Abstract
We investigated the phenotypic and genetic diversity (by ribotyping) of Streptococcus pneumoniae isolates recovered from French children, by blood culture from meningitis-free patients (n = 244) and by cerebrospinal fluid culture from patients with meningitis (n = 154). Isolates belonging to serotypes associated with carriage and penicillin-resistant isolates were significantly more frequent in children under 2 years of age than in older children. The seven-valent pneumococcal conjugate vaccine covered 68% of strains associated with bacteremia and 61% of strains associated with meningitis in children under 2 years. Although some serotypes were recovered more frequently from children with bacteremia than from those with meningitis, no difference in the genetic backgrounds of the two groups of strains was found.
Streptococcus pneumoniae is an important cause of morbidity and mortality in children. In particular, it is the most common cause of pediatric acute otitis media, sinusitis, and pneumonia. Since the advent of polysaccharide-protein conjugate vaccines against Haemophilus influenzae type b, S. pneumoniae has become the leading cause of bacterial meningitis and bacteremia in children less than 2 years old (1). Significant variability in serotype patterns and antimicrobial susceptibility have been observed among different countries (13). Recently, a conjugate pneumococcal vaccine was licensed for use in young children. It is immunogenic in young infants, but the number of different serotypes that such vaccines can cover is limited (3). A clear picture of the population structure of invasive S. pneumoniae is thus needed before launching of mass vaccination with a conjugate pneumococcal vaccine. In this study, we used complementary methods to obtain information on the penicillin susceptibility, capsule locus, and genetic background of S. pneumoniae isolates recovered from children with isolated bacteremia or meningitis in France.
MATERIALS AND METHODS
Bacterial strains.
We studied 244 consecutive pneumococcal isolates recovered by blood culture from meningitis-free children age 0 to 14 years between March 1996 and December 1997 in towns located throughout France (134 isolates were recovered from children under 2 years old and 110 were recovered from children over 2 years old). We also studied 154 pneumococcal isolates recovered from pediatric cerebrospinal fluid (CSF) samples between 1992 and 1997 (100 isolates were recovered from children under 2 years old and 54 from children over 2 years old). The latter isolates were not randomly selected, but we have respected the frequency of each serogroup recovered in the national collection and the rates of penicillin resistance. All of these isolates belong to the collection of the Centre National de Référence des Pneumocoques (directed by P. Geslin).
Penicillin MICs were determined by the dilution method on Mueller-Hinton agar supplemented with 5% sheep blood, as previously reported (6). Serotyping was performed by coagglutination with antiserum-coated latex particles.
For genetic analysis, we selected 109 of the blood isolates and 86 of the CSF isolates that were representative with respect to the patients' ages, the serotype distribution, and penicillin susceptibility. In addition, two penicillin-susceptible nontypeable reference strains (R6 and the type strain of the species, ATCC 33400), and members of Spanish clones recognized by the Pneumococcal Molecular Epidemiology Network (Spain23F-1, strain SP456 [penicillin MIC, 2 mg/liter] and Spain6B-2 [penicillin MIC, 1 mg/liter]) (15) were used for comparison.
DNA extraction.
Isolates were grown in 20 ml of TGY broth (Bio-Rad, Steenvoorde, France) for 4 h. After centrifugation, DNA was prepared from the bacterial pellet as previously described (4).
Restriction fragment length polymorphism (RFLP) analysis of rRNA (rrn) genes (ribotyping).
DNA was digested with HindIII and EcoRI (Boehringer, Mannheim, Germany) according to the manufacturer's instructions and analyzed by Southern blotting with a chemiluminescent ribosomal probe as previously described (4).
Statistical analysis.
