Abstract
Background
The optimal duration of steroid therapy for patients with COVID-19 remains unclear. This study compared clinical outcomes between early steroid withdrawal (EW; ≤10 days) and prolonged steroid tapering (PT; >10 days) in patients with severe COVID-19 requiring oxygen support.
Methods
This retrospective, single-center cohort study included adult patients with COVID-19 and WHO-CPS scores of 6–9 admitted to a tertiary hospital in Seoul, Republic of Korea. After 1:1 propensity score matching, 68 patients were included in each group. Primary outcomes were 28-day and 60-day mortality. Secondary outcomes included clinical aggravation, rebound pneumonia, infectious complications, readmission or emergency department (ED) revisits, duration of oxygen support, and lengths of hospitalization and ICU stay.
Results
Baseline characteristics were well balanced after matching. No significant differences were observed in 28-day mortality (5.9% vs. 10.3%, HR 0.54, 95% CI 0.16–1.84, p = 0.32) or 60-day mortality (14.7% vs. 11.8%, HR 1.22, 95% CI 0.48–3.10, p = 0.67) between PT and EW groups. Rates of clinical aggravation, rebound pneumonia, infectious complications, and readmission or ED revisit were also comparable. However, the PT group had significantly longer durations of oxygen support (17.5 vs. 13.0 days, p = 0.001), hospitalization (20.0 vs. 14.0 days, p = 0.001), and ICU stay (5.0 vs. 1.0 days, p = 0.01).
Conclusions
Prolonged steroid therapy beyond 10 days did not improve survival or other clinical outcomes in patients with severe COVID-19, suggesting that early steroid withdrawal may be appropriate for selected patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-025-03674-1.
Keywords: SARS-CoV-2, Severe pneumonia, Steroid, Mortality, Rebound
Introduction
Cytokine dysregulation, resulting from uncontrolled replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and aberrant immunologic responses, is a primary factor in the development of acute respiratory distress syndrome in coronavirus disease 2019 (COVID-19) patients. Dexamethasone and other steroids, known for their anti-inflammatory effects, have therefore become key treatments for COVID-19 patients requiring oxygen support [1–3]. Unlike in COVID-19, steroid use in viral infections such as influenza and respiratory syncytial virus has shown no clear benefit [4–6], whereas in Pneumocystis jirovecii pneumonia, steroids help reduce inflammation and lung damage [7]. Despite mixed outcomes in non-COVID-19 pneumonia, the benefit of steroids in COVID-19 is well-established [3].
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial revealed that 6 mg of dexamethasone daily for up to 10 days significantly reduced 28-day mortality in oxygen-requiring patients [3], supporting the current COVID-19 treatment guidelines [3, 8, 9]. However, the optimal duration of steroid therapy beyond 10 days remains undetermined and there is currently no definitive guidance on whether to abruptly cease or gradually taper dexamethasone in such patients. This decision often relies on the clinician’s judgment and the patient’s clinical progress.
Case reports have described rebound COVID-19 pneumonia following abrupt cessation of dexamethasone after 10 days [10, 11]. One study suggested that prolonged steroid use may prevent rebound COVID-19 pneumonia [12]. However, extended steroid use may increase the risk of bacterial or fungal infections [13–15]. Additionally, high doses of steroids have been associated with increased 28-day mortality and delayed SARS-CoV-2 clearance [16]. The decision to cease or taper steroids is a critical clinical consideration that can impact patient recovery. Considering conflicting evidence, this study aimed to compare clinical outcomes, specifically focusing on mortalities and the incidence of rebound pneumonia, between patients who discontinued steroids within 10 days and those who continued with tapering beyond 10 days.
Methods
Study setting and participants
This single-center, retrospective cohort study was conducted at a 2,800-bed tertiary hospital in Seoul, Republic of Korea. Adult patients (≥ 18 years) diagnosed with initial COVID-19 infection within 7 days of hospital admission between September 1, 2020 and May 31, 2022 were included. Eligible patients had World Health Organization Clinical Progression Scale (WHO-CPS) scores between 6 and 9 and received oxygen support via high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), mechanical ventilation (MV), or extracorporeal membrane oxygenation (ECMO) [17]. COVID-19 diagnosis was confirmed via nasopharyngeal swab polymerase chain reaction (PCR) tests and all patients received 6 mg dexamethasone daily or equivalent for at least 5 days [18].
We excluded patients who had been taking ≥ 5 mg of oral prednisolone (or equivalent) for over 4 weeks [19] due to conditions such as rheumatologic or pulmonary diseases and solid organ transplantation. These patients were already on steroids before their COVID-19 infection and maintained steroid therapy after recovery, complicating the determination of tapering duration. Moreover, almost all these patients underwent steroid tapering, making comparisons between early withdrawal and prolonged tapering groups challenging. Additionally, we excluded patients with WHO-CPS scores < 6, those treated with steroids for < 5 days, or those who died within 10 days of admission.
Clinical information was collected from electronic medical records (EMRs) of all patients. This data included age, sex, body mass index, vaccination status against SARS-CoV-2, comorbidities, the severity of COVID-19 pneumonia, Sequential Organ Failure Assessment (SOFA) score, treatments, such as pronation, remdesivir, baricitinib, tocilizumab, and steroids, and laboratory results. During the study period, study participants received one of the following vaccines: Comirnaty® (Pfizer Inc., Manhattan, New York, United States), Spikevax® (Moderna Inc., Cambridge, Massachusetts, United States), or Vaxzevria™ (AstraZeneca plc., Cambridge, United Kingdom). Patients were classified based on their vaccination status as none or partially vaccinated (0–1 dose), fully vaccinated (2 doses), or boosted (≥ 3 doses). All identifiable information was anonymized before being accessed by the authors. Due to the retrospective design, informed consent from patients was waived. This study was approved by the institutional review board of Asan Medical Center (IRB No. 2022 − 1431).
