Abstract
Dietary calcium supplements can prevent osteoporosis, and our previous investigation demonstrated a notable increase in duodenal calcium absorption due to the impact of estrogen. The decrease of estrogen level in postmenopausal women is significantly associated with an increased incidence of osteoporosis. In this study, we further investigated the role of estrogen regulating duodenal calcium absorption in osteoporosis and elucidated its underlying molecular mechanism. We recruited ten young women in the prefollicular stage and ten menopausal women for the study. Furthermore, we performed trials on mice, as well as human duodenal epithelial cells and SCBN cells. The measurement of calcium absorption in the duodenum of mice was conducted through single-pass perfusion in vivo. We used a calcium imaging system to evaluate calcium absorption in SCBN cells. The bone mineral density was measured using small animal computed tomography and a bone densitometer. Furthermore, The expression levels of calcium transport proteins, namely plasma membrane calcium pump (PMCA1b) and transient receptor potential cation channel (TRPV6), were evaluated using western blot analysis. Compared to young women, postmenopausal women exhibited significant reductions in estrogen levels, bone mineral density, and the expression of PMCA1b and TRPV6 in duodenal mucosal tissues (P < 0.05). A positive correlation was also observed between estrogen levels, the expression of PMCA1b and TRPV6, and bone mineral density (P < 0.05). The estrogen levels, the expression of PMCA1b and TRPV6 in the duodenal mucosa, calcium absorptions, and bone mineral density were observed to be decreased in ovariectomized mice based on in vivo animal experiments (P < 0.05). However, estrogen upplementation can enhance duodenal calcium absorptions and ameliorate osteoporosis in ovariectomized mice, with its primary mechanism of action being the regulation of PMCA1b expression and function (P < 0.05). The findings from SCBN cells further confirm that estrogen enhances duodenal calcium absorption through the effect of ERβ on PMCA1b. Estrogen enhances the expression and functionality of PMCA1b in duodenal mucosal cells via ERβ, promoting duodenal calcium absorption and ameliorating postmenopausal osteoporosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-00605-2.
Subject terms: Diseases, Endocrinology, Gastroenterology, Medical research, Pathogenesis
Introduction
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mass, microstructural deterioration of bone tissue, heightened vulnerability to fractures, and increased fragility of bones. The most severe outcome of osteoporosis is the occurrence of fragile fractures, which not only rank among the primary causes for surgical interventions but also contribute significantly to mortality rates in the elderly population1,2. The epidemiological investigations revealed a significant decrease in estrogen levels among postmenopausal women, accompanied by a notable increase in the incidence of osteoporosis3–5. Estrogen replacement therapy can effectively prevent and treat osteoporosis by attenuating bone calcium loss through inhibiting osteoclast activity6,7. While estrogen replacement therapy has been linked to an elevated breast cancer risk, the utilization of extremely low doses of estrogen (0.014 mg/day) has exhibited effectiveness in averting decreased bone mineral density among postmenopausal women without inducing uterine hyperplasia. Consequently, these ultra-low doses have gained approval from the Food and Drug Administration (FDA) for the prevention of osteoporosis8.
Osteoporosis is closely related to decreased calcium absorption and increased bone calcium loss9,10. The duodenum serves as the primary site for calcium absorption, and the absorption of dietary calcium is an essential physiological process to maintain the body’s calcium balance and bone health. The active transport pathway of duodenal epithelial cells is responsible for absorbing and transporting calcium from food into the blood. TRPV6, a calcium ion channel, and Ca2+-ATP enzyme (PMCA1b) play essential roles in this process11. Studies have shown that the supplementation of dietary calcium exhibits a preventive effect against osteoporosis and contributes to a reduction in the incidence rate of bone fractures12,13. Therefore, in addition to its role in reducing bone calcium loss, can estrogen effectively prevent the occurrence of postmenopausal osteoporosis by enhancing intestinal calcium absorption? Studies have shown that estrogen can promote the secretion of chloride ions and bicarbonate in duodenal mucosal epithelial cells, indicating that estrogen regulates the function of duodenal mucosal epithelial cells14.
Our previous study revealed that estrogen significantly enhanced the expression and functionality of calcium transporters PMCA1b and TRPV6 in the duodenal mucosa, thereby facilitating efficient calcium absorption in the duodenum15. In this study, we further explored whether estrogen-promoted duodenal calcium absorption improves the occurrence of osteoporosis and investigated the possible mechanism of action in older women, thereby providing a theoretical basis for the treatment of osteoporosis in clinical practice.