Ribotyping data were summarized as two-way tables (two tables for RFLP analysis of rRNA genes with the two endonucleases). Each table contains one row for each of the 199 isolates and a number of columns corresponding to the number of DNA fragments detected by ribotyping. In each column the DNA fragment was coded in binary manner (present = 2, absent = 1). Factor analysis of correspondence was applied with SPAD 4.0 software (Cisia Ceresta, Montreuil, France) to the ribotyping data obtained from the combination of the two endonuclease patterns. FAC is a multidimensional statistical analysis based on the main axes of inertia of a scatter plot associated with strains described by the variables (14, 21). This technique describes the dispersion and shape of a cloud of n objects or p variates in a multidimensional space by replacing the original data set by a new set of orthogonal linear coordinates in a space of significantly lower dimension. The first axis of the analysis accounts for the greatest part of variability in the new space, and the second axis, orthogonal to (independent of) the first, accounts for the greatest part of variability which is not accounted by the first. The explained variances of the elements of the data set are in decreasing order of magnitude with respect to these new coordinates. The dispersion of the sample on the plane indirectly expresses the diversity of the sample.
The χ2 test was used to compare the rates of penicillin resistance and the serotype distribution of the isolates.
RESULTS
Bacteriological data.
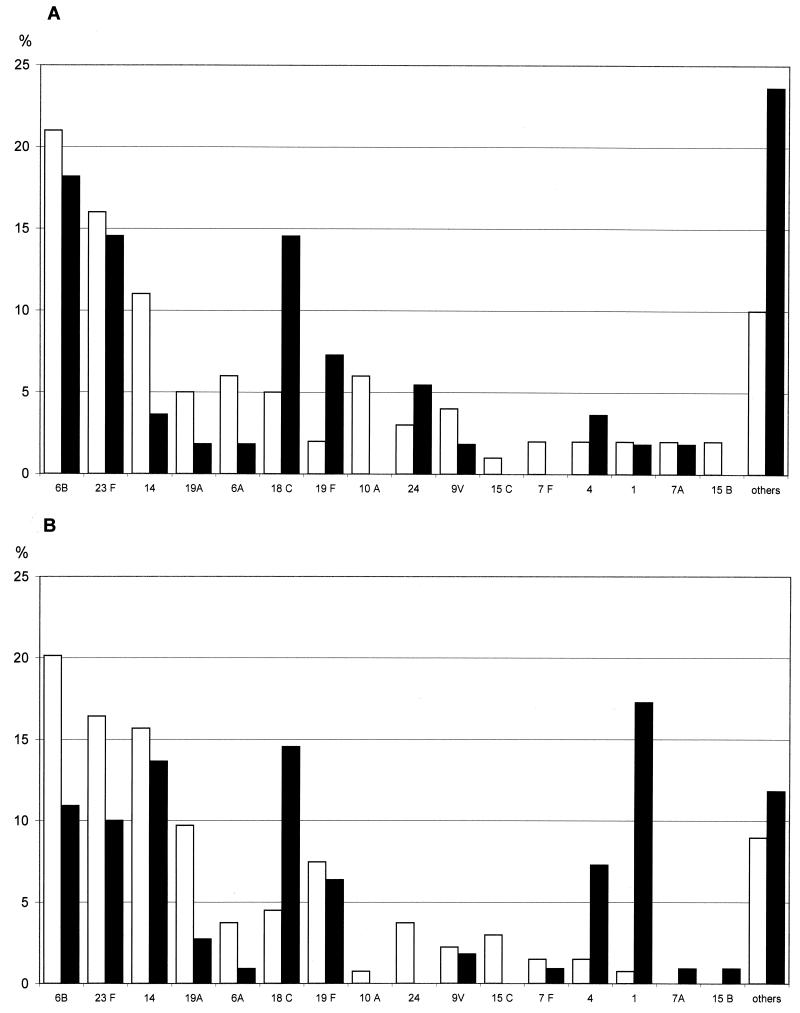
The clinical isolates belonged to 38 different serotypes. According to NCCLS guidelines (17), 102 of the 244 blood isolates (42%) were penicillin nonsusceptible (56 resistant and 46 intermediate), and 65 of the 154 CSF isolates (42%) were penicillin nonsusceptible (32 resistant and 33 intermediate). The penicillin-resistant isolates (penicillin MICs of 2 to 4 mg/liter) belonged to four serotypes (6B, 14, 19A, and 23F). The penicillin-intermediate isolates (penicillin MICs of 0.12 to 1 mg/liter) belonged to 12 serotypes (6A, 6B, 9V, 12A, 14, 15A, 15B, 15C, 19A, 19F, 23F, and 24). Isolates recovered from children under 2 years of age and those recovered from children over 2 years had different serotype distributions (Fig. 1) and penicillin resistance pattern. Penicillin-nonsusceptible isolates were significantly more frequent in children