During the study period, three variants of concern successively predominated in the Republic of Korea: Alpha (September 2020–July 2021), Delta (August 2021–January 2022), and Omicron (February 2022–May 2022) [20]. Patient variant exposure was categorized by these timeframes. Baricitinib and tocilizumab were approved for use on April 1, 2021 (Alpha-dominant period) and March 15, 2022 (Omicron-dominant period), respectively, and were included as matching variables to isolate the effect of steroids.
Clinical outcomes and definitions
Patients were divided into two groups: early withdrawal (EW, ≤ 10 days of steroid use without tapering) and prolonged tapering (PT, > 10 days with tapering), following the RECOVERY trial protocol [3]. The primary outcome was 28-day mortality. Secondary outcomes included 60-day mortality, aggravation (e.g., intubation after HFNC/NIV or ECMO after MV) following initial dexamethasone administration for 7–10 days, rebound pneumonia, infectious complications, steroid-induced hyperglycemia or psychiatric problems, emphysema or pneumothorax, readmission or emergency department (ED) revisits within three months after discharge, length of ICU stay or hospitalization, and duration of oxygen or MV support.
Rebound pneumonia was defined by: (i) initial clinical improvement with dexamethasone therapy; (ii) subsequent WHO-CPS increase or clinical worsening after steroid cessation/reduction; and (iii) no other bacterial, fungal, or viral infections confirmed by microbiologic examinations, such as respiratory specimen cultures and PCR tests [12, 21]. Infectious complications included all bacterial, fungal, or viral infections during or after steroid therapy. Steroid-induced hyperglycemia or psychiatric events were defined by at least one specialist consultation for glucose or symptom control 7–10 days after starting dexamethasone. Emphysema or pneumothorax was confirmed via chest X-ray or computed tomography (CT) in the same timeframe. Readmissions or ED revisits were defined as hospital returns due to dyspnea or pneumonia. Oxygen support duration was defined from the start of WHO-CPS ≥ 5 to recovery at WHO-CPS ≤ 4.
Statistical analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) and were compared using the Mann–Whitney U test. Categorical variables were expressed as frequencies with percentages and were analyzed using chi-square or Fisher’s exact test, as appropriate.
To assess the effect of prolonged steroid therapy, Cox proportional hazards models and Kaplan-Meier curves were used to compare mortalities between the two groups after propensity score matching. The coxph() function with the cluster() option in R was used to adjust the clustering effects within matched pairs. The proportional hazards assumption was tested using Schoenfeld residuals for each predictor, confirming no significant violations.
Given potential selection bias (e.g., more severe patients receiving prolonged steroids), 1:1 propensity score matching was performed using the nearest-neighbor method with a caliper of 0.2 times the standard deviation of the logit of the propensity score. Confounding factors were identified via logistic regression and matching was conducted based on the calculated propensity scores. Matching variables included age, sex, comorbidities, SARS-CoV-2 variant period, vaccination status, specific laboratory results (e.g., elevated lactate dehydrogenase level or lymphopenia), treatments (e.g., pronation, remdesivir, baricitinib, or tocilizumab), oxygen support methods (e.g., HFNC, NIV, MV, or ECMO), and SOFA scores. Balance was assessed via standardized mean differences (< 0.10 considered acceptable). The effectiveness of the matching was further evaluated by comparing the baseline characteristics of the matched groups using chi-square or Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables, as appropriate.
Outcomes were analyzed in the matched dataset, with hazard ratios (HRs) and 95% confidence intervals (CIs) reported for mortality. Time-to-event data were censored at the last follow-up date for those lost. Subgroup analyses were also performed based on the following stratifications: age (< 65 vs. ≥65 years), obesity, oxygen delivery method, SOFA score (< 4 vs. ≥4), and SARS-CoV-2 variant.
Univariate and multivariate Cox proportional hazards regression were conducted to identify risk factors for 28-day mortality. Similarly, univariate and multivariate logistic regression identified risk factors for rebound pneumonia. For predictive model development, variables with a p-value < 0.20 in univariate analysis were included in multivariate analyses. Backward elimination method was applied to refine the final model, retaining variables with p-values < 0.10.
All significance tests were two-sided, with p values of less than 0.05 considered statistically significant. Statistical analyses and graphic presentations were conducted using R Studio version 4.4.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the study participants
During the study period, 312 patients with severe COVID-19 were admitted. After excluding 65 patients (due to prior steroid use, short steroid course, or early death within 10 days of admission), 247 were eligible (Fig. 1). The baseline characteristics of these patients before matching are presented in Table 1. Following 1:1 propensity score matching, 68 patients from each group (EW and PT) were included in the final analysis (Fig. 1). Among the 136 matched patients, seven patients (all in the EW group) were lost to follow-up. Of them, four were lost before day 28 and three between days 29 and 60.
Fig. 1.
Flowchart of the study participants. Abbreviations: COVID-19, coronavirus disease 2019; WHO-CPS, World Health Organization-Clinical Progression Scale; n, number
Table 1.
