Abstract
We describe a case of keratitis caused by a new species of the hyphomycetous genus Sarcopodium, S. oculorum. The corneal ulcer developed after 5 months of treatment with corticosteroids in a Brazilian boy diagnosed with allergic conjunctivitis. Fungal hyphae and conidia were detected in corneal scrapings, and repeated cultures were positive for this fungus. The infection was resolved with natamycin and ketoconazole. Eleven antifungals were tested against this fungus, and all except flucytosine and fluconazole showed in vitro activity.
Filamentous fungi are frequent causes of keratomycoses in humans (1). Multiple fungal species are reported to infect the human cornea, and so far more than 30 fungal genera have been involved in these illnesses (1). New fungi are frequently added to the list of microorganisms that are able to cause keratitis, e.g., we recently reported two very rare fungi, both of which were from Brazil (6, 7), but species of Candida, Aspergillus, and Fusarium are generally the most common ones. The prevalence of individual pathogens largely depends on geographical and climatic factors. Keratomycoses occur mainly in warm climates and coincide with seasonal increases in temperature and humidity (1). Many predisposing factors have been mentioned for keratomycosis. These are, for example, the use of systemic or topical steroids, trauma (particularly with vegetable material or soil), preexisting ocular surface disorders that may have produced a devitalized surface, the use of antibiotics, and systemic illnesses (1, 4). Devastating ocular consequences commonly occur if the corneal infections are not diagnosed early and treated properly (4).
Here we report the first case of human infection caused by a species of Sarcopodium, a genus of hyphomycetes usually found on plant debris. To our knowledge, the strain responsible for the infection belongs to an undescribed species of this genus and is proposed here as new.
Case report.
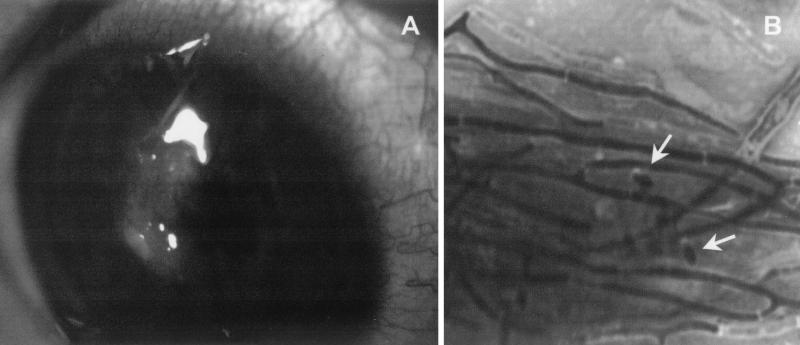
A 12-year-old Brazilian boy, with a vernal conjunctivitis, attended the Ophthalmology Department of the Escuela Paulista de Medicina of São Paulo, Brazil. He complained of a pain in his right eye and felt the presence of a foreign body. The patient was diagnosed with keratoconjunctivitis, which was treated with specific antiallergic drugs, topical dexamethasone (0.1%) and prednisone (20 mg). The conjunctivitis improved after 3 months of treatment. A month and a half after treatment, the patient developed a corneal ulcer as a consequence of the allergic process (Fig. 1A). He was again treated with corticosteroids, the frequency of dosage being increased to five times daily. Five months later the patient, still under corticoid treatment, showed redness of the eye with inflammatory infiltrate and a suspicion of infection. Deep corneal scrapings were collected with a sterile scalpel blade for direct mounts and culture. Direct examination of Gram-stained mounts of the scrapings revealed the presence of numerous septate and branched hyphae and a few ellipsoidal conidia (Fig. 1B). The corneal scrapings were directly inoculated onto Sabouraud dextrose agar (Oxoid, Basingstoke, England) and incubated at 25, 30, and 37°C. After 4 to 5 days, several colonies of a single, darkly pigmented fungus appeared in all cultures. This fungus was tentatively identified as Phoma sp. Corneal scrapings were again collected for direct examination and culture, and an identical fungus was grown. The isolates grew well at 25 and 30°C, but the growth was sparse at 37°C. Cytological examination of corneal scrapings stained with Giemsa stain revealed the presence of numerous septate hyphae among clusters of degenerated neutrophils, some degenerated epithelial cells, and scarce mononuclear cells. Results of routine bacteriological cultures were negative.