Results
Estrogen regulates duodenal calcium absorption through PMCA1b
We initially established ovariectomized and estrogen replacement treatment models in female mice, followed by assessing duodenal calcium absorption capacity in mice two weeks post-ovariectomy. The results demonstrated a significant reduction in estrogen levels among the ovariectomized mice compared to the other experimental groups (Fig. 1A). The ovariectomized mice exhibited a significant decrease in duodenal calcium absorption compared to the control group (P < 0.05). However, the calcium absorption reverted to the control level after 17β-estradiol replacement therapy (P > 0.05). The ovariectomized group of mice was administered with 17β-estradiol + PMCA1b inhibitor TFP, significantly reducing duodenal calcium absorption (P < 0.05). Inhibition of TRPV6 with 2-APB partially reduced duodenal calcium absorption in OVX + E2 mice (P > 0.05), though this effect did not reach statistical significance, suggesting PMCA1b plays a dominant role in estrogen-mediated calcium absorption (Fig. 1B).
Fig. 1.
Regulation of estrogen on duodenal calcium absorption in mice. (A) Notable changes in serum estradiol levels in the control and experimental groups. (B) Significant differences in duodenal calcium absorption of the mice in the control and experimental groups. E2, 17β-estradiol; OVX, ovariectomized; TFP: PMCA1b inhibitor; 2-APB: TRPV6 inhibitor. *P < 0.05, #P > 0.05, compared with controls.
Estrogen improves postmenopausal osteoporosis by up-regulating PMCA1b expression and function
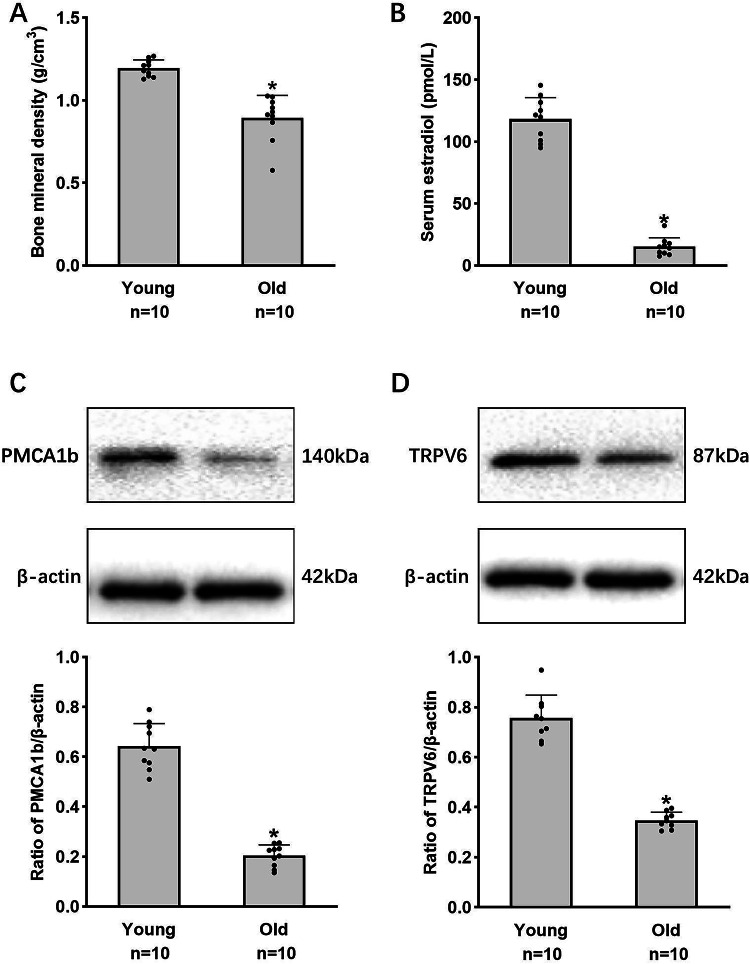
Subsequently, we investigated the impact of estrogen-mediated modulation of PMCA1b on duodenal calcium absorption in relation to osteoporosis. We recruited ten prefollicular young women and ten postmenopausal older women to assess their serum estrogen levels, bone mineral density, and the expression of PMCA1b and TRPV6 in the duodenal mucosa. The study revealed a significant decrease in estrogen levels and bone mineral density (BMD) among postmenopausal women, with a positive correlation between estrogen levels and BMD (Fig. 2A, B). The decrease in estrogen levels was accompanied by a significant reduction in the expression of PMCA1b and TRPV6 in duodenal mucosal tissues (Fig. 2C, D).
Fig. 2.
Serum estradiol levels, bone mineral density, and the expression of PMCA1b and TRPV6 in the duodenal mucosa in prefollicular young and postmenopausal older women. (A) Measurement of serum estradiol levels. (B) Measurement of bone mineral density. (C) Duodenal mucosal PMCA1b protein expression. (D) Duodenal mucosal TRPV6 protein expression. Young: prefollicular young women (20–29 years), Old: postmenopausal older women (60–69 years). *P < 0.05, compared with controls.