under 2 years than in children over 2 years (48.5 versus 31.5% [P < 0.001]; 51 versus 31% for blood culture isolates [P < 0.01] and 47 versus 33% for CSF isolates [not significant]). Likewise, serotypes associated with carriage (6A, 6B, 19A, 19F, and 23F) were globally more frequent in children under 2 years than in older children (P < 0.001), while serotypes 1, 4, and 18C were significantly more frequent in children over 2 years (P < 0.001, P = 0.02, and P = 0.001, respectively). Among children over 2 years, serotypes 1 and 14 were recovered significantly more frequently from those with isolated bacteremia than from those with meningitis (P < 0.01 and P = 0.05, respectively). For children under 2 years, who are the target population for the seven-valent pneumococcal conjugate vaccine, only 66% of the strains studied here were covered (4, 6B, 9V, 14, 18C, 19F, and 23F) (68% of strains isolated from blood and 61% of those isolated from CSF).
FIG. 1.
Distribution (percentage) of serotypes of pneumococcal isolates recovered by CSF culture (A) and blood culture (B) from French children according to age group. □, <2 years; ▪, >2 years.
rrn RFLP data.
Digestion with HindIII yielded four to nine ribosomal DNA fragments per isolate. A total of 30 distinct fragments were obtained. They generated 50 different patterns for the 109 blood culture isolates and 42 patterns for the 86 CSF isolates studied. EcoRI yielded 6 to 10 ribosomal DNA fragments per isolate and a total of 27 distinct fragments. This yielded 51 different patterns for the 109 blood culture isolates and 39 patterns for the 86 CSF isolates studied. Combination of the results obtained with the two endonucleases generated 64 ribotypes for the blood culture isolates and 53 ribotypes for the CSF isolates (14 ribotypes were common to the blood and CSF isolates).
Statistical analysis.
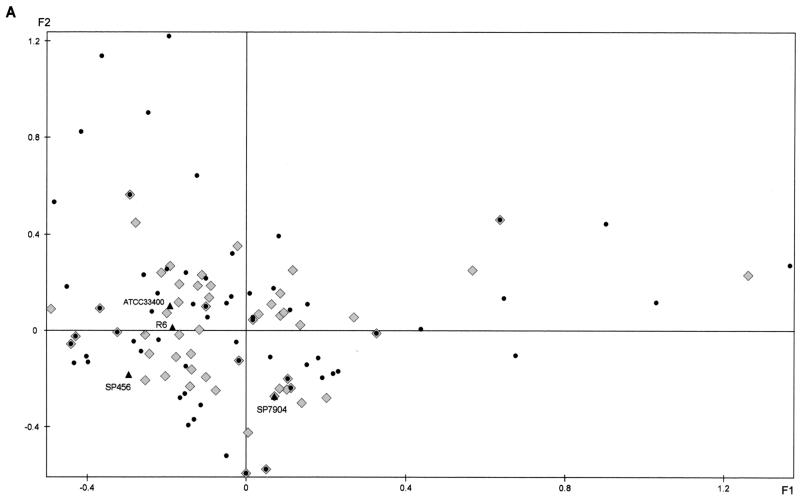
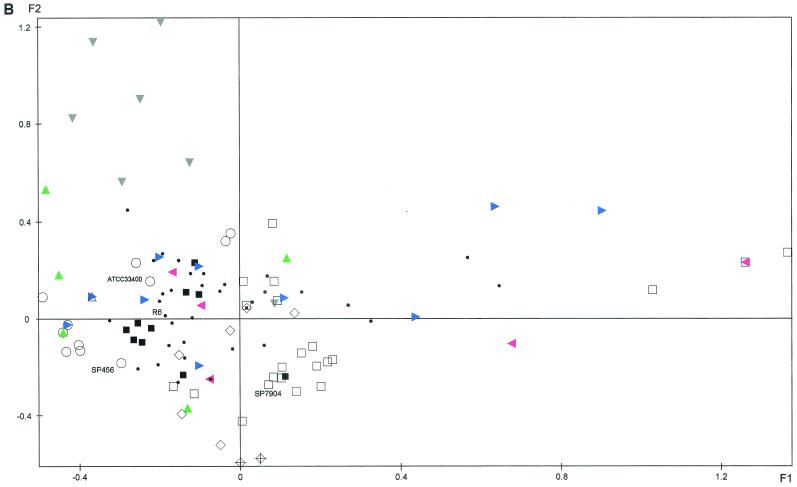
FAC was applied to the ribotyping data resulting from the combination of the two endonuclease patterns obtained with the 109 clinical isolates recovered from blood, the 86 isolates recovered from CSF, and the 4 reference strains, yielding plane F1/F2, which accounted for 29.8% of the total variance (Fig. 2). The two groups of isolates (bacteremia and meningitis) were widely distributed throughout the plane, and their genetic diversities were comparable (Fig. 2A). Isolates belonging to some