Clinical characteristics of the study patients before and after propensity score matching
| Characteristic | Unmatched | Matched | ||||||
|---|---|---|---|---|---|---|---|---|
| Early steroid withdrawal, n = 111 (%) |
Prolonged steroid tapering, n = 136 (%) |
P value | Total, n = 136 (%) |
Early steroid withdrawal, n = 68 (%) |
Prolonged steroid tapering, n = 68 (%) |
P value | ||
| Age, years, median (IQR) | 64.0 (55.0–72.0) | 65.0 (56.5–74.0) | 0.21 | 65.0 (52.5–73.0) | 64.5 (52.5–72.5) | 65.0 (52.5–74.0) | 0.99 | |
| Male sex | 72 (64.9) | 79 (58.1) | 0.34 | 87 (64.0) | 44 (64.7) | 43 (63.2) | > 0.99 | |
| Predominant variant of concern at diagnosis | 0.74 | 0.94 | ||||||
| Alpha | 40 (36.0) | 53 (39.0) | 52 (38.2) | 25 (36.8) | 27 (39.7) | |||
| Delta | 60 (54.1) | 73 (53.7) | 72 (52.9) | 37 (54.4) | 35 (51.5) | |||
| Omicron | 11 (9.9) | 10 (7.4) | 12 (8.8) | 6 (8.8) | 6 (8.8) | |||
| Vaccination against SARS-CoV-2 | 0.89 | 0.96 | ||||||
| None or partial (0–1) | 93 (83.8) | 115 (84.6) | 113 (83.1) | 56 (82.4) | 57 (83.8) | |||
| Full (2) | 14 (12.6) | 15 (11.0) | 15 (11.0) | 8 (11.8) | 7 (10.3) | |||
| Boosted (≥ 3) | 4 (3.6) | 6 (4.4) | 8 (5.9) | 4 (5.9) | 4 (5.9) | |||
| Days from symptom onset to admission, median (IQR) | 7.0 (4.0–9.0) | 7.0 (4.0–9.0) | 0.36 | 7.0 (4.0–9.0) | 7.0 (2.5–9.0) | 7.0 (5.0–8.5) | 0.91 | |
| Days from symptom onset to steroid use, median (IQR) | 7.0 (3.0–9.0) | 6.0 (3.0–7.0) | 0.05 | 6.0 (3.0–8.0) | 6.5 (3.0–9.0) | 6.0 (3.0–7.0) | 0.41 | |
| Comorbidities | ||||||||
| Diabetes mellitus | 39 (35.1) | 40 (29.4) | 0.41 | 41 (30.1) | 21 (30.9) | 20 (29.4) | > 0.99 | |
| Hypertension | 62 (55.9) | 65 (47.8) | 0.26 | 70 (51.5) | 35 (51.5) | 35 (51.5) | > 0.99 | |
| Obesity | 49 (44.1) | 66 (48.5) | 0.58 | 66 (48.5) | 33 (48.5) | 33 (48.5) | > 0.99 | |
| Cardiovascular disease | 19 (17.1) | 25 (18.4) | 0.93 | 25 (18.4) | 12 (17.6) | 13 (19.1) | > 0.99 | |
| Chronic kidney disease | 12 (10.8) | 9 (6.6) | 0.34 | 12 (8.8) | 6 (8.8) | 6 (8.8) | > 0.99 | |
| Chronic lung disease | 3 (2.7) | 6 (4.4) | 0.71 | 6 (4.4) | 3 (4.4) | 3 (4.4) | > 0.99 | |
| Chronic liver disease | 3 (2.7) | 10 (7.4) | 0.18 | 6 (4.4) | 3 (4.4) | 3 (4.4) | > 0.99 | |
| Rheumatic disease | 1 (0.9) | 4 (2.9) | 0.50 | 1 (0.7) | 0 (0.0) | 1 (1.5) | > 0.99 | |
| Solid malignancy | 5 (4.5) | 9 (6.6) | 0.66 | 8 (5.9) | 4 (5.9) | 4 (5.9) | > 0.99 | |
| Hematologic malignancy | 2 (1.8) | 7 (5.1) | 0.29 | 4 (2.9) | 2 (2.9) | 2 (2.9) | > 0.99 | |
| Severity of illness | < 0.001 | > 0.99 | ||||||
| WHO-CPS 6 (HFNC or NIV) | 88 (79.3) | 48 (35.3) | 89 (65.4) | 45 (66.2) | 44 (64.7) | |||
| WHO-CPS 7–9 (MV) | 23 (20.7) | 88 (64.7) | 47 (34.6) | 23 (33.8) | 24 (35.3) | |||
| Pronation | 5 (4.5) | 54 (39.7) | < 0.001 | 11 (8.1) | 5 (7.4) | 6 (8.8) | > 0.99 | |
| SOFA score, median (IQR) | 3.0 (2.0–5.0) | 5.0 (3.0–7.0) | < 0.001 | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | 0.91 | |
Laboratory data, mean (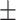 SD) SD) |
||||||||
Leukocyte,  103/uL 103/uL |
8.2 (± 3.7) | 8.3 (± 4.4) | 0.87 | 8.3 (± 4.1) | 8.5 (± 3.7) | 8.0 (± 4.4) | 0.49 | |
| Hemoglobin, g/dL | 14.1 (± 2.5) | 13.1 (± 1.9) | 0.42 | 13.0 (± 2.0) | 12.7 (± 2.0) | 13.2 (± 2.0) | 0.18 | |
Platelet, 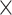 103/uL 103/uL |
224.3 (± 86.5) | 205.9 (± 86.4) | 0.10 | 218.3 (± 94.3) | 223.2 (± 91.5) | 213.3 (± 97.1) | 0.54 | |
| Lymphocyte, count/uL | 786.6 (± 370.8) | 725.1 (± 418.0) | 0.15 | 774.2 (± 371.4) | 787.4 (± 351.3) | 760.9 (± 391.5) | 0.69 | |
| C-reactive protein, mg/dL | 10.0 (± 7.9) | 11.4 (± 9.9) | 0.22 | 10.0 (± 7.5) | 10.5 (± 7.6) | 9.5 (± 7.4) | 0.46 | |
| D-dimer, ug/mL, median (IQR) | 1.1 (0.5–2.8) | 1.4 (0.6–5.8) | 0.22 | 1.0 (0.5–3.7) | 1.1 (0.6–3.2) | 1.0 (0.6–4.5) | 0.97 | |
| Lactate dehydrogenase, IU/L | 466.4 (± 156.8) | 563.0 (± 235.6) | < 0.001 | 514.3 (± 208.4) | 485.4 (± 168.8) | 543.2 (± 248.0) | 0.12 | |
| Received therapies | ||||||||
| Remdesivir | 100 (90.1) | 123 (90.4) | > 0.99 | 124 (91.2) | 62 (91.2) | 62 (91.2) | > 0.99 | |
| Baricitinib | 18 (16.2) | 14 (10.3) | 0.24 | 21 (15.4) | 11 (16.2) | 10 (14.7) | > 0.99 | |
| Tocilizumab | 55 (49.5) | 72 (52.9) | 0.69 | 71 (52.2) | 36 (52.9) | 35 (51.5) | > 0.99 | |
| Use of steroids | ||||||||
| Duration of dexamethasone, days, median (IQR) | 9.0 (7.0–10.0) | 10.0 (8.0–10.0) | 0.07 | 10.0 (7.0–10.0) | 10.0 (7.5–10.0) | 10.0 (7.0–10.0) | 0.52 | |
| Duration of tapering, days, median (IQR) | - | 14.0 (8.0–27.5) | - | - | - | 13.0 (7.0–21.0) | - | |
| Daily average dose of tapering steroidsa, mg, median (IQR) | - | 22.3 (16.8–30.7) | - | - | - | 19.5 (14.6–25.0) | - | |
Data are presented as the number (%) of patients unless otherwise indicated
Abbreviations: n, number; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; WHO-CPS, World Health Organization-Clinical Progression Scale; HFNC, high-flow nasal cannula; NIV, noninvasive ventilation; MV, mechanical ventilation; SOFA, sequential organ failure assessment
a Equivalent dose in methylprednisolone
Significant differences in WHO-CPS scores, SOFA scores, pronation, and serum lactate dehydrogenase levels were balanced after matching (Table 1). The distributions of the propensity scores and standardized mean differences for the covariates before and after matching are presented in Table S1 and Fig. S1.