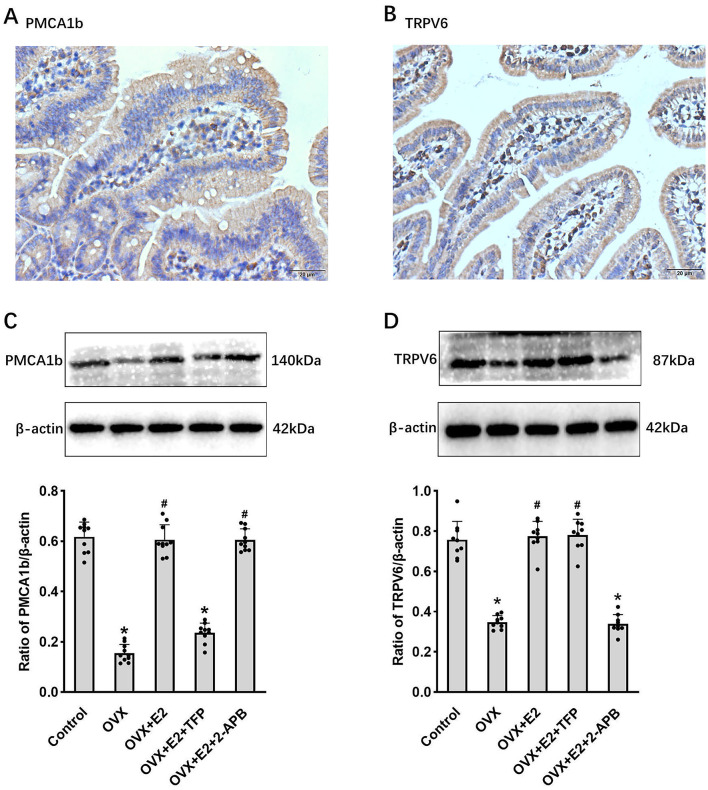
We conducted further investigations to elucidate the impact of estrogen regulating the expression and functionality of PMCA1b and TRPV6 on osteoporosis through animal experimentation. Initially, we investigated the distribution of PMCA1b and TRPV6 expression in the duodenal mucosa. PMCA1b and TRPV6 were expressed in the membranes of duodenal epithelial cells (Fig. 3A, B). The findings revealed a reduction in estrogen levels, as well as decreased expression of duodenal mucosal PMCA1b and TRPV6 in the ovariectomized mice; replenishment of 17β-estradiol effectively restored the expression levels of PMCA1b and TRPV6 to their normal levels. After the administration of 17β-estradiol and the PMCA1b inhibitor TFP, there was a significant decrease in the expression of PMCA1b in the duodenal mucosa, while no notable change was observed in TRPV6 expression. The administration of 17 β-estradiol and TRPV6 inhibitor 2-APB reduced TRPV6 expression but did not significantly affect the expression of PMCA1b (Fig. 3C, D). The experimental group exhibited lower bone density, trabecular number, and bone thickness but higher bone separation than the control group (P < 0.05). However, after 17 β-estradiol replacement treatment, these parameters returned to normal levels (P > 0.05). The ovariectomized mice treated with 17β-estradiol + PMCA1b inhibitor TFP significantly reduced bone mineral density, trabecular number, trabecular thickness, and increased trabecular separation (P < 0.05). However, treatment with 17β-estradiol + TRPV6 inhibitor 2-APB did not significantly affect these parameters in the ovariectomy group (P > 0.05) (Fig. 4). The results suggest that estrogen supplementation can relieve osteoporosis in ovariectomized mice and improve it by regulating PMCA1b expression and function.
Fig. 3.
Regulation of estrogen on duodenal PMCA1b and TRPV6 expression in mice. (A,B) The expression distribution of PMCA1b and TRPV6 in normal duodenal mucosa of mice was determined using immunohistochemistry. (C,D) PMCA1b and TRPV6 expression in the duodenal mucosa of the mice in each group. *P < 0.05, #P > 0.05, compared with controls.
Fig. 4.
The impact of estrogen regulating the expression of PMCA1b and TRPV6 on osteoporosis. (A1–A5) Changes in bone mineral density (BMD) in control group (A1), OVX group (A2), OVX + E2 group (A3), OVX + E2 + TFP group (A4), and OVX + E2 + 2-APB group (A5), respectively. (B–E) Statistical plots of the change in BMD (B), trabecular number (C), bone thickness (D), and bone separation (E) in each group, respectively. E2, 17β-estradiol; OVX, ovariectomized; TFP: PMCA1b inhibitor; 2-APB: TRPV6 inhibitor; The red arrows indicate trabeculae. *P < 0.05, #P > 0.05, compared with controls.
