Abstract
Fluconazole susceptibility among 800 clinical Candida isolates (60% C. albicans) and two control strains (C. krusei ATCC 6258 and C. parapsilosis ATCC 22019) was tested with the NCCLS M27-A method (gold standard) and six commercial products (Candifast, disk, Etest, Fungitest, Integral System Yeasts, and Sensititre YeastOne). Results were classified as susceptible, susceptible-dose dependent, or resistant using M27-A breakpoints or, for Fungitest, Integral System Yeasts, and Candifast, as susceptible, intermediate, or resistant, according to the manufacturers' instructions. Concordance with NCCLS M27-A results was analyzed with the χ2 test. Intra- and interlaboratory reproducibility was also evaluated. NCCLS M27-A (90.1%), Etest (93.1%), Sensititre YeastOne (93.1%), disk (96.7%), Fungitest (92.6%), Integral System Yeasts (40.6%), and Candifast (6.0%) classified the indicated percentages of C. albicans isolates as susceptible. Among non-C. albicans strains, the percentages of susceptible isolates were as follows: NCCLS M27-A, 74.0%; Etest, 83.8%; Sensititre YeastOne, 64.1%; disk, 60.6%; Fungitest, 76.6%; Integral System Yeasts, 28.3%; and Candifast, 27.4%. All methods except Candifast and Integral System Yeasts showed good agreement with NCCLS M27-A results for both C albicans and non-C. albicans isolates. Intralaboratory reproducibility was excellent for NCCLS M27-A, Etest, Sensititre YeastOne, disk, and Fungitest (88 to 91%). Similar results emerged from the interlaboratory reproducibility evaluation. Our findings indicate that some commercial methods can be useful for fluconazole susceptibility testing of clinical Candida isolates. Those characterized by a lack of medium standardization and/or objective interpretative criteria should be avoided. Particular caution is necessary when testing is being done for clinical and epidemiological purposes.
Fluconazole is frequently used for prophylaxis and treatment of fungal infections in various clinical situations, including AIDS and bone marrow and organ transplant patients (13-16). As recently stressed by Rex et al. (14), demonstration of correlation between in vitro azole (especially fluconazole) susceptibility test results for Candida spp. and therapeutic outcomes of certain forms of candidiasis requires the use of reliable and reproducible in vitro methods.
In 1997, the National Committee for Clinical Laboratory Standards (NCCLS) (7) defined a standard reference broth microdilution method for testing the susceptibility of yeasts to antifungal drugs. This method is both economical and reliable. It provides validated breakpoints for interpretive classification of in vitro fluconazole, flucytosine, and itraconazole susceptibility data. Despite the advantages, however, many clinical laboratories prefer to use commercially available products (1, 4, 5, 9, 10, 14, 17), which claim to be easier and more rapid alternatives to the method recommended by the NCCLS. However, some of these products, at least in Italy, can be marketed without prior demonstration of their reliability.
In 1999, we conducted an early pilot study to compare the results of five of these commercial methods with the NCCLS broth microdilution method (12). Analysis of fluconazole susceptibility test results for 100 consecutively isolated clinical yeasts and two quality control strains revealed significant discrepancies between some of the commercial methods tested and the NCCLS M27-A reference method.
The present study was undertaken (i) to verify the results of the pilot study, using a statistically significant number of yeast isolates; (ii) to extend testing to all parts of the national territory (southern, central, and northern Italy); (iii) to identify commercial methods that can be considered reliable and reproducible alternatives to the reference method; (iv) to establish an experienced national group for the in vitro study of antifungal compounds (Italian Group for In Vitro Study of Antifungals); and (v) to create a national-regional network of reference laboratories for the surveillance of in vitro susceptibility of yeasts to antifungal agents.
(Portions of this work were presented in Chicago at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2001.)
MATERIALS AND METHODS
Isolates.