FIG. 1.
(A) Corneal ulcer. (B) Gram stain showing segmented hyphae and conidia (arrows). Magnification, ×1,280.
Antifungal treatment was initiated with topical natamycin (5%) hourly and ketoconazole at 200 mg/day. The patient also continued to take corticosteroids twice daily and the antiallergic drugs. Eleven days later, the lesion improved dramatically and there was much less inflammation. The natamycin therapy was reduced to every 2 h, and ketoconazole was maintained. Further improvements were made, and after 20 days the lesion had healed. The patient continued with natamycin four times daily, and cyclosporine and dexafenicol were added. On the 24th day, the patient was considered cured of the infection and corticoids were given again. On the 37th day, a corneal erosion was detected and the allergy worsened. The doses of dexafenicol and dexamethasone were increased to eight times daily, and there was an improvement in a week. After 2 months the patient was left with a visual acuity of 0.1 in the affected eye. Two isolates collected at different times were sent to the Microbiology Unit of the Rovira i Virgili University in Reus, Spain, to be identified and to test antifungal susceptibility.
Morphological study.
The two isolates developed the same peculiar conidiomata (fruiting bodies containing conidia) with identical conidiophores and conidia, which indicates that both were undoubtedly the same fungus (Fig. 2). For identification purposes, the fungus was subcultured on potato dextrose agar (PDA; Difco Laboratories, Detroit, Mich.), potato carrot agar (20 g of potatoes, 20 g of carrot, 18 g of agar, 1,000 ml of tap water), and oatmeal agar (30 g of oat flakes, 1 g of MgSO4 · 7H2O, 1.5 g of KH2PO4, 15 g of agar, 1,000 ml of tap water) and incubated at 25, 37, and 40°C in the dark. Colonies on PDA at 25°C attained a diameter of 39 to 40 mm after 14 days. At first they were flat, mucous, and cream colored, but they soon become radially folded, brownish gray, and granulose due to the abundant production of conidiomata (Fig. 3A) and grayish white toward the periphery with sparse aerial mycelium; the reverse was colorless to brown. On oatmeal agar and potato carrot agar at 25°C, the colonies were very similar and grew more rapidly than on PDA, attaining a diameter of up to 48 and 45 mm, respectively, after 14 days. These colonies were granulose at the center and smooth toward the periphery, with whitish, soft cottony aerial mycelium and brownish gray submerged hyphae; the reverse was brownish gray and paler toward the periphery. Colonies on PDA at 37°C attained a diameter of 14 to 15 mm after 20 days. They were elevated and cerebriform, with abundant sporulation, but no conidiomata developed. The fungus did not grow at 40°C.
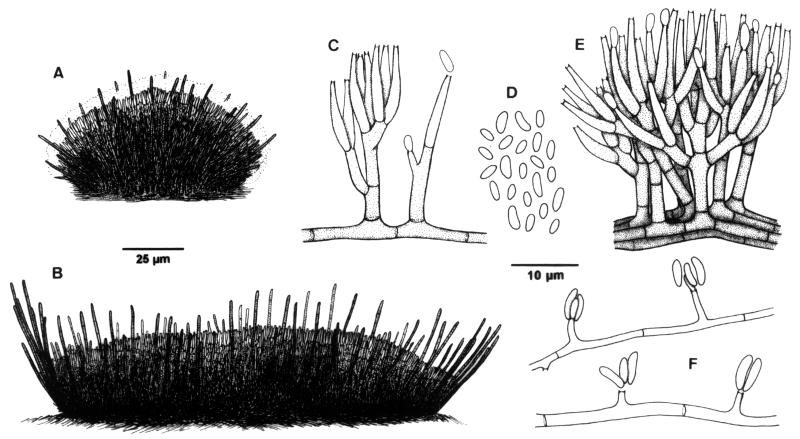
FIG. 2.