Estrogen enhances duodenal calcium absorption and alleviates osteoporosis by the effect of Estrogen receptor ER Β on PMCA1b
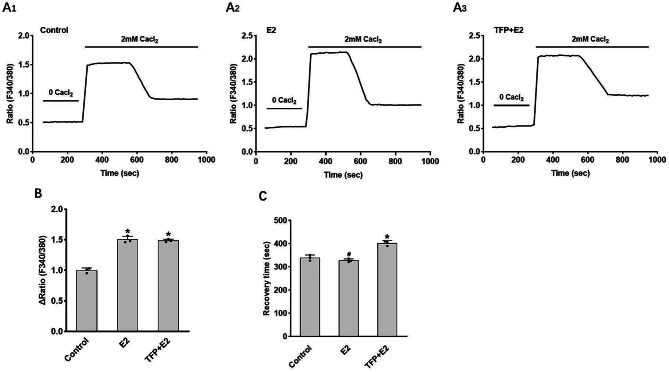
We further investigated the underlying mechanism through which estrogen regulates PMCA1b to enhance duodenal calcium absorption and improve osteoporosis. Compared with the control group, the results indicate that stimulation with 17β-estradiol (E2) increases cellular calcium influx but does not significantly affect the intracellular calcium recovery time in SCBN cells. Treatment with the PMCA1b inhibitor TFP does not significantly alter cellular calcium influx. However, it significantly prolongs the intracellular calcium recovery time in SCBN cells (Fig. 5).
Fig. 5.
Regulation of calcium absorption in SCBN cells by 17β-estradiol and the pivotal role of PMCA1b. (A) Representative time course of changes in intracellular calcium levels under different conditions. (B) Comparisons of the increase in intracellular calcium levels among different groups, emphasizing the influence of PMCA1b. (C) Comparisons of the recovery time for intracellular calcium levels among different groups. E2, 17β-estradiol; TFP, PMCA1b inhibitor. ∆Ratio refers to maximal value minus base value. Recovery time refers to the time from the maximum value to the stable value. *P < 0.05, #P > 0.05, compared with controls.
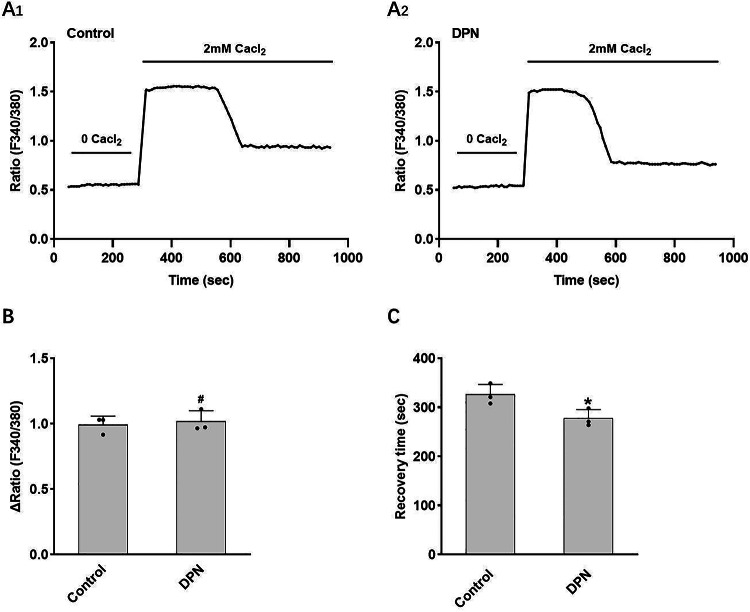
Next, we further explored the mechanism by which estrogen-regulated PMCA1b affects calcium absorption. The result shows that the interference with ERβ expression in SCBN cells significantly decreased PMCA1b expression (Fig. 6). Compared with the control group, a significant enhancement in cellular calcium influx was observed following E2 stimulation of SCBN cells, while no notable alteration was detected in intracellular calcium recovery time. The cellular calcium influx significantly increased, and the intracellular calcium recovery time was prolonged after E2 stimulation in SCBN cells with ERβ expression interference (Fig. 7). Compared with the control group, there was no significant change in the cellular calcium influx of SCBN cells stimulated by ERβ agonist DPN, but the intracellular calcium recovery time was significantly shortened (Fig. 8). These findings suggest that estrogen up-regulates the expression and functionality of PMCA1b via ERβ, facilitating duodenal calcium absorption and ameliorating osteoporosis.
Fig. 6.
Impact of ERβ silencing in SCBN cells on PMCA1b expression. (A) SCBN cells were transfected with ERβ shRNA. (B) The silencing of ERβ in SCBN cells was verified by WB detection. (C) PMCA1b protein expression in each group. E2, 17β-estradiol; ERβ, estrogen receptor β. *P < 0.05, compared with controls.
Fig. 7.
Regulation of ERβ suppression on calcium absorption in SCBN cells. (A) Representative time course of changes in intracellular calcium levels under different conditions. (B) Comparisons of the increase in intracellular calcium levels among different groups. (C) Comparisons of the recovery time for intracellular calcium levels among different groups. E2, 17β-estradiol; ERβ, estrogen receptor β; shERβ, ERβ shRNA. ∆Ratio refers to maximal value minus base value. Recovery time refers to the time from the maximum value to a stable value. *P < 0.05, #P > 0.05, compared with controls.
Fig. 8.