Testing was performed on a total of 800 Candida isolates (480 C. albicans, 133 C. glabrata, 67 C. tropicalis, 54 C. parapsilosis, 26 C. krusei, and 40 others, including C. dublinensis, C. famata, C. guilliermondii, C. inconspicua, C. kefyr, C. lusitaniae, and C. pelliculosa) which had been consecutively isolated from blood and genital and respiratory tract specimens between November 2000 and January 2001. The isolates were identified by standard procedures (18), i.e., morphology on cornmeal agar plates, germ tube production in serum, and biochemical patterns based on findings with the Vitek system, API 20CAUX, or ATB 32C panels (Bio-Merieux, Rome, Italy). We also tested the quality control strains listed in the NCCLS M27-A document, C. krusei ATCC 6258 and C. parapsilosis ATCC 22019. Prior to testing, each isolate was subcultured at least twice on Sabouraud dextrose agar to ensure viability, purity, and optimal growth characteristics. All yeasts were maintained at −70°C.
Susceptibility testing.
Each isolate, grown overnight on Sabouraud dextrose agar at 35°C, was suspended in 5 ml of sterile distilled water and vortexed thoroughly to achieve a smooth suspension. Turbidity (read at a wavelength of 530 nm) was adjusted to 0.5 McFarland standard with water. This suspension (approximately 1 × 106 to 5 × 106 CFU/ml) was used with the reference method, Etest, disk, Sensititre YeastOne, and Fungitest (after appropriate dilution according to the manufacturer's instructions and/or standardized protocol). According to the manufacturers' instructions, the inoculum suspensions used with Candifast and Integral System Yeasts were made by direct suspension of a single colony of the isolate in the medium supplied with the product and subsequent adjustment of turbidity to a number 1 McFarland standard.
Fluconazole susceptibility testing methods investigated.
The materials used for testing were prepared centrally by the study coordinator (reference method) or the product manufacturer. Each of the eight centers used products and materials that came from the same lot. For the fluconazole E-test (AB Biodisk, Solna, Sweden; lot BA2097), we used modified Casitone agar (Eurolab, Avezzano, Italy; lot 1130013), as recommended by the manufacturer, to minimize the “trailing endpoints” phenomenon that can occur with azole derivatives (10). For the fluconazole disk diffusion test, we used 25-μg fluconazole disks and Mueller-Hinton agar (Eurolab; lot 1116004) with 2% glucose (which accelerates growth of some species, e.g., C. glabrata) and 0.5 μg of methylene blue (which improves edge definition of inhibition zones) per ml (6). Fluconazole Sensititre YeastOne colorimetric antifungal panel (Trek Diagnostic Systems Ltd., East Grinstead, England; lot B0114A) plates contained dried fluconazole (concentrations ranging from 0.125 to 128 μg/ml from rows 2 to 12) with incorporated Alamar Blue, which changes from blue to pink in the presence of microbial growth.
The Fungitest panel (Sanofi Diagnostics Pasteur, Paris, France; lot 0E076R; now Bio-Rad SDP), a 16-well microplate, allows susceptibility testing of yeasts for six antifungal drugs at two different concentrations in modified RPMI 1640 in the presence of a redox indicator. The Candifast (International Microbio/Stago Group, Diagnostic International Distribution, Milan, Italy; lot 44030/ADM H17280) consists of a panel with 16 wells arranged in two rows of eight. The first row allows identification of the yeasts. The wells of the second row contain glucose (well 1) or glucose plus one of the following antifungal drugs (wells 2 to 8): amphotericin B (4 μg/ml), nystatin (200 U/ml), 5-flucytosine (35 μg/ml), econazole (16 μg/ml), ketoconazole (16 μg/ml), miconazole (16 μg/ml), and fluconazole (16 μg/ml). Integral System Yeasts (Liofilchem Diagnostics, L'Aquila, Italy; lot 928007) consists of a panel containing three rows of six wells. In the first two rows, yeast isolates are identified; the wells of the third row (numbers 13 to 18) contain the following antifungal drugs: nystatin (200 U/ml), amphotericin B (200 μg/ml), 5-flucytosine (20 μg/ml), econazole (100 μg/ml), ketoconazole (100 μg/ml), and fluconazole (100 μg/ml). All tests were performed according to the manufacturer's instructions or protocol M27-A recommendations.