Among the 136 matched patients, 87 (64.0%) were male, with a median age of 65.0 years (IQR 52.5–73.0). More than half (52.9%) were diagnosed with COVID-19 infection during the delta variant-dominant period. Most (65.4%) received HFNC or NIV, while the remainder were supported by MV. Median SOFA score was 3.0 (IQR 2.0–6.0) and the median time from symptom onset to initial steroid use was 6.0 days (IQR 3.0–8.0). In the PT group, the median duration of the tapering periods was 13.0 days (IQR 7.0–21.0), with the median daily dose equivalent to 19.5 mg of methylprednisolone (IQR 14.6–25.0) (Table 1).
Risk factors associated with In-Hospital 28-Day mortality and rebound pneumonia in patients with severe COVID-19
Univariate analysis of the matched severe COVID-19 cohort identified age, obesity, chronic kidney disease, solid malignancy, hematologic malignancy, SOFA score, and D-dimer level as potential predictors associated with 28-day mortality. Subsequent multivariate analysis using backward elimination produced a refined model that included age (adjusted HR (aHR) 1.08, 95% CI 1.02–1.15, p = 0.01), solid malignancy (aHR 8.87, 95% CI 2.44–32.20, p < 0.01), and hematologic malignancy (aHR 13.09, 95% CI 1.33–128.72, p = 0.03) as significant predictors of 28-day mortality (Table 2).
Table 2.
Risk factors associated with 28-day mortality in the matched patients with severe COVID-19
| Variable | Univariate model | Multivariate model | Refined model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | Adjusted HR | 95% CI | P value | Adjusted HR | 95% CI | P value | |||
| Age | 1.09 | 1.03–1.15 | < 0.01 | 1.06 | 0.99–1.14 | 0.12 | 1.08 | 1.02–1.15 | 0.01 | ||
| Variant of concern | 1.80 | 0.70–4.66 | 0.22 | - | - | - | - | - | - | ||
| Vaccination status | 1.15 | 0.42–3.15 | 0.79 | - | - | - | - | - | - | ||
| Obesity | 0.22 | 0.05–1.03 | 0.05 | 0.40 | 0.07–2.20 | 0.29 | - | - | - | ||
| Diabetes | 0.83 | 0.22–3.14 | 0.79 | - | - | - | - | - | - | ||
| Hypertension | 1.07 | 0.33–3.51 | 0.91 | - | - | - | - | - | - | ||
| Cardiovascular disease | 1.64 | 0.43–6.18 | 0.47 | - | - | - | - | - | - | ||
| Chronic lung disease | 2.66 | 0.34–20.77 | 0.35 | - | - | - | - | - | - | ||
| Chronic kidney disease | 6.26 | 1.83–21.41 | < 0.01 | 1.97 | 0.42–9.12 | 0.39 | - | - | - | ||
| Solid malignancy | 11.86 | 3.47–40.62 | < 0.01 | 10.97 | 2.16–55.67 | < 0.01 | 8.87 | 2.44–32.20 | < 0.01 | ||
| Hematologic malignancy | 4.03 | 0.52–31.50 | 0.18 | 11.69 | 1.14-119.46 | 0.04 | 13.09 | 1.33-128.72 | 0.03 | ||
| Mechanical ventilation (WHO-CPS 7–9) | 1.57 | 0.48–5.15 | 0.45 | - | - | - | - | - | - | ||
| SOFA score | 1.18 | 0.95–1.48 | 0.14 | 1.11 | 0.82–1.50 | 0.51 | - | - | - | ||
| Pronation | 1.07 | 0.14–8.35 | 0.95 | - | - | - | - | - | - | ||
| Lymphopenia | 0.89 | 0.24–3.35 | 0.86 | - | - | - | - | - | - | ||
| CRP level | 1.01 | 0.94–1.09 | 0.74 | - | - | - | - | - | - | ||
| Lactate dehydrogenase level | 1.00 | 0.99–1.01 | 0.52 | - | - | - | - | - | - | ||
| D-dimer level | 1.05 | 1.01–1.09 | 0.02 | 0.98 | 0.92–1.04 | 0.49 | - | - | - | ||
| Baricitinib | 1.17 | 0.25–5.43 | 0.84 | - | - | - | - | - | - | ||
| Tocilizumab | 0.52 | 0.15–1.76 | 0.29 | - | - | - | - | - | - | ||
| Prolonged steroid therapy | 0.54 | 0.16–1.84 | 0.32 | - | - | - | - | - | - | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; WHO-CPS, World Health Organization-Clinical Progression Scale; SOFA, sequential organ failure assessment; CRP, C-reactive protein
Additionally, univariate analysis for rebound pneumonia identified age, obesity, chronic kidney disease, solid malignancy, mechanical ventilation (WHO-CPS 7–9), pronation, D-dimer level, and use of remdesivir as potential predictors. Multivariate analysis with backward elimination yielded a refined model comprising obesity (adjusted odds ratio (aOR) 0.20, 95% CI 0.05–0.81, p = 0.02), chronic kidney disease (aOR 4.27, 95% CI 0.91-20.00, p = 0.07), solid malignancy (aOR 7.24, 95% CI 1.19–44.18, p = 0.03), mechanical ventilation (aOR 4.24, 95% CI 1.09–16.53, p = 0.04), and remdesivir use (aOR 0.20, 95% CI 0.04–0.90, p = 0.04). Table S2 presents the results from logistic regression on rebound pneumonia.