serotypes seemed to be genetically homogeneous: for example, with serotype 1, seven different patterns were obtained for 14 isolates, six of which were closely projected on the plane; with serotype 18C, 12 different patterns were obtained for 17 strains, of which 11 were closely projected on the plane. Others were very heterogeneous: with serotype 19F, 9 different patterns were obtained for 11 isolates, and with serogroup 6B, 21 different patterns were obtained for 42 isolates; both these sets of isolates were broadly dispersed on the plane (Fig. 2B). Identical ribotypes were found for penicillin-resistant isolates of different serotypes (serotype 9V and 14 isolates, serotype 6A and 6B isolates, serotype 19A and 19F isolates, and serotype 19A and 23F isolates) corresponding to the major resistant clones previously observed in France (7).
FIG. 2.
Factorial analysis of correspondence of S. pneumoniae bacteremia isolates (n = 109), CSF isolates (n = 86), and reference strains based on RFLP of rRNA genes. (A) Projection of the bacteremia isolates (•), the CSF isolates (♦), and the reference strains (▴). (B) Projection of serotype 1 (▾; grey), 6A (◂; pink), 6B (□), 9V (+), 14 (⋄), 18C (▪), 19A (▴; green), 19F (▸; blue), 23F (○) and other (•) isolates. For clarity, when several isolates are projected on the same point of the plane, only one is indicated.
DISCUSSION
The incidence of invasive pneumococcal infection in the French population has been relatively stable over the past decade, with estimated incidences of 8.5 to 10.5 per 100,000 inhabitants (i.e., 6,000 cases per year since 1991, including 600 cases of meningitis) and of 45 per 100,000 children under 1 year of age (5). The rate of invasive pneumococcal infection in children is three to eight times higher in the United States than in western Europe, although this may be due at least in part to differences in blood culture practice guidelines (10). Although all 90 recognized serotypes of S. pneumoniae appear to be pathogenic, their involvement in invasive infections varies among countries and among age groups. Classically, some serotypes are more frequent in children with both carriage and infection, while others are rarely found in carriers but are frequent in patients with invasive disease. Few studies have focused on the epidemiology of pneumococcal infection in children under 2 years of age. In France, no data are available for this age group (9).