Reading and interpretation of results.
All results were read after 24 h (first reading) and 48 h (second reading) of incubation, with the exception of the Fungitest panels, which were read after 48 and 72 h as recommended by the manufacturer. The lowest drug concentration that had reduced turbidity to ≥80% of that in the control well was recorded as the MIC in the reference method (3). Results for each isolate were recorded on the electronic data report form and immediately submitted to the Data Management Unit, where they were transferred to a database. A separate electronic data report form was used to record results for the two quality control strains tested in each test session.
Results obtained with methods that furnish actual MICs, either directly (the reference method, Etest, and Sensititre YeastOne) or indirectly (disk diffusion), were classified as susceptible, susceptible-dose dependent, or resistant according to the MIC breakpoints recommended for fluconazole in the M27-A protocol (7). For the remaining systems tested, the isolates were classified as susceptible, intermediate, or resistant in accordance with the individual manufacturer's instructions. For the purpose of analysis, isolates classified with the conceptually similar term intermediate by Fungitest, Candifast, and Integral System Yeasts were included in the susceptible-dose dependent category.
Statistical analysis.
In accordance with the manufacturers' instructions, the results of the first reading were used in our analyses for all commercial methods unless this reading showed no growth. In this case, the second reading was used. For the reference microdilution broth method, the second reading was considered, in compliance with the NCCLS protocol. The chi-square test was used to compare the results obtained with each of the commercial methods with those provided by the reference method. Concordance between a given test method and the reference method was defined as the percentage of isolates classified in the same category by both methods. Discrepancies were considered major if an isolate classified as susceptible by the reference method was classified as resistant by the commercial method and very major if an isolate classified as resistant by the reference method was classified as susceptible by the commercial method. Minor errors were considered when susceptible versus susceptible-dose dependent, resistant versus susceptible-dose dependent, susceptible-dose dependent versus susceptible, or susceptible-dose dependent versus resistant results emerged.
Intra- and interlaboratory reproducibility.
In the analysis of intralaboratory reproducibility, repeat testing results for a given isolate (or quality control strain) were considered in agreement with those of the original under the following conditions: for methods that provided actual MICs, the resulting MICs were identical or differed by no more than two dilutions. If, however, the discrepancy resulted in contradictory classifications of the isolate (e.g., susceptible versus susceptible-dose dependent) using the proposed breakpoints (7), the repeat test result was considered discordant with the first. For the other methods (disk, Fungitest, Candifast, and Integral System Yeasts), the isolate had to be classified within the same category originally reported. For analysis of interlaboratory reproducibility, results reported by the eight centers for a given isolate were considered concordant when the susceptibility categories were identical.
Study design.
The eight independent laboratories that took part in this study were uniformly distributed throughout the national territory (southern, central, and northern Italy). Each was instructed to collect 100 consecutively isolated Candida spp. with the following limits: no more than 60% of the isolates could be C. albicans, and no more than 20% could be collected from immunocompromised hosts.
For each isolate, fluconazole susceptibility testing was to be evaluated simultaneously with the NCCLS standard broth microdilution method (reference method) and the six commercial products described above (see fluconazole susceptibility testing methods above). The same inoculum suspension was to be used with all seven methods, and two quality control strains (C. krusei ATCC 6258 and C. parapsilosis ATCC 22019) were to be included in each testing session. Results for each isolate were to be recorded on an electronic data report form and sent to the Data Management Unit (M. M. Borelli from Dimensione Ricerca, Rome, Italy); a separate electronic data report form was used to transmit susceptibility data for the two quality control strains.
To assess intralaboratory reproducibility, each center was instructed to retest its own isolates (100 per center) with all seven methods between May and June 2001 (i.e., 2 to 3 months after the original tests). A second electronic data report form was used to transmit these results to the Data Management Unit. Interlaboratory reproducibility was evaluated as follows. At the end of the study, eight isolates (four C. albicans and four C. glabrata) were randomly selected from the original group of 800. These isolates were sent to all participating centers, where each was tested twice with all seven methods.