Effect of ERβ agonists on intracellular calcium in SCBN cells. (A) Representative time course of changes in intracellular calcium levels under different conditions. (B) Comparisons of the increase in intracellular calcium levels between groups. (C) Comparisons of the recovery time for intracellular calcium levels between groups. E2, 17β-estradiol; ERβ, estrogen receptor β; DPN, selective ERβ agonist. ∆Ratio refers to maximal value minus base value. Recovery time refers to the time from the maximum value to a stable value. *P < 0.05, #P > 0.05, compared with controls.
Discussion
Our study provides the first evidence that estrogen enhances duodenal calcium absorption and ameliorates postmenopausal osteoporosis through ERβ-mediated upregulation of PMCA1b expression and function. While previous studies established the role of estrogen in regulating TRPV6 and PMCA1b expression15, our work uniquely identifies ERβ as the critical receptor mediating PMCA1b functionality and directly links this mechanism to improvements in bone mineral density.
Estrogen is produced by the female ovary and serves various crucial physiological functions. The incidence of fracture and osteoporosis in postmenopausal women is significantly increased due to estrogen deficiency16–18. Estrogen deficiency can stimulate osteoclastogenesis and enhance bone resorption, resulting in calcium loss and decreased bone mineral density, ultimately leading to osteoporosis19–21. Calcium is paramount in the human body as a vital nutritional component. It predominantly exists in the form of hydroxyapatite, primarily found within bones and teeth, playing a crucial role in maintaining optimal bone health and facilitating numerous essential physiological functions22. Calcium supplementation mainly relies on calcium absorption from food by the duodenum, which plays a pivotal role in maintaining calcium homeostasis23,24. The process of absorbing calcium in the duodenum involves three consecutive steps: Calcium primarily enters the brush border membrane of duodenal epithelial cells via the TRPV6 calcium channel, followed by its transfer from the cellular brush border membrane to the basolateral membrane. Finally, calcium is transported into the bloodstream predominantly through the plasma membrane Ca2+-ATPase (PMCA1b/Ca2+-pump). Studies have demonstrated that dietary calcium supplementation has significantly ameliorated the prevalence of osteoporosis and bone fractures among postmenopausal women25,26. Our previous study showed that estrogen exerts a promoting effect on duodenal calcium absorption15. However, the precise impact of estrogen’s regulation of duodenal calcium absorption on osteoporosis and its underlying molecular mechanisms remains elusive. In this study, we indicated that ovariectomized mice significantly reduced estrogen levels and duodenal mucosal calcium absorption. However, estrogen replacement therapy restored the duodenal calcium absorption to normal levels. Inhibition of PMCA1b expression and function significantly attenuates the promoting effect of estrogen replacement therapy on duodenal calcium absorption. However, the inhibition of TRPV6 expression and function only partially reduces the promoting effect of estrogen replacement therapy on duodenal calcium absorption. These results provide further evidence that estrogen promotes calcium absorption in the mouse duodenum mainly by regulating PMCA1b expression and function. Although fluid absorption markers were not employed, the consistent reduction in calcium absorption observed in OVX mice and its restoration by estrogen replacement strongly support the physiological relevance of PMCA1b in duodenal calcium transport.
TRPV6 and PMCA1b predominantly regulate the transport of calcium ions, yet there is a lack of research investigating their impact on osteoporosis. We initially confirmed a positive correlation between estrogen levels, the expression of duodenal PMCA1b, TRPV6, and changes in bone mineral density among young and postmenopausal women. We explored the effect of estrogen regulating the function and expression of PMCA1b and TRPV6 on osteoporosis. The findings indicated that ovariectomized mice exhibited diminished PMCA1b and TRPV6 expression levels in the duodenal mucosa, reduced bone mineral density, decreased trabecular number, diminished trabecular thickness, and increased trabecular separation. The replacement therapy of 17β-estradiol upregulates the expression of PMCA1b and TRPV6, restoring these parameters to normal levels. Repression of PMCA1b expression and function significantly inhibits the efficacy of estrogen replacement therapy in ameliorating osteoporosis in mice. However, the inhibition of TRPV6 expression and function only partially attenuated the beneficial effects of estrogen replacement therapy on osteoporosis in mice, with no significant statistical difference observed. The above findings suggest that estrogen supplementation can ameliorate osteoporosis in ovariectomized mice, with its primary mechanism of action being the regulation of PMCA1b expression and function. While TFP and 2-APB are widely used inhibitors of PMCA1b and TRPV6, their specificity limitations are noted. However, our findings align with genetic knockdown models15, and the concordance between PMCA1b expression changes and functional calcium absorption strongly supports the specificity of their effects in this context.