Effects of prolonged steroid therapy on clinical outcomes
Before matching, no significant differences were observed between the groups regarding in-hospital 28-day mortality, aggravation rate, readmission or ED revisits within three months after discharge, the incidence of rebound pneumonia, and steroid-induced hyperglycemia or psychiatric problems. However, 60-day mortality and the incidence of infectious complications were significantly higher in the PT group than in the EW group, with rates of 19.9% vs. 7.2% (p = 0.01) and 17.6% vs. 8.1% (p = 0.04), respectively (Table 3).
Table 3.
Clinical outcomes for the study patients before and after 1:1 propensity score matching
| Characteristic | Unmatched | Matched | |||||
|---|---|---|---|---|---|---|---|
| Early steroid withdrawal, n = 111 (%) |
Prolonged steroid tapering, n = 136 (%) |
P value | Early steroid withdrawal, n = 68 (%) |
Prolonged steroid tapering, n = 68 (%) |
P value | ||
| Length of hospitalization, days, median (IQR) | 13.0 (10.0–17.0) | 24.0 (17.0–39.0) | < 0.001 | 14.0 (11.0–18.5) | 20.0 (14.0–29.5) | 0.001 | |
| Duration of oxygen support, days, median (IQR) | 11.0 (9.0–17.0) | 22.0 (15.0–37.0) | < 0.001 | 13.0 (9.0–17.0) | 17.5 (14.0–25.5) | 0.001 | |
| Length of ICU stay, days, median (IQR) | 0.0 (0.0-5.5) | 12.0 (4.5–21.5) | < 0.001 | 1.0 (0.0–7.0) | 5.0 (0.0-13.5) | 0.01 | |
| Duration of MV support, days, median (IQR) | 0.0 (0.0–0.0) | 8.5 (0.0-16.5) | < 0.001 | 0.0 (0.0–4.0) | 0.0 (0.0-9.5) | 0.21 | |
| Aggravation after use of dexamethasone b | 1 (0.9) | 6 (4.4) | 0.21 | 1 (1.5) | 1 (1.5) | > 0.99 | |
| 28-day mortality | 7 (6.3) | 12 (8.8) | 0.62 | 7 (10.3) | 4 (5.9) | 0.53 | |
| 60-day mortality | 8 (7.2) | 27 (19.9) | 0.01 | 8 (11.8) | 10 (14.7) | 0.80 | |
| Readmission or revisit to the emergency department c | 3 (2.7) | 9 (6.6) | 0.26 | 2 (2.9) | 6 (8.8) | 0.27 | |
| Incidence of rebound pneumonia | 9 (8.1) | 13 (9.6) | 0.86 | 6 (8.8) | 10 (14.7) | 0.43 | |
| Infectious complications | 9 (8.1) | 24 (17.6) | 0.04 | 6 (8.8) | 11 (16.2) | 0.30 | |
| Steroid-induced hyperglycemia | 7 (6.3) | 15 (11.0) | 0.28 | 4 (5.9) | 8 (11.8) | 0.36 | |
| Steroid-induced psychiatric problems | 10 (9.0) | 15 (11.0) | 0.76 | 9 (13.2) | 8 (11.8) | > 0.99 | |
| Emphysema or pneumothorax | 0 (0.0 | 24 (17.6) | < 0.001 | 0 (0.0) | 7 (10.3) | 0.02 | |
Data are presented as the number (%) of patients unless otherwise indicated
Abbreviations: n, number; IQR, interquartile range; ICU, intensive care unit; MV, mechanical ventilator
a Until recovery to a WHO-CPS score of 4 or lower
b Intubation rates among patients requiring HFNC or NIV and ECMO rates among patients requiring MV, following the initial use of dexamethasone for 7–10 days
c Within 3 months after discharge
After matching, no significant differences were observed in 28-day and 60-day mortalities, aggravation rate, readmission/ED revisits, rebound pneumonia, infections, and steroid-induced complications (Table 3). Cox regression analyses revealed no significant differences in 28-day mortality (HR 0.54, 95% CI 0.16–1.84, p = 0.32) or 60-day mortality (HR 1.22, 95% CI 0.48–3.10, p = 0.67) between the groups. Kaplan-Meier curves for these results are presented in Fig. 2. Table S3 presents the results of subgroup analyses for factors such as age, obesity, oxygenation methods, SOFA scores, and SARS-CoV-2 variants. It similarly showed that prolonged steroid use was not significantly associated with 28-day and 60-day mortalities, consistent with the main analysis findings.
Fig. 2.
Survival analysis according to steroid tapering in patients with critical COVID-19. (A) 28-day mortality. (B) 60-day mortality
The lengths of hospitalization, ICU stays, and oxygen support were significantly longer in the PT group compared to the EW group, regardless of the matching process. Additionally, the incidence of emphysema or pneumothorax was significantly higher in the PT group than in the EW group, also irrespective of the matching process. Before matching, the duration of MV support was significantly longer in the PT group. However, after matching, there was no significant difference in this outcome between the two groups (Table 3).
Discussion
This study examined patients with severe COVID-19 who received oxygen support via HFNC, NIV, MV, or ECMO and who had SOFA scores 2.0–6.0. We found that prolonged steroid use beyond 10 days did not significantly reduce 28-day or 60-day mortality. Early steroid withdrawal without tapering was also not associated with increased rebound pneumonia, readmission, or ED revisit. Multivariable analysis showed consistent results, indicating no significant association between prolonged steroid therapy and reduced mortality. These findings provide valuable insights into the clinical management of severe COVID-19, especially regarding the optimal duration of steroid therapy.