RESULTS
Fluconazole susceptibilities.
Results were analyzed for only 793 clinical isolates (478 C. albicans and 315 non-C. albicans) because seven isolates were not available for the repeat testing carried out in May-June 2001. Fluconazole susceptibility data for the 793 Candida spp. isolates are summarized in Table 1. The majority of isolates displayed full susceptibility to fluconazole with all methods except Candifast and Integral System Yeasts, which indicated that most of the isolates were resistant (Candifast) or had only intermediate susceptibility (Integral System Yeasts) to the drug. The percentages of isolates classified as susceptible by two other commercial methods, Etest (89.39%) and Fungitest (86.29%), were higher than those indicated by the reference method.
TABLE 1.
Percentages of isolates determined to be fluconazole susceptible, susceptible-dose dependent, and resistant among the 793 Candida spp. tested as determined by the NCCLS microdilution broth method and six commercial systemsa
| Method and species (no. of isolates) | % of isolates
|
||
|---|---|---|---|
| Susceptible | Susceptible-dose dependent | Resistant | |
| Microdilution broth | |||
| All isolates | 83.61 | 8.07 | 8.32 |
| C. albicans (478) | 90.1 | 3.2 | 6.7 |
| Non-C. albicans (315) | 74.0 | 15.7 | 10.3 |
| Sensititre YeastOne | |||
| All isolates | 81.69 | 12.94 | 5.46 |
| C. albicans (478) | 93.1 | 3.6 | 3.4 |
| Non-C. albicans (315) | 64.1 | 27.2 | 8.7 |
| Etest | |||
| All isolates | 89.39 | 3.91 | 6.69 |
| C. albicans (478) | 93.1 | 1.0 | 5.9 |
| Non-C. albicans (315) | 83.8 | 8.3 | 8.0 |
| Disk diffusion | |||
| All isolates | 82.35 | 10.34 | 7.31 |
| C. albicans (478) | 96.7 | 2.1 | 1.3 |
| Non-C. albicans (315) | 60.6 | 22.9 | 16.5 |
| Fungitest | |||
| All isolates | 86.29 | 9.01 | 4.70 |
| C. albicans (478) | 92.6 | 5.3 | 2.1 |
| Non-C. albicans (315) | 76.6 | 14.7 | 8.7 |
| Candifast | |||
| All isolates | 14.54 | 17.07 | 68.39 |
| C. albicans (478) | 6.1 | 17.4 | 76.5 |
| Non-C. albicans (315) | 27.4 | 16.6 | 56.1 |
| Integral System Yeasts | |||
| All isolates | 35.69 | 57.76 | 6.56 |
| C. albicans (478) | 40.6 | 58.2 | 1.3 |
| Non-C. albicans (315) | 28.3 | 57.1 | 14.6 |
Results of the reference method, Etest, Sensititre YeastOne, and disk diffusion were classified as susceptible, susceptible-dose dependent, or resistant according to the MIC breakpoints recommended for fluconazole in the M27-A protocol (7). For the remaining systems tested, the isolates were classified as susceptible, intermediate, or resistant in accordance with the individual manufacturer's instructions. For the purpose of analysis, the isolates classified with the conceptually similar term intermediate by Fungitest, Candifast, and Integral System Yeasts were included in the susceptible-dose dependent category.
When the results for C. albicans and non-C. albicans isolates were analyzed separately, slight differences emerged. In general, C. albicans isolates showed high susceptibility rates in all systems except Candifast and Integral System Yeasts. Greater discrepancy with the reference method was detected in the results for non-C. albicans isolates. In this case, the Sensititre YeastOne and disk methods as well as Candifast and Integral System Yeasts methods performed poorly. Once again, the highest rates of susceptibility emerged with the Etest and Fungitest methods (Table 1). The results reported for the two quality control strains, which were included in each testing session, did not reveal any significant intra- or interlaboratory variability with any of the seven methods tested, and all were within the accepted limits (C. krusei ATCC 6258 MIC range, 16 to 128 μg/ml; C. parapsilosis ATCC 22019 MIC range, 1 to 4 μg/ml) (2).