Subsequently, we conducted cellular experiments further to elucidate the molecular mechanism underlying estrogen-mediated regulation of PMCA1b, thereby facilitating duodenal calcium absorption. Estrogen exerts its physiological effects mainly through estrogen receptors ERα and ERβ27,28. Our research indicated that in an in vitro experiment using human duodenal epithelial cell line SCBN cells, E2 treatment increased the expression of PMCA1b protein, and ERβ shRNA reduced ERβ expression in cells and decreased PMCA1b protein expression. ERβ shRNA did not change the E2 effect on cellular calcium influx, but prolonged the intracellular calcium recovery time in SCBN cells with ERβ expression interference, which would prolong the time of intracellular calcium retention and thereby reduce calcium absorption in duodenal epithelial cells. Compared with the control group, there was no significant change in the cellular calcium influx of SCBN cells stimulated by the ERβ agonist DPN, but the intracellular calcium recovery time was significantly shortened, which would decrease the time of intracellular calcium retention and thus increase calcium absorption in duodenal epithelial cells. The shortened calcium recovery time in ERβ agonist-treated cells (Fig. 8) functionally corroborates enhanced PMCA1b activity, consistent with its upregulated expression. Future studies employing PMCA1b-specific activity assays or basolateral membrane isolation techniques could further validate its functional role in calcium extrusion. Our findings provide further evidence that estrogen enhances the expression and functionality of PMCA1b in duodenal mucosal cells via ERβ, thereby facilitating duodenal calcium absorption.
In conclusion, this study provides evidence that estrogen enhances the expression and functionality of PMCA1b in duodenal mucosal cells via ERβ, thereby promoting duodenal calcium absorption and ameliorating postmenopausal osteoporosis. Estrogen plays a pivotal role in the pathogenesis of postmenopausal osteoporosis. In addition to its role in bone metabolism, this study affirms the significance of estrogen’s regulation of duodenal calcium absorption in the pathogenesis of osteoporosis. This elucidates a novel mechanism through which estrogen influences the development of osteoporosis, offering insights into the clinical management of postmenopausal osteoporosis.
Materials and methods
Reagents
17β-estradiol, Diarylpropionitrile (DPN), 2-aminoethoxydiphenyl borate (2-APB), trifluoperazine dihydrochloride (TFP), and Fura-2 acetoxymethyl ester (Fura-2 AM) were purchased from MedChemExpress (MCE). The TRPV6 antibody was purchased from Affinity Biosciences, while the PMCA1b antibody was acquired from Antibody Online.
Animal experiments
C57BL/6J female mice, aged six weeks, were purchased from the Experimental Animal Center of the Army Military Medical University (Chongqing, China), experimental animal production License No.: SCXK (Chongqing) 20,170,002. The mice were categorized into five groups: the control group (sham operation), the OVX group (ovariectomy), the OVX + E2 group (ovariectomy with replacement treatment of 17β-estradiol), the OVX + E2 + TFP group (ovariectomy with replacement treatment of both 17β-estradiol and TFP), and the OVX + E2 + 2-APB group (ovariectomy with replacement treatment of both 17β-estradiol and 2-APB). Murine ovariectomy was conducted following Kim et al.’s method29. The OVX + E2 mice were given a daily subcutaneous injection of 17β-estradiol at a dose of 0.1 mg/kg body weight, starting from the day following the operation. In the OVX + E2 + TFP group, the mice received a daily subcutaneous injection of both 17β-estradiol (0.1 mg/kg body weight) and TFP (0.14 mg/kg body weight) from the second day after the operation. Similarly, The mice in the OVX + E2 + 2-APB group were administered a combined subcutaneous injection of 17β-estradiol (0.1 mg/kg body weight) and 2-APB (0.36 mg/kg body weight) daily, starting from the second-day post-operation. Six mice in each group were selected for the bone mineral density determination either post-operation or following administration for two months. Ten mice in each group were chosen for the in vivo measurement of duodenal calcium absorption post-operation or after administration for two weeks. Upon completion of the experiment, the mice were euthanized by cervical dislocation and duodenal mucosal tissue was obtained for subsequent analysis using immunohistochemistry and western blot techniques. Serum samples were also collected before killing to measure estradiol levels using a competitive immunoassay kit (estradiol EIA test; Diagnostic Systems Laboratories Inc. Webster, TX, USA) per the manufacturer’s instructions. The mice all showed an absence of ovarian tissue and atrophy in the uterine horns, confirming the successful ovariectomy.
Detection of duodenal calcium absorption in mice in vivo
The mice underwent a 12-h fasting period. Then, the duodenal segment was dissected under anesthesia induced by intraperitoneal injection of 50 mg/kg ketamine and 10 mg/kg xylazine. The gastric wall and duodenum were cannulated intraluminally through two small incisions. One incision was positioned near the pyloric sphincter in the stomach, allowing for gentle insertion of a polyethylene tube into the stomach. The tube was then securely fastened around the outer part of the pyloric sphincter using a silk suture. Another incision was created in the duodenum, where a second polyethylene tube was inserted and firmly secured just before its junction with the bile duct but distal to its blood supply. Following surgery, an isotonic saline solution was used to wash out the isolated duodenal segment for 15 min at a rate of 0.2 ml/min. Subsequently, a constant rate of 0.1 ml/min of isotonic saline containing 2 mM CaCl2 was perfused through the duodenal segment. Effluent solution samples were collected every 15 min for 30 min after initiating perfusion. The inflow and outflow volumes and calcium concentration for each 15-min interval were measured. After the experiment, the perfused duodenal segment was excised, and its length was measured before being preserved in liquid nitrogen for subsequent investigations. Duodenal calcium absorption was calculated using the same method as before15. While our perfusion method provides functional insights into relative calcium absorption across groups, future studies using calcium isotope tracers could further validate absolute flux quantification.