S. oculorum IMI 387421. (A and B) Sporodochia. (C to E) Conidiophores and conidia from sporodochia. (F) Undifferentiated hyphae with conidiogenous cells and conidia.
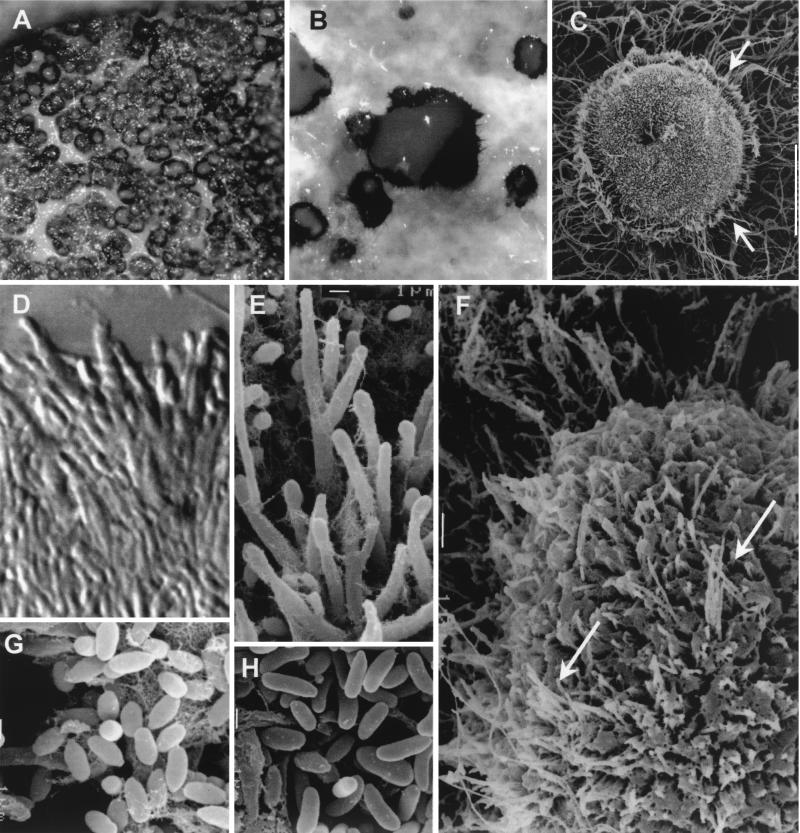
FIG. 3.
S. oculorum IMI 387421. (A and B) Sporodochia from the colony growing on PDA after 3 weeks of incubation at 25°C. (C) Sporodochium with setae (arrows). (D and E) Conidiophores from sporodochia. (F) Part of a sporodochium showing setae (arrows) among conidiophores. (G) Conidia from sporodochia. (H) Conidia from undifferentiated hyphae. Magnifications: ×120 (A), ×400 (B), ×200 (C), ×2,130 (D), ×3,010 (E), ×600 (F), and ×4,000 (G and H).