Our findings align with previous studies that reported limited survival benefits from extended steroid therapy. A meta-analysis of seven randomized clinical trials and twenty observational studies showed no additional survival benefit beyond 6–7 days of steroid use [22]. Another retrospective study revealed that prolonged steroids (> 10 days) significantly increased in-hospital mortality and infectious complications [23]. Although our study did not show a significant association between prolonged steroid use and mortality or infection rates, overall mortality in that study was higher (35.9%) compared to our study (13.2%) [23]. This discrepancy may stem from the higher proportion of mechanically ventilated patients in the previous study and the inclusion of Omicron variant cases in our study, which are generally less severe than Delta infections [24].
However, most of our cohort was admitted during the Delta wave, with only 8.8% from the Omicron period. Notably, after matching, the severity of patients in the matched PT group was significantly reduced. In this group, over 60% of patients required only HFNC or NIV, rather than MV. Furthermore, the median SOFA score was 3.0 (IQR 2.0–6.0) and only 8.8% of patients underwent pronation. Therefore, the observed lack of benefit from prolonged steroid therapy may apply primarily to patients with less severe critical illness, such as those with SOFA scores below 6.0 or without acute respiratory distress syndrome. The impact of prolonged steroid therapy on mortality in more critically ill patients cannot be conclusively determined from our study.
Aggravated cases were limited to just two cases, with one in each treatment group. No significant differences were observed in the clinical aggravation or duration of MV support. These findings are consistent with a randomized controlled trial of patients with COVID-19 requiring low-flow oxygen, HFNC, or NIV, which showed no significant differences in tracheostomy (2.4% vs. 2.6%, p = 0.82), ICU referral (12.2% vs. 13.2%, p = 0.68), and MV-free days at day 28 (28.0 vs. 28.0, p = 0.92) between prolonged methylprednisolone treatment and early dexamethasone withdrawal groups [25]. Thus, the effect of prolonged steroid therapy on clinical deterioration and prognosis in critical COVID-19 pneumonia remains uncertain. However, this effect might have been underestimated due to the lower severity of matched patients. In patients with higher SOFA scores and more critical illness, the impact of prolonged steroid therapy may differ.
A retrospective study of COVID-19 patients infected with the Omicron variant found that prolonged high-dose steroid therapy significantly reduced overall mortality in those requiring HFNC, NIV, MV, or ECMO [26]. However, this study had a notably higher mortality rate (47.1%) compared to our study (14.2%), which may have exaggerated the perceived benefit of steroids. Interestingly, although our cohort included more patients requiring MV (34.6% vs. 17.6%), our mortality rate was lower. This discrepancy may be partly explained by the significantly lower use of remdesivir in that study (45.2% vs. 91.2%), as remdesivir has been associated with improved outcomes in severe COVID-19 pneumonia [27].
Durations of oxygen support, hospitalization, and ICU stay were all significantly longer in the PT group than in the EW group. This may be attributed to longer steroid treatment durations in the PT group, which were individualized based on clinical responses. Despite propensity score matching, more severe patients may have been included in the PT group, leading to longer oxygen support and ICU care. Additionally, the incidence of emphysema or pneumothorax was significantly higher in the PT group, potentially reflecting the inclusion of patients requiring longer or more intensive respiratory support [28, 29].
We found no significant association between prolonged steroid use and rebound pneumonia. A prior study reported that age, oxygen requirements, lymphopenia, and elevated soluble IL-2 receptor levels were predictive of rebound pneumonia, while steroid duration and timing were not significantly different between patients with and without rebound phenomena [21]. Another study suggested that early initiation and abrupt cessation of steroids may trigger rebound events [12]. We conducted univariate and multivariate analyses to identify risk factors for rebound pneumonia. The final refined model included obesity, chronic kidney disease, solid malignancy, mechanical ventilation, and remdesivir use. Notably, remdesivir use was significantly associated with a reduced risk of rebound phenomena. Remdesivir may mitigate viral replication and reduce viral loads, thereby decreasing the likelihood of rebound pneumonia, consistent with previous findings [30]. Therefore, prolonged steroid use may not effectively prevent rebound phenomena in severe COVID-19 patients, as rebound may be more strongly influenced by factors beyond inflammation, such as increased viral loads and impaired immune responses [30, 31].
Long-term systemic steroid use may increase the risk of bacterial and fungal infections [32, 33]. Before propensity score matching, the PT group had a significantly higher rate of infectious complications, which became nonsignificant after matching. The higher WHO-CPS and SOFA scores in the PT group before matching suggest that the increased rate of infections could be attributed to the severity of the disease. Thus, infectious complications may reflect not only prolonged steroid use but also the underlying severity of illness [34]. Similarly, there were no significant differences between groups in other steroid-induced adverse events, such as hyperglycemia and psychiatric problems. These events may also be related to illness severity and extended ICU stays, in addition to prolonged steroid administration [35, 36].
Our study provides important insights into managing severe COVID-19 patients requiring oxygen support. Specifically, patients with SOFA scores of 2.0 to 6.0 who required oxygen through HFNC, NIV, or MV may not benefit from steroid therapy beyond 10 days. However, several limitations should be considered. First, the retrospective, single-center design and small sample size may limit generalizability and may introduce selection bias. Although no significant differences were observed between the groups, the study may have been underpowered to detect modest effects due to the small sample size, as reflected in the wide confidence intervals. Moreover, though matching was used, unmeasured or residual confounding may remain. Second, we excluded patients previously on steroids, which precludes an analysis of prolonged steroid therapy effects on immunocompromised patients who have undergone solid organ or hematopoietic stem cell transplantation. Third, seven patients were lost to follow-up (all in the EW group), which may introduce bias despite censoring in survival analysis. Fourth, our cohort excluded patients on low-flow oxygen, as few of these received prolonged steroid therapy. Fifth, although we used the criteria from the previous studies to define rebound pneumonia, the diagnosis relied on subjective clinical judgment and may be prone to misclassification. However, we endeavored to distinguish these conditions using microbiological studies, providing more objective evidence for classification. Lastly, not all patients underwent chest CT or had their imaging reviewed, raising the possibility that steroid-responsive conditions like organizing pneumonia were underrepresented [37, 38]. However, as most cases of organizing pneumonia occur 2–3 weeks after symptom onset [37–39] and the median time to steroid initiation in our cohort was 6 days, the likelihood of widespread inclusion of such cases is low. Furthermore, the absence of significant differences in readmissions or ED revisits after discharge supports this conclusion. To better establish the optimal duration of steroid treatment in patients with severe COVID-19, further randomized controlled trials with larger, more diverse cohorts and comprehensive chest imaging reviews are necessary.