In our study, isolates belonging to serogroups associated with carriage were more frequent in invasive disease in children under 2 years than in older children, probably because the younger children had not yet developed antibodies against these common serotypes (19). As penicillin resistance is found mainly in serotypes associated with carriage, the prevalence of resistant strains was significantly higher in the younger children.
In children less than 2 years old, who form the target population for the seven-valent vaccine, only 66% of the invasive isolates studied here (68% of blood isolates and 61% of CSF isolates) would have been covered. In contrast, in the United States, 82% of invasive isolates recovered from children less than 2 years old correspond to the vaccinal serotypes (18). This difference could be explained by better diagnosis in the United States of occult pneumococcal bacteremia, in which the prevalence of the vaccinal serotypes is particularly high (2). In our study, the low rate of coverage was principally due to the high prevalence of serotype 19A (18 of the 30 serogroup 19 isolates) and serotype 6A (11 of the 59 serogroup 6 isolates). If the vaccine provides cross-protection against serotypes 6A and 19A, a further 12.4% of invasive infections (bacteremia, 13.4%; meningitis, 11%) would be covered, bringing the rate to 77.4% in France. However, cross-protection within a given serogroup is not fully ensured (8, 16, 22). The addition of serotypes 1 and 5 in the 9-valent vaccine or serotypes 1, 3, 5, and 7F in the 11-valent vaccine would not significantly increase the potential coverage rate in France (to 67 and 70%, respectively).
Most serotypes were isolated with similar frequencies from blood and CSF. The only differences in the serotype distribution between the two samples concerned patients over 2 years old, in whom serotypes 1 and 14 were significantly more frequent in blood than in CSF. This has previously been reported by Kaltof et al. for Danish children (11) and by Hausdorff et al. in a meta-analysis of pediatric studies (10) and could be related to these strains' inability to sustain high numbers in blood and/or to cross the blood-brain barrier (12).
Ribotyping had good discriminatory power, as the 109 blood isolates and the 86 CSF isolates tested yielded 64 and 53 different ribotypes, respectively. FAC showed similar genetic diversity in the two groups of strains (blood and CSF isolates), and identical ribotypes were found for some blood and CSF isolates. We found no difference in the genetic background of the two groups of strains. As previously reported (7), the genetic diversity of isolates within a given serotype was variable. Isolates belonging to invasive serotypes seem to be genetically more homogeneous than isolates belonging to serotypes associated with carriage. This could be related to the fact that carriage isolates are more amenable to intraspecies genetic exchanges than are invasive isolates. Isolates belonging to the major resistant clones are recovered from both blood and CSF. Some of them, such the Spanish-French 9V/14 clone and the 23F/19 clone, have disseminated worldwide (15).
Vaccination may change the epidemiology of pneumococcal disease in children in the coming decade. A clear picture of the epidemiologic situation before mass vaccination with the conjugate vaccine, which has an impact on pneumococcal carriage, is crucial to further evaluate the impact of this vaccine. The detection of closely related clones of different serotypes in our study suggests that serotype switching may occur in vivo. Indeed, pneumococcal capsular type switching under selective pressure exerted by capsule-based vaccines, together with replacement of vaccine serotypes by nonvaccine serotypes in the nasopharynx, has been described (20). Further studies of S. pneumoniae capsular types and genetic diversity among invasive and nasopharyngeal isolates recovered from vaccinated children are required.