The microscopic characteristics of the case strain were determined by making wet mounts with lactic acid, which were then examined under a light microscope (Leitz Dialux 20). Photomicrographs were also obtained by scanning electron microscopy (JEOL JSM-6400). One of the most distinctive features of this fungus, apart from the peculiar conidiomata, was the presence of two types of conidia. One was produced from sporodochial conidiomata (cushion-like structures on which numerous compact short conidiophores, which produce the conidial mass, are born) (Fig. 2A to D), and one was produced from undifferentiated hyphae (Fig. 2F). Sporodochia were superficial, solitary, gregarious or confluent, sessile, applanate to cupulate, or pulvinate, subhyaline to dark brown, setose, and up to 400 μm in diameter (Fig. 3B). Numerous sterile hyphae (setae) were present. These formed a frill at the margin of the sporodochium (Fig. 3C) but were also interspersed with the conidiophores (Fig. 3F). They were single or formed small fascicles of three to five hyphae and were erect, unbranched or slightly branched toward the base, straight or flexuose, septate, subhyaline to dark brown, smooth walled, thin to slightly thick walled, cylindrical, and up to 65 μm long by 1.5 to 2.5 μm wide, and their apices were obtuse. The conidiophores were well differentiated, straight or flexuose, subhyaline to pale brown, smooth walled, and up to 35 μm long. They were irregularly branched, and each branch usually bore a single terminal group of slightly appressed conidiogenous cells (Fig. 3D and E). The conidiogenous cells were enteroblastic, monophialidic, terminal or lateral, hyaline to subhyaline, smooth walled, subcylindrical, 8 to 13 μm long by 1 to 1.8 μm wide, rarely intercalary with a cylindrical and lateral projection, and up to 6 μm long by 1.5 μm wide. These intercalary conidiogenous cells were predominantly found on undifferentiated hyphae (Fig. 2F). The conidia were aggregated in cream-colored slimy masses, which remained attached to the upper part of the conidioma and covered all the surface (Fig. 3B). The individual conidia were subhyaline, aseptate, smooth and thin walled, ellipsoid, navicular or slightly allantoid, and 1.2 to 3 μm long by 0.8 to 1.5 μm wide (Fig. 3G). They tended to be longer, reaching up to 5 μm, and cylindrical or allantoid when they emerged from undifferentiated hyphae (Fig. 3H).
The presence of numerous, dark sporodochia covered by a wet mass of cylindrical, hyaline conidia can cause some confusion with the typical conidiomata (pycnidia) of Phoma or other similar coelomycetous fungi, mainly when the colonies are observed under a stereomicroscope. However, when these structures are observed at high magnification, they are easily differentiated. Some species of Phoma have also occasionally been reported to cause keratomycosis (2). Unlike the sporodochia, the pycnidia are closed structures that are generally spherical or obpyriform and open only at the apical part by an ostiole. Also, the pycnidia have a pseudoparenchymatous wall that is absent in the sporodochia. Inside the pycnidia and lining the internal cavity, numerous conidiophores are formed. Other coelomycetous fungi, which develop acervular conidiomata (cup-shaped fruiting bodies) on natural substrates, turn sporodochial in vitro. Some of these fungi, such as a few species of Colletotrichum, have also been described elsewhere as agents of keratitis (2).
The morphology of many fungi, especially the plant pathogens, is different when they grow on natural substrates from when they are grown in culture. This makes it very difficult to identify them in vitro. To verify the stability of the fertile structures of the case strain, we inoculated a conidial suspension of the isolate onto sterilized plant material according to the technique described in the work of Gené and Guarro (5). Under these conditions, the fungus developed conidiomata identical to those observed in the different culture media. On the basis of the above-mentioned characteristics, this clinical strain was therefore identified as a Sarcopodium sp. This hyphomycetous genus is characterized by the presence of sporodochia with sterile, smooth or ornamented, often coiled pale brown setae arising from among branched conidiophores and producing slimy conidia from phialides. The conidia are hyaline, aseptate, and fusiform to ellipsoid or cylindrical (3, 11). The genus Sarcopodium currently encompasses 11 species (13), none of which has all of the morphological features observed for this case strain, which we therefore describe below as a new Sarcopodium species. It is named after the infection site.
Sarcopodium oculorum Gené et Guarro, sp. nov.