Conclusions
In patients with severe COVID-19 requiring HFNC, NIV, or MV and SOFA scores between 2.0 and 6.0, prolonged steroid therapy beyond 10 days did not improve 28-day or 60-day mortality, the incidence of rebound pneumonia, or the rate of readmission or ED revisits. These findings suggest that extended steroid use with tapering may be unnecessary in similarly severe cases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None to be made.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- RECOVERY
Randomized Evaluation of COVID-19 Therapy
- WHO-CPS
World Health Organization Clinical Progression Scale
- HFNC
High-flow nasal cannula
- NIV
Noninvasive ventilation
- MV
Mechanical ventilation
- ECMO
Extracorporeal membrane oxygenation
- PCR
Polymerase chain reaction
- EMR
Electronic medical record, SOFA, sequential organ failure assessment
- ED
Emergency department
- IQR
Interquartile range
- HR
Hazard ratio
- CI
Confidence interval
- CT
Computed tomography
Author contributions
Concept and Design: E. C., S. K. Acquisition, analysis, or interpretation of data: J. H., E. C. Drafting of manuscript: J. H., E. C. Critical revision of the manuscript for important intellectual content: S. B., J. J., M. J. K., Y. P. C., S. C., S. L., Y. S. K., E. C., S. K. Statistical analyses: J. H., E. C. Obtained funding: S. K. Administrative, technical or material support: E. C., S. K. Supervision: E. C., S. K.
Funding
This study was supported by a grant from the National Research Foundation of Korea from the Ministry of Science and ICT, South Korea (grant number RS-2023-00219002).
Data availability
J. H. and E. C. had full access to data for the study and were responsible for the integrity of the data and the accuracy of analyses. The data that support the findings of this study are not openly available due to reasons of sensitivity.
Declarations
Ethics approval and consent to participate
This retrospective cohort study received approval from the institutional review board of the Asan Medical Center (IRB No. 2022 − 1431) and its ethics committee. No personalized information of patients was exposed and no direct contact were made. The ethics committee waived determined that it was unnecessary to obtain informed consent due to the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ma S, Xu C, Liu S et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther. 2021;6:83. Available at: 10.1038/s41392-021-00521-7 [DOI] [PMC free article] [PubMed]
- 2.Wang W, Snell LB, Ferrari D et al. Real-world effectiveness of steroids in severe COVID-19: a retrospective cohort study. BMC Infect Dis. 2022;22:776. Available at: 10.1186/s12879-022-07750-3 [DOI] [PMC free article] [PubMed]
- 3.The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 2021;384:693–704. Available at: http://www.nejm.org/doi/10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed]
- 4.Lee FEH, Walsh EE, Falsey AR. The effect of steroid use in hospitalized adults with respiratory syncytial virus-related illness. Chest. 2011;140:1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damlaj M, Bartoo G, Cartin-Ceba R et al. Corticosteroid use as adjunct therapy for respiratory syncytial virus infection in adult allogeneic stem cell transplant recipients. Transplant Infectious Disease. 2016;18:216–226. Available at: 10.1111/tid.12513 [DOI] [PubMed]
- 6.Lansbury LE, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Shen Lim W. Corticosteroids as Adjunctive Therapy in the Treatment of Influenza: An Updated Cochrane Systematic Review and Meta-analysis. Crit Care Med. 2020;48. Available at: https://journals.lww.com/ccmjournal/fulltext/2020/02000/corticosteroids_as_adjunctive_therapy_in_the.29.aspx [DOI] [PubMed]
- 7.Mundo W, Morales-Shnaider L, Tewahade S et al. Lower Mortality Associated With Adjuvant Corticosteroid Therapy in Non-HIV-Infected Patients With Pneumocystis jirovecii Pneumonia: A Single-Institution Retrospective US Cohort Study. Open Forum Infect Dis. 2020;7:ofaa354. Available at: 10.1093/ofid/ofaa354 [DOI] [PMC free article] [PubMed]
- 8.Investigators TWC for the R-C. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020;324:1317–1329. Available at: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed]
- 9.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/
- 10.Chen PH, Cheng CY, Li LF, Yu CC. Pneumonia rebound after stopping steroid in a patient with COVID-19: A case report. Respirol Case Rep 2021;9. [DOI] [PMC free article] [PubMed]
- 11.Tran H, Vu VH, Phan TT, Nguyen KD, Truong BQ. A case of rebound inflammation in a 38-Year-Old man with severe COVID-19 pneumonia following cessation of dexamethasone therapy. Am J Case Rep 2022;23. [DOI] [PMC free article] [PubMed]
- 12.Imai R, Ro S, Tomishima Y, Nishimura N. Steroid resistance and rebound phenomena in patients with COVID-19. Respir Investig. 2021;59:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R, Cho SY, Lee DG, et al. Risk factors and clinical impact of COVID-19-associated pulmonary aspergillosis: multicenter retrospective cohort study. Korean J Intern Med. 2022;37:851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Hong JY, Bae S et al. Risk factors for coronavirus disease 2019 (COVID-19)-Associated pulmonary aspergillosis in critically ill patients: A nationwide, multicenter, retrospective cohort study. J Korean Med Sci 2022;37. [DOI] [PMC free article] [PubMed]
- 15.Dupper AC, Malik Y, Cusumano JA et al. Longer Steroid Treatment Increases Secondary Bloodstream Infection Risk Among Patients With COVID-19 Requiring Intensive Care. Infectious Diseases in Clinical Practice. 2022;30. Available at: https://journals.lww.com/infectdis/fulltext/2022/07000/longer_steroid_treatment_increases_secondary.7.aspx
- 16.Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Group TCS 2 T. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021;326:1807–1817. Available at: 10.1001/jama.2021.18295 [DOI] [PMC free article] [PubMed]
- 19.Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6:e000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Ae KyungAU - Lee HyeokjinAU - Kim JunyoungAU - Kim Dong HyeokAU. - Kim Jeong-AhAU - No Jin SunAU - Lee Chae youngAU - Woo SangHeeAU - Lee JaeheeAU - Rhee JeeEunAU - Kim Eun-Jin I-H-P. July 2021 status and characteristics of the COVID-19 variant virus outbreak in the Republic of Korea. Public Health Weekly Report 2021;14:2547–2560. Available at: https://phwr.org/journal/view.html?doi=
- 21.Murakami K, Sano H, Tode N et al. Clinical features of COVID-19 patients with rebound phenomenon after corticosteroid therapy. BMJ Open Respir Res 2022;9.