Agreement and discrepancy between reference microdilution broth method and each of the commercial systems tested.
The overall rates of agreement between the reference method and the commercial systems investigated for the 793 Candida spp. isolates were 82% for Etest, 81.4% for Sensititre YeastOne, 77.7% for Fungitest, 77.6% for disk diffusion, 37.6% for Integral System Yeasts, and 22.1% for Candifast. Table 2 summarizes the percentages of agreement for the five most representative species studied. In general, Etest, Sensititre YeastOne, Fungitest, and disk results showed high percentages of agreement with the reference method results for C. albicans and C. parapsilosis isolates, while for C. glabrata, C. krusei, and C. tropicalis isolates, relatively poor concordance with the reference method was observed for all six methods (with the exception of Etest and Sensititre YeastOne for C. tropicalis). In contrast, Candifast and Integral System Yeasts results were characterized by very low rates of agreement with the reference method except in the case of C. parapsilosis isolates.
TABLE 2.
Agreement with the reference microdilution broth method for six commercial systems for fluconazole susceptibility testing of Candida spp., with details for the five most representative species tested
| Method | % Agreementa
|
|||||
|---|---|---|---|---|---|---|
| All isolates | C. albicans (n = 478) | C. glabrata (n = 133) | C. tropicalis (n = 67) | C. parapsilosis (n = 54) | C. krusei (n = 26) | |
| Sensititre YeastOne | 81.4 | 88.4 | 50.8 | 83.1 | 88.9 | 66.7 |
| Etest | 82 | 86 | 68.4 | 81.5 | 92.6 | 58.3 |
| Disk diffusion | 77.6 | 88.3 | 50.4 | 66.2 | 88.9 | 62.5 |
| Fungitest | 77.7 | 83.8 | 65.2 | 58.5 | 92.6 | 58.3 |
| Candifast | 22.1 | 12.8 | 19.5 | 21.5 | 72.5 | 47.8 |
| Integral System Yeasts | 37.6 | 39.1 | 24.8 | 12.3 | 77.8 | 45.8 |
Agreement rates reflect the percentage of isolates classified in the same category by both the commercial and reference methods.
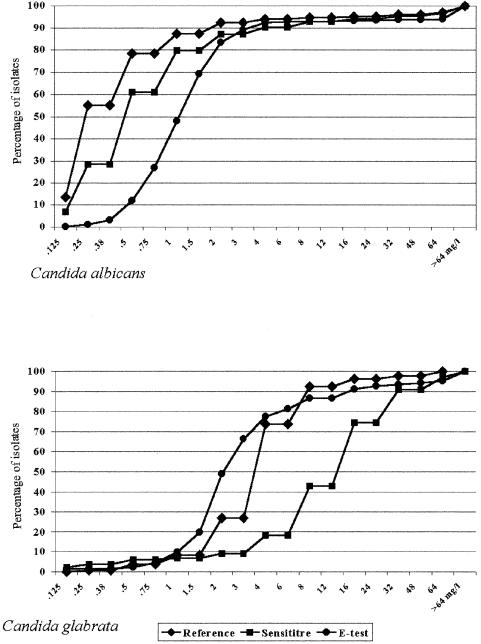
Table 3 shows the MIC ranges and the MICs for 50% and 90% of isolates (MIC50 and the MIC90, respectively) obtained for the most representative species of Candida with the three methods that provide actual MICs (reference method, Etest, and Sensititre YeastOne). Although the MICs of fluconazole obtained with these methods were quite similar on the whole (MIC90s for broth microdilution, Etest, and Sensititre YeastOne: for C. albicans, 2, 4, and 4 μg/ml, respectively; for C. glabrata, 8, 16, and 32 μg/ml, respectively; for C. tropicalis, 4, 4, and 16 μg/ml, respectively; for C. parapsilosis, 4, 4, and 2 μg/ml, respectively; and for C. krusei, 64, >256, and 64 μg/ml, respectively), slight differences were observed with the single MICs according to the species, especially C. glabrata (Fig. 1).