Bone mineral density measurement in mice
Small animal CT (BRUKER micro CT Skyscan 1272, Germany) was provided by the central laboratory of the First Affiliated Hospital of Army Medical University (Chongqing, China). After two months, the mice were euthanized, and the right femur of the mice was harvested for three-dimensional reconstruction to observe the changes in bone trabeculae. The femur bone mineral density was measured by small animal computed tomography (Skyscan CT analyzer software, Germany).
Human study
The research included healthy Chinese females aged 20–29 and 60–69 with no history of gastrointestinal disorders, diabetes, or other medical conditions. The research adhered to the guidelines outlined in the Helsinki Declaration and received ethical approval from the ethics committee at the Second Affiliated Hospital of Army Military Medical University in Chongqing, China. After two weeks of refraining from using oral contraceptives and hormonal medications, the participants provided the written informed consent. The gastroscopic biopsy samples of duodenal mucosal tissue were cryogenically preserved in liquid nitrogen for future analysis of TRPV6 and PMCA1b expressions. Furthermore, each participant provided 2 ml of venous blood samples to determine the estradiol concentration. The subjects’ bone mineral density was measured using a GE LUNAR DPX-NT bone densitometer (USA).
Cell culture
The experiments used SCBN cells, which are derived from a human patient and belong to a non-cancerous duodenal epithelial cell line30. These cells were obtained as a generous gift from Dr. Hui Dong at the University of California, San Diego. Before the experiments, the cryopreserved SCBN cells were subjected to thawing and subsequently cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 50 mg/ml penicillin-streptomycin for two days.
Cell transfection
The human ERβ shRNA and SMARTvector non-targeting control particles used in this study were purchased from Dharmacon. SCBN cells were transfected according to the manufacturer’s instructions. Western blot analysis was conducted to confirm successful silencing by examining the protein expression levels of ERβ. The detailed steps of the experiment were conducted based on our previous method15.
Detection of cellular calcium influx
SCBN cells were exposed to 10 nM of 17β-estradiol, a selective ERβ agonist DPN at a concentration of 100 nM, and the PMCA1b inhibitor TFP at 10 µM for 24 h. The cells were cultured on coverslips and loaded with Fura-2/AM at a concentration of 5 µM for half an hour at 37 °C in a physiological saline solution. The coverslip was placed in an open perfusion chamber and initially perfused with calcium-free physiological saline solution (PSS). Real-time images were captured using an epifluorescence Nikon Eclipse Ti microscope and EasyRatioPro software. The fluorescence ratio of 340/380 was recorded. Once the baseline reached stability, the coverslip was continuously perfused with PSS containing calcium chloride (CaCl2) at a concentration of 2 mM. The time required for intracellular calcium levels to return from their peak rise to stable baseline levels was also measured.
Western blotting
Duodenal mucosal tissues from both humans and mice were collected. SCBN cells, including those with ERβ-knockdown, underwent a treatment of 17β-estradiol (10 nM) for 24 h. Subsequently, the collected SCBN cells were processed. The lysis buffer was used to homogenize the human and murine duodenal mucosal tissues or SCBN cells at a temperature of 4℃. Protein expression analysis was conducted using western blotting analysis following previously described methods in the literature31. Primary antibodies including anti-TRPV6 (1:100), anti-PMCA1b (1:200), anti-ERβ (1:1000), and anti-β-actin (1:1000) were utilized. The protein expression levels of PMCA1b, TRPV6, and ERβ were relatively quantified using β-actin as a reference.
Immunohistochemistry
Duodenal tissue of female mice in the control group was collected. The tissue was then fixed using formalin and embedded in paraffin. Immunohistochemical analysis was performed on the tissue samples embedded to investigate the distribution and localization of TRPV6 or PMCA1b within the duodenal mucosal epithelial cells. The specific steps refer to the previous method32.
Statistical analysis
SPSS 26.0 software was used for statistical analysis, and all outcomes were presented as mean ± standard error. A single-factor ANOVA examined differences among multiple groups, while a non-parametric rank sum test assessed variations between groups that did not meet normal distribution and homogeneity of variance criteria. T-tests compared two groups to determine statistical significance. The criterion for statistical significance was set at a level of P < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
J-Y.B. and X-B.N. conceived and designed research. Y-Y.W., X.G., and A-R.J. performed experiments and collected data. Y-Y.W., J-Y.B., and X-B.N. analyzed data. Y-Y.W. wrote the main manuscript text and prepared all figures. J-Y.B. and X-B.N. edited and revised the manuscript. All authors reviewed the manuscript and approved the final version of the manuscript.