- 22.Ssentongo P, Yu N, Voleti N et al. Optimal duration of systemic corticosteroids in coronavirus disease 2019 treatment: A systematic review and Meta-analysis. Open Forum Infect Dis 2023;10. [DOI] [PMC free article] [PubMed]
- 23.Viana MV, Pellegrini JAS, Perez AV et al. Association between prolonged corticosteroids use in COVID-19 and increased mortality in hospitalized patients: a retrospective study with inverse probability of treatment weighting analysis. Crit Care 2023;27. [DOI] [PMC free article] [PubMed]
- 24.Hyams C, Challen R, Marlow R et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, united Kingdom. Lancet Reg Health - Europe 2023;25. [DOI] [PMC free article] [PubMed]
- 25.Salton F, Confalonieri P, Centanni S et al. Prolonged higher dose Methylprednisolone versus conventional dexamethasone in COVID-19 pneumonia: a randomised controlled trial (MEDEAS). Eur Respir J 2023;61. [DOI] [PMC free article] [PubMed]
- 26.Fan J, Xie H, Wang Y et al. Longer duration of high-dose corticosteroids provides benefit for hospitalized COVID-19 patients with high oxygen requirement. Heliyon 2024;10. [DOI] [PMC free article] [PubMed]
- 27.Mozaffari E, Chandak A, Gottlieb RL et al. Lower Mortality Risk Associated With Remdesivir + Dexamethasone Versus Dexamethasone Alone for the Treatment of Patients Hospitalized for COVID-19. Clinical Infectious Diseases. 2025;80:63–71. Available at: 10.1093/cid/ciae477 [DOI] [PMC free article] [PubMed]
- 28.Turan YB. Risk factors affecting the development of pneumothorax in patients followed up in intensive care with a diagnosis of COVID-19. BMC Infect Dis. 2024;24:1243. Available at: 10.1186/s12879-024-10147-z [DOI] [PMC free article] [PubMed]
- 29.Ioannidis G, Lazaridis G, Baka S et al. Barotrauma and pneumothorax. J Thorac Dis. 2015;7:S38-43. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25774306 [DOI] [PMC free article] [PubMed]
- 30.Lai CC, Hsueh PR. Coronavirus disease 2019 rebounds following Nirmatrelvir/ritonavir treatment. J Med Virol. 2023;95. [DOI] [PMC free article] [PubMed]
- 31.Hay JA, Kissler SM, Fauver JR et al. Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: A retrospective cohort study. Elife 2022;11. [DOI] [PMC free article] [PubMed]
- 32.Rostaing L, Malvezzi P. Steroid-Based therapy and risk of infectious complications. PLoS Med 2016;13. [DOI] [PMC free article] [PubMed]
- 33.Klein NC, Go CH-U, Cunha BA, Infections associated with steroid use. Infectious Disease Clinics. 2001;15:423–432. Available at: 10.1016/S0891-5520(05)70154-9 [DOI] [PubMed]
- 34.Komori A, Iriyama H, Kainoh T, Aoki M, Naito T, Abe T. The impact of infection complications after trauma differs according to trauma severity. Sci Rep. 2021;11:13803. Available at: 10.1038/s41598-021-93314-5 [DOI] [PMC free article] [PubMed]
- 35.Le VT, Ha QH, Tran MT, Le NT, Le VT, Le MK. Hyperglycemia in Severe and Critical COVID-19 Patients: Risk Factors and Outcomes. Cureus. 2022;14:e27611. Available at: 10.7759/cureus.27611 [DOI] [PMC free article] [PubMed]
- 36.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: A prospective cohort study. Crit Care 2009;13. [DOI] [PMC free article] [PubMed]
- 37.Segala FV, Sgalla G, Salvati F et al. Adjunctive corticosteroid treatment for organizing pneumonia in COVID-19 patients with persistent respiratory failure. Respir Med. 2021;187. [DOI] [PMC free article] [PubMed]
- 38.Tonon CR, Tanni SE, Rocha J, et al. Organizing pneumonia and COVID-19. Am J Med Sci. 2023;366:458–63. [DOI] [PubMed] [Google Scholar]
- 39.de Oliveira Filho CM, Vieceli T, de Fraga Bassotto C, da Rosa Barbato JP, Garcia TS, Scheffel RS. Organizing pneumonia: A late phase complication of COVID-19 responding dramatically to corticosteroids. The Brazilian Journal of Infectious Diseases. 2021;25:101541. Available at: https://www.sciencedirect.com/science/article/pii/S1413867021000040 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
J. H. and E. C. had full access to data for the study and were responsible for the integrity of the data and the accuracy of analyses. The data that support the findings of this study are not openly available due to reasons of sensitivity.