TABLE 3.
In vitro fluconazole susceptibility, as determined by the reference broth microdilution method, Etest, and Sensititre YeastOne, for the five most representative Candida species studied
| Species (no. of isolates) | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference method
|
Etest
|
Sensititre YeastOne
|
|||||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |
| C. albicans (478) | 0.125->64 | 0.25 | 4 | 0.125->256 | 1.5 | 4 | 0.125->128 | 0.5 | 4 |
| C. glabrata (133) | 0.25-64 | 4 | 8 | 0.125->256 | 3 | 16 | 0.125->128 | 16 | 32 |
| C. tropicalis (67) | 0.125-64 | 0.5 | 4 | 0.25->256 | 1 | 6 | 0.5->128 | 1 | 32 |
| C. parapsilosis (54) | 0.125-32 | 2 | 4 | 0.5-16 | 1.5 | 4 | 0.125->128 | 1 | 2 |
| C. krusei (26) | 1->64 | 32 | 64 | 0.75->256 | >256 | >256 | 0.25-128 | 32 | 64 |
FIG. 1.
Cumulative percentages of strains exhibiting different fluconazole MICs determined by the reference broth microdilution, Etest, and Sensititre YeastOne methods for 478 C. albicans (top) and 133 C. glabrata (bottom) isolates.
Discrepancies between each method and the reference method are detailed in Table 4. Greater discrepancies emerged among the results for non-C. albicans isolates, especially C. glabrata and C. tropicalis. In this case, all of the commercial methods except the Etest performed poorly. Major discrepancies (in which the reference method classified an isolate as susceptible and the commercial system as resistant) were most common with Candifast (42.3% of all isolates) and least frequent with Sensititre YeastOne (1.6%). Very major errors occurred at a low percentage (2.1 to 5.8%) with all methods except Candifast, especially among C. albicans isolates. The highest percentage of minor errors occurred with the Integral System Yeasts method (56.4%), followed by the Candifast (22.9%), disk (13.8%), Fungitest (13.7%), Sensititre YeastOne (13.3%), and Etest (9.4%) methods.
TABLE 4.
Discrepancies between the reference method and commercial systems in the classification of 793 Candida isolates based on NCCLS-defined breakpoints for fluconazole susceptibilitya
| Method and organisms tested (no. of isolates) | No. of isolates with discordant results
|
|||||
|---|---|---|---|---|---|---|
| Minor errors
|
Major errors | Very major errors | ||||
| S vs. SDD | SDD vs. S | SDD vs. R | R vs. SDD | |||
| Sensititre YeastOne | ||||||
| C. albicans (478) | 11 | 10 | 2 | 7 | 7 | 22 |
| Non-C. albicans (315) | 49 | 17 | 5 | 9 | 6 | 7 |
| Etest | ||||||
| C. albicans (478) | 3 | 13 | 1 | 1 | 22 | 27 |
| Non-C. albicans (315) | 11 | 33 | 7 | 6 | 5 | 14 |
| Disk diffusion | ||||||
| C. albicans (478) | 9 | 12 | 2 | 0 | 2 | 31 |
| Non-C. albicans (315) | 52 | 18 | 14 | 3 | 20 | 12 |
| Fungitest | ||||||
| C. albicans (478) | 23 | 11 | 2 | 0 | 8 | 33 |
| Non-C. albicans (315) | 23 | 32 | 6 | 12 | 13 | 13 |
| Candifast | ||||||
| C. albicans (478) | 79 | 0 | 11 | 4 | 325 | 0 |
| Non-C. albicans (315) | 43 | 7 | 36 | 4 | 11 | 0 |
| Integral System Yeasts | ||||||
| C. albicans (478) | 248 | 6 | 1 | 22 | 4 | 10 |
| Non-C. albicans (315) | 134 | 9 | 11 | 17 | 26 | 7 |
Discrepancies were considered major errors if an isolate classified as susceptible (S) by the reference method was classified as resistant (R) by the commercial method, very major errors if an isolate classified as resistant by the reference method was classified as susceptible by the commercial method, and minor when susceptible versus susceptible-dose dependent (SDD), resistant versus susceptible-dose dependent, susceptible-dose dependent versus susceptible, or susceptible-dose dependent versus resistant results emerged.