Funding
This study was funded by the Chongqing Natural Science Foundation (cstc2021jcyj-msxmX0118) and the Chongqing Health Appropriate Technology Promotion Project (2022jstg033). The funders had no role in design, data collection, or statistical analysis.
Data availability
Data is provided within the manuscript or supplementary information.
Declarations
Competing interests
The authors declare no competing interests.
Ethics
All experimental protocols were approved by the Second Affiliated Hospital of Army Military Medical University. All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. The study followed the principles of the Helsinki Declaration and was approved by the ethics committee at the Second Affiliated Hospital of Army Military Medical University in Chongqing, China.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianying Bai and Xubiao Nie contributed equally.
Contributor Information
Jianying Bai, Email: drbaijy9909@163.com.
Xubiao Nie, Email: niexubiao@tmmu.edu.cn.
References
- 1.Ensrud, K. E. & Crandall, C. J. Osteoporosis. Ann. Intern. Med.177, C1–C16 (2024). [DOI] [PubMed]
- 2.Alam, F. et al. Guidelines for fracture risk assessment and management of osteoporosis in postmenopausal women and men above the age of 50 in Qatar. Arch. Osteoporos.19, 34 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, C. Osteoporosis: Staying strong. Nature550, S15–S17 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Walker, M. D. & Shane, E. Postmenopausal osteoporosis. N. Engl. J. Med.389, 1979–1991 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Lu, L. & Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: The role and mechanisms of Estrogen. J. Endocrinol. 259 (2023). [DOI] [PubMed]
- 6.Levin, V. A., Jiang, X. & Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int.29, 1049–1055 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Powell, K. M. et al. 6′-Methoxy Raloxifene-analog enhances mouse bone properties with reduced Estrogen receptor binding. Bone Rep.12, 100246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khosla, S. & Hofbauer, L. C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol.5, 898–907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet393, 364–376 (2019). [DOI] [PubMed]
- 10.Kanis, J. A. et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int.30, 3–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz, D. B. G., Guizzardi, S. & Tolosa, D. T. N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol.21, 7142–7154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capozzi, A., Scambia, G. & Lello, S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas140, 55–63 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Balk, E. M. et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int.28, 3315–3324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuo, B. et al. Estrogen regulation of duodenal bicarbonate secretion and sex-specific protection of human duodenum. Gastroenterology141, 854–863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie, X. et al. Estrogen regulates duodenal calcium absorption through differential role of estrogen receptor on calcium transport proteins. Dig. Dis. Sci.65, 3502–3513 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli, R. Postmenopausal osteoporosis: Assessment and management. Best Pract. Res. Clin. Endocrinol. Metab.32, 739–757 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Si, L., Winzenberg, T. M., Jiang, Q., Chen, M. & Palmer, A. J. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos. Int.26, 1929–1937 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Geng, Q., Gao, H., Yang, R., Guo, K. & Miao, D. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int. J. Biol. Sci.15, 58–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, L. T., Chen, L. R. & Chen, K. H. Hormone-related and drug-induced osteoporosis: A cellular and molecular overview. Int. J. Mol. Sci.24 (2023). [DOI] [PMC free article] [PubMed]
- 20.Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell. Dev. Biol.123, 14–21 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Khosla, S., Oursler, M. J. & Monroe, D. G. Estrogen and the skeleton. Trends Endocrinol. Metab.23, 576–581 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamdy, R. C. Bone health, calcium, vitamin D metabolism, and gastro-intestinal diseases. J. Clin. Densitom. 23, 153–154 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Fang, A. & Li, K. Calcium deficiency: Where does the diagnostic criterion come from and by what is bone health influenced? Chin. Med. J. (Engl). 127, 4161–4163 (2014). [PubMed] [Google Scholar]
- 24.Vannucci, L. et al. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 10 (2018). [DOI] [PMC free article] [PubMed]
- 25.Liu, C. et al. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct.11, 10817–10827 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Bhattarai, H. K., Shrestha, S., Rokka, K., Shakya, R. & Vitamin, D. calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J. Osteoporos. 2020, 9324505 (2020). [DOI] [PMC free article] [PubMed]
- 27.Barton, M. et al. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol.176, 4–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrtacnik, P., Ostanek, B., Mencej-Bedrac, S. & Marc, J. The many faces of estrogen signaling. Biochem. Med. (Zagreb). 24, 329–342 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, H. Y., Alarcon, C., Pourteymour, S., Wergedal, J. E. & Mohan, S. Disruption of claudin-18 diminishes ovariectomy-induced bone loss in mice. Am. J. Physiol. Endocrinol. Metab.304, E531–E537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang, G. et al. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology111, 8–18 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Xu, J. et al. Expression and functional role of vacuolar H(+)-ATPase in human hepatocellular carcinoma. Carcinogenesis33, 2432–2440 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Nie, X. et al. VEPH1 suppresses the progression of gastric cancer by regulating the Hippo-YAP signalling pathway. Dig. Liver Dis.56, 187–197 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information.