REFERENCES
- 1.Adams, W. G. 1993. Decline of childhood H. influenzae type b disease in the Hib vaccine era. JAMA 269:221-226. [PubMed] [Google Scholar]
- 2.Alpern, E. R., E. A. Alessandri, K. L. McGowan, L. M. Bell, and K. N. Shaw. 2001. Serotype prevalence of occult pneumococcal bacteremia. Pediatrics 108:E23.. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Committee on Infectious Diseases. 2000. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine and antibiotic prophylaxis. Pediatrics 106:362-366. [DOI] [PubMed] [Google Scholar]
- 4.Bingen, E., E. Denamur, N. Lambert-Zechovsky, et al. 1992. Mother-to-infant vertical transmission and cross-colonization of Streptococcus pyogenes confirmed by DNA restriction fragment length polymorphism analysis. J. Infect. Dis. 165:147-150. [DOI] [PubMed] [Google Scholar]
- 5.de Benoist, A. C., V. Goulet, and E. Laurent. 1999. Infections invasives à Haemophilus influenzae, Listeria monocytogenes, méningocoque, pneumocoque, streptocoques A et B en France en 1997, p. 156-160. In Annual Epidemiological Report. Infectious Diseases Epidemiology in France in 1997. Réseau National de Santé Publique, Saint Maurice, France.
- 6.Doit, C., S. Bonacorsi, A. Frémaux, et al. 1994. In vitro killing activity of antibiotics at clinically achievable cerebrospinal fluid concentrations against penicillin-resistant Streptococcus pneumoniae isolated from children with meningitis. Antimicrob. Agents Chemother. 38:2655-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doit, C., B. Picard, C. Loukil, P. Geslin, and E. Bingen. 2000. Epidemiologic survey of penicillin-susceptible and -resistant Streptococcus pneumoniae recovered from patients with meningitis in France. J. Infect. Dis. 181:1971-1978. [DOI] [PubMed] [Google Scholar]
- 8.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käythi, P. Karma, R. Kohberger, G. Siber, and H. Mäkelä. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, and G. R. Siber. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff, W. P., G. Siber, and P. R. Paradiso. 2001. Geographical difference in invasive pneumococcal disease rates and serotype frequency in young children. Lancet 357:950-952. [DOI] [PubMed] [Google Scholar]
- 11.Kaltoft, M. S., N. Zeuthen, and H. B. Konradsen. 2000. Epidemiology of invasive pneumococcal infections in children aged 0-6 years in Denmark: a 19-year nationwide surveillance study. Acta Paediatr. 89(Suppl.):3-10. [DOI] [PubMed] [Google Scholar]
- 12.Kim, K. S. 2001. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 69:5217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klugman, K. P., and I. R. Friedland. 1995. Antibiotic-resistant pneumococci in pediatric disease. Microb. Drug Resist. 1:5-8. [DOI] [PubMed] [Google Scholar]
- 14.Lebart, L., A. Morineau, and K. M. Warwick. 1984. Multivariate descriptive analysis: correspondence analysis and related techniques for large matrices. Wiley-Interscience, New York, N.Y.
- 15.McGee, L., J. McDougal, J. Zhou, et al. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1994. Performance standards for antimicrobial susceptibility testing. Fifth informational supplement M100-S5. NCCLS publication M100-S6. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 18.Robinson, K. A., W. Baughman, G. Rothrock, et al. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998. Opportunities for prevention in the conjugate vaccine era. JAMA 285:1729-1735. [DOI] [PubMed] [Google Scholar]
- 19.Soininen, A., H. Pursiainen, T. Kilpi, and H. Käyhty. 2001. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 184:569-576. [DOI] [PubMed] [Google Scholar]
- 20.Spratt, B., and B. M. Greenwood. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter. Lancet 356:1210-1211. [DOI] [PubMed] [Google Scholar]
- 21.Tenehaus, M., and F. W. Young. 1985. An analysis and synthesis of multiple correspondence analysis, optimal scaling, dual scaling, homogeneity analysis and other methods for quantifying categorical multivariate data. Psychometrika 50:91-119. [Google Scholar]
- 22.Väkeväinen, M., C. Eklund, J. Eskola, and H. Käyhty. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789-793. [DOI] [PubMed] [Google Scholar]