Coloniae in vitro granulosae, griseo fuscae. Sporodochia superficialia, subhyalina vel atrobrunnea. Setae erectae, subhyalinae vel atrobrunneae, plerumquam non ramosae, usque ad 65 μm longae, 1.5-2.5 μm latae. Conidiophora dense aggregata, ramosa, subhyalina vel pallide brunneae, usque ad 35 μm longa. Cellulae conidiogenae enteroblasticae, hyalinae vel subhyalinae, subcylindricae, 8-13 × 1-1.8 μm. Conidia in massas mucosas aggregata, subhyalina, unicellularia, ellipsoidea, leviter navicularia vel allantoidea, 1.2-3(5) × 0.8-1.5 μm. Holotypus: IMI 387421, ex keratomycosis humanum, Brazil.
Sarcopodium spp. are reported frequently on dead herbaceous stems and dead wood of different trees in many parts of the world. They are also reported to infect plants but have never been found in soil, air, or animals. S. oculorum is morphologically close to S. circinatum, the type species of the genus. Both fungi have sessile sporodochia, unbranched setae, and ellipsoid or cylindrical conidia, but S. circinatum has flexuous or circinate and verrucose setae up to 6 μm wide and larger (7 to 10 μm long by 2 μm wide) conidia that are never navicular or allantoid. Another similar species is S. tortuosum, but this is easily differentiated by its orange slimy conidial masses and branched setae. S. oculorum also has some morphological resemblance to Myrothecium spp. (12) and other conidial states of Hypocreales (Ascomycota) such as the anamorph of Stephanonectria keithii (10). However, the sporodochial sterile hyphae in Myrothecium are hyaline to subhyaline and confined to the edge of the sporodochium, and in S. keithii they are absent. Moreover, the conidial masses are green in the former and brown in the latter. Living cultures of the two isolates of the case strain are kept in the culture collection at the Faculty of Medicine in Reus as FMR 6632 and FMR 7190. Ex-type cultures are also deposited in the CABI Bioscience collection in England (IMI387421) and in the Centraalbureau voor Schimmelcultures in The Netherlands (CBS 110031).
As well as several important pathogenic fungi, e.g., anthropophilic dermatophytes, there are a number of fungi, such as that involved in the present case, which are found exclusively in humans. These fungi are Rhizopus schipperae, Phoma cruris-hominis, Phoma dennissii var. oculo-hominis, Pseudochaetosphaeronema larense, Botryomyces caespitosus, Cladophialophora devriesii, Cladophialophora modesta, Cylindrocarpon cyanescens, Cyphellophora laciniata, Cyphellophora pluriseptata, Dissitimurus exedrus, Emmonsia pasteuriana, Exophiala bergeri, Exserohilum mcginnissi, Hormographiella verticillata, Polycytella hominis, and Ramichloridium mackenziei (2). As few isolates of these species exist, it is difficult to say whether humans are the exclusive habitat, and it would be very interesting to study this aspect.
Antifungal susceptibility testing.
The new fungus was tested to determine its susceptibility to 11 antifungal drugs (Table 1). Tests were carried out by a previously described microdilution method (9), mainly according to the guidelines of the National Committee for Clinical Laboratory Standards for testing molds (8). We used RPMI 1640 medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS), an inoculum of 8.8 × 105 CFU/ml, and an incubation temperature of 30°C. Readings were taken at 48 and 96 h, and an additive drug dilution procedure was performed. Except for flucytosine and fluconazole, MICs were very low, which demonstrates the in vitro activity of most of the antifungals tested.
TABLE 1.