Intra- and interlaboratory reproducibility.
The overall intralaboratory concordance rates were 90% for the reference method, 90% for the Etest, 88% for the Sensititre YeastOne, 91% for the disk diffusion, 88% for the Fungitest, 77% for the Candifast, and 71% for the Integral System Yeast method. Interlaboratory reproducibility was excellent for the reference method, Etest, Sensititre YeastOne, and disk (97 to 100% for C. albicans isolates, 77 to 90% for C. glabrata isolates). High variability emerged for the remaining three methods, Fungitest, Integral System Yeasts, and Candifast, which was once again the method that displayed the poorest results.
DISCUSSION
The primary aim of the present study was to verify the reliability and reproducibility of several commercial products that are currently available on the Italian market for in vitro antifungal susceptibility testing. The gold standard used for comparison was the standardized M27-A microdilution broth method, which is recommended by the NCCLS (7). Fluconazole was chosen because, due to its high bioavailability and its suitability for both oral and intravenous administration, it is the antifungal agent most widely used in clinical practice for treatment of deep and mucocutaneous forms of candidiasis as well as for empirical therapy of suspected disseminated candidiasis in nonneutropenic patients (13).
Our findings confirm fluconazole's overall efficacy against Candida isolates, although species-related variability exists. Although most of the C. glabrata isolates were classified as susceptible with the NCCLS M27-A method, the MICs for these isolates were at the upper limits of this category (4 to 8 μg/ml). This finding indicates that close monitoring of clinical C. glabrata isolates with in vitro antifungal susceptibility testing is particularly important, especially when the patient is already receiving antifungal drugs (11, 16). The innate resistance to fluconazole of the C. krusei isolates was confirmed. The interpretative breakpoints provided by the M27-A method should also be useful in identifying resistant C. albicans strains, which are particularly common in human immunodeficiency virus-positive patients with mucosal candidiasis, and to study the molecular mechanisms involved in this resistance (8).
For C. albicans and C. parapsilosis, all but two of the commercial methods (Candifast and Integral System Yeasts) showed good rates of concordance (range, 83.8% to 92.6%) with the reference method. Comparison of the single MICs of the three methods that provide such data showed a marked difference for C. albicans, especially at low concentrations (Fig. 1), as demonstrated by the different MIC50s (Table 3). For other clinically important species (C. glabrata and C. krusei), all six test methods performed poorly (concordance range, 24.8% to 68.4%), and for C. tropicalis isolates good correlation was demonstrated only for the Sensititre YeastOne and Etest methods (83.1% and 81.5%, respectively). This behavior was dramatically evident for C. glabrata isolates when we considered the single MICs instead of the breakpoint categories, especially for Sensititre YeastOne (Fig. 1). Also, the disk diffusion method, recently endorsed for fluconazole by NCCLS, showed poor correlation for non-C. albicans isolates. The results would not change if we used the NCCLS zone interpretative criteria for susceptible (diameter, ≥19 mm), resistant (diameter, ≤14 mm), or susceptible-dose dependent (diameter between 15 and 18 mm). These results are probably responsible for the relatively poor performance of the commercial methods (based on concordance with the reference method) in testing non-C. albicans isolates.
Our data indicate that Etest, Sensititre YeastOne, disk diffusion, and Fungitest can be considered useful for in vitro evaluation of fluconazole sensitivity among Candida spp. isolates in clinical laboratories, although the Fungitest method provides a limited number of drug concentrations (4, 14). Similar conclusions have emerged from other recent studies (1, 4-6, 9, 10, 12, 14, 17) involving the direct comparison of these commercial products with the M27-A reference method. The other two systems tested, Candifast and Integral System Yeasts, are characterized by subjective criteria for reading results and a lack of standardization in preparation of the inoculum and/or the growth medium. For these reasons alone, we and others (12) believe that they should be avoided for both clinical and epidemiological studies. Both methods performed satisfactorily with the two quality control strains, but as Barry et al. have pointed out, this does not guarantee accuracy with clinical isolates (2).
