411p.
- 74.Taylor, J. A. & Ivry, R. B. Flexible cognitive strategies during motor learning. PLoS Comput. Biol.7, e1001096 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hadjiosif, A. M., Morehead, J. R. & Smith, M. A. A double dissociation between savings and long-term memory in motor learning. Plos Biol.21, e3001799 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadjiosif, A. M., Gibo, T. L. & Smith, M. A. The cerebellum acts as the analog to the medial Temporal lobe for sensorimotor memory. Proc. Natl. Acad. Sci.121, e2411459121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alhussein, L. & Smith, M. A. Motor planning under uncertainty. eLife10, e67019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDougle, S. D., Bond, K. M. & Taylor, J. A. Implications of plan-based generalization in sensorimotor adaptation. J. Neurophysiol.118, 383–393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code for this paper’s analyses is maintained at https://github.com/AlkisMH/Latency.