Antifungal susceptibilities of the clinical isolates of S. oculorum
| Antifungal agent | MICs (μg/ml)
|
|
|---|---|---|
| 78 h | 96 h | |
| Amphotericin B | 0.5 | 1 |
| Flucytosine | >128 | >128 |
| Itraconazole | 0.5 | 0.5 |
| Ketoconazole | 0.5 | 0.5 |
| Miconazole | 0.06 | 0.06 |
| Voriconazole | 0.25 | 0.25 |
| Fluconazole | >16 | >16 |
| Terbinafine | 1 | 1 |
| UR-9825 | 0.25 | 0.5 |
| Clotrimazole | 1 | 2 |
| Sertaconazole | 0.25 | 0.5 |
The prolonged use of corticosteroids was probably the predisposing factor that provoked the fungal infection in this case. Corticosteroids are not known to have a direct stimulating effect on the growth of fungi, but it has been argued elsewhere that they suppress the endogenous immune defense and so facilitate fungal proliferation (1). In this case, as well as the numerous hyphae present, direct examination showed that there were some conidia. Although this is not frequent, it indicates that the fungus is sporulating in tissue, thus allowing the progress of the infection.
As many fungal species have been involved in keratomycoses in recent years, the infections are often difficult to diagnose. Some of these fungi sometimes fail to sporulate in culture, and sometimes they are confused with contaminants. The main problem in the diagnosis seems to be a failure to suspect the infection. Without doubt the key element in diagnosing fungal infection of the cornea is the clinical suspicion by the ophthalmologist (4). In this case, a very rare fungus, which had not previously been found in any other substrate, proliferated in the debilitated cornea and caused an infection that responded to combined therapy with ketoconazole and natamycin. Ketoconazole also showed a good activity in vitro. This fungus must be added to the long list of species that can produce corneal infections in humans (1, 2, 4).
Acknowledgments
We are indebted to G. Samuels and A. Y. Rossman (Systematic Botany and Mycology Laboratory, Beltsville, Md.), K. Seifert (Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, Research Branch, Ottawa, Canada), and H.-J. Schroers (Centraalbureau voor Schimelcultures, Utrecht, The Netherlands) for their comments on identifying the fungus.
REFERENCES
- 1.Behrens-Braumann, W. 1999. Mycosis of the eye and its adnexa. Dev. Ophthalmol. 32:i-ix, 1-201. [PubMed] [Google Scholar]
- 2.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, and the Rovira i Virgili University, Reus, Spain.
- 3.Ellis, M. B. 1976. More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, United Kingdom.
- 4.Foster, C. S. 1992. Fungal keratitis. Infect. Dis. Clin. N. Am. 6:851-857. [PubMed] [Google Scholar]
- 5.Gené, J., and J. Guarro. 1996. A new Chaetomium from Thailand. Mycol. Res. 100:1005-1009. [Google Scholar]
- 6.Guarro, J., T. Akiti, R. Almada-Horta, L. A. M. Leite-Filho, J. Gené, S. Ferreira-Gomes, C. Aguilar, and M. Ortoneda. 1999. Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J. Clin. Microbiol. 37:4170-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarro, J., L. A. Vieira, D. de Freitas, J. Gené, L. Zaror, A. L. Hofling-Lima, O. Fischman, C. Zorat-Yu, and M. J. Figueras. 2000. Phaeoisaria clematidis as a cause of keratomycosis. J. Clin. Microbiol. 38:2434-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Pujol, I., J. Guarro, C. Llop, L. Soler, and J. Fernández. 1996. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob. Agents Chemother. 40:2106-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroers, H.-J., G. J. Samuels, and W. Gams. 1999. Stephanonectria, a new genus of the Hypocreales (Bionectricaceae), and its sporodochial anamorph. Sydowia 51:114-126. [Google Scholar]
- 11.Sutton, B. C. 1981. Sarcopodium and its synonyms. Trans. Br. Mycol. Soc. 76:97-102. [Google Scholar]
- 12.Tulloch, M. 1972. The genus Myrothecium Tode ex Fr. Mycol. Pap. 130:1-42. [Google Scholar]
- 13.Watanabe, T. 1993. Sarcopodium araliae sp. nov. on root of Aralia elata from Japan. Mycologia 85:520-526. [Google Scholar]