Based on our intra- and interlaboratory agreement data, reproducibility was excellent for the reference method, disk, Etest, and Sensititre YeastOne methods, good for Fungitest, and, once more, very poor for the two remaining methods. The use of lot-identical products and supplies (as well as of frozen microdilution plates for M27-A prepared in a single center) undoubtedly contributed to the positive results by reducing the number of variable factors, but this advantage was not sufficient to compensate for the major shortcoming of the Candifast and Integral System Yeasts methods, which involve a high level of subjectivity in interpretation of the colorimetric results.
In conclusion, our study fulfilled its objectives and has established a foundation for the creation of a network of experienced laboratories (regional and/or national) for surveillance of the in vitro susceptibility of yeasts to antifungal agents.
Acknowledgments
This study was made possible by the financial support of Pfizer Italia, SpA.
We thank Marian Kent for revision of the English.
REFERENCES
- 1.Arthington-Skaggs, B. A., M. Motley, D. W. Warnock, and C. J. Morrison. 2000. Comparative evaluation of PASCO and National Committee for Clinical Laboratory Standards M27-A broth microdilution methods for antifungal drug susceptibility testing of yeasts. J. Clin. Microbiol. 38:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormican, M. G., and M. A. Pfaller. 1996. Standardization of antifungal susceptibility testing. J. Antimicrob. Chemother. 38:561-578. [DOI] [PubMed] [Google Scholar]
- 4.Davey, K. G., A. D. Holmes, E. M. Johnson, A. Szekely, and D. W. Warnock. 1998. Comparative evaluation of FUNGITEST and broth microdilution methods for antifungal drug susceptibility testing of Candida species and Cryptococcus neoformans. J. Clin. Microbiol. 36:926-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey, K. G., A. Szekely, E. M. Johnson, and D. W. Warnock. 1998. Comparison of a new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J. Antimicrob. Chemother. 42:439-444. [DOI] [PubMed] [Google Scholar]
- 6.Mers, J., M. Petron, J. Bille, D. Ellis, D. Gibbs, and the Global Antifungal Surveillance Group. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Diagn. Microbiol. Infect. Dis. 36:213-223. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martinez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., S. A. Messer, S. J. Hollis, A. Espinel-Ingroff, M. A. Ghannoum, H. Plavan, S. B. Killian, and C. C. Knapp. 1998. Multisite reproducibility of MIC results by the Sensititre YeastOne colorimetric antifungal susceptibility panel. Diagn. Microbiol. Infect. Dis. 31:543-547. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., S. A. Messer, A. Karlsson, and A. Bolmstrom. 1998. Evaluation of the E-test method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the Sentry Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posteraro, B., L. Romano, M. Sanguinetti, L. Masucci, G. Morace, and G. Fadda. 2000. Commercial systems for fluconazole susceptibility testing of yeasts: comparison with the broth microdilution method. Diagn. Microbiol. Infect. Dis. 38:29-36. [DOI] [PubMed] [Google Scholar]
- 13.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-672. [DOI] [PubMed] [Google Scholar]
- 14.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strahilevitz, J., A. M. Sugar, and D. Engelhard. 2000. Fluconazole in transplant recipients: options and limitations. Transpl. Infect. Dis. 2:62-71. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez, J. A., G. Peng, J. D. Sobel, L. Steele-Moore, P. Schuman, W. Holloway, and J. D. Neaton for the Terry Beirn Community Programs for Clinical Research on AIDS. 2001. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis. Clin. Infect. Dis. 33:1067-1075. [DOI] [PubMed] [Google Scholar]
- 17.Warnock, D. W., E. M. Johnson, and T. R. Rogers. 1998. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. BSAC Working Party on Antifungal Chemotherapy. J. Antimicrob. Chemother. 42:321-331. [DOI] [PubMed] [Google Scholar]
- 18.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray et al. (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.