Abstract
Exposure to valproic acid (VPA), a common anti-seizure medication, in utero is a risk factor for autism spectrum disorder (ASD). People with ASD often display changes in the cerebellum, including volume changes, altered circuitry, and changes in Purkinje cell populations. ASD is also characterized by changes in the medial prefrontal cortex (mPFC), where excitatory/inhibitory balance is often altered. This study exposed rats to a high dose of VPA during gestation and assessed cognition and anxiety-like behaviors during young adulthood using a set-shifting task and the elevated plus maze. Inhibitory parvalbumin-expressing (PV +) neuron counts were assessed in the mPFC and cerebellar lobules VI and VII (Purkinje cell layers), which are known to modulate cognition. VPA males had increased PV + counts in crus I and II of lobule VII. VPA males also had decreased parvalbumin-expressing neuron counts in the mPFC. It was also found that VPA-exposed rats, regardless of sex, had increased parvalbumin-expressing Purkinje cell counts in lobule VI. In males, this was associated with impaired intra-dimensional shifting on a set-shifting task. Purkinje cell over proliferation may be contributing to the previously observed increase in volume of Lobule VI. These findings suggest that altered inhibitory signaling in cerebellar-frontal circuits may contribute to the cognitive deficits that occur within ASD.
Keywords: Valproic acid, Medial prefrontal cortex, Cerebellum, Parvalbumin, Purkinje cell, Set-shifting, Autism spectrum disorder
Introduction
The cerebellum is a structure that is impacted across a wide array of neurodevelopmental and neurological disorders including schizophrenia (Gill and Sillitoe 2019), autism spectrum disorder (ASD) (van der Heijden et al. 2021), and Down syndrome (Shapiro 2001). In ASD, the cerebellum has been found to be underdeveloped (Courchesne et al. 1994a; Cauda et al. 2011). In humans with ASD, symptomatology has been linked to changes in cerebellar structure and function (D’Mello et al. 2015). The cerebellum also plays a role in cognitive flexibility (Schmahmann 2019), and those with ASD have executive function deficits (Leung et al. 2016). It has been suggested that altered cellular morphology in the cerebellum may play a role in some cognitive impairments (D’Mello and Stoodley 2015). Individuals with ASD have altered major white matter structures within the cerebellum (Sivaswamy et al. 2010; Mosconi et al. 2015; Wolff et al. 2017), Purkinje cell loss (Bailey et al. 1998; Kern 2003; Whitney et al. 2008, 2009), and reduced cerebellar cortex volume (Courchesne et al. 1988, 1994a; Townsend et al. 1999; Allen et al. 2004; D’Mello et al. 2015; Liu et al. 2017; Crucitti et al. 2020). Furthermore, those with ASD have reduced activation of regions of the cerebellum important for attentional tasks (Allen et al. 2004) and increased crus I volumes are associated with increased sensorimotor response variability (McKinney et al. 2022). Understanding more about these changes in the cerebellum, their links to cognition, and their variation by biological sex may be beneficial to identify interventions.
The VPA model
Gestational exposure to the anticonvulsant medication valproic acid (VPA) is associated with significantly increased risk of ASD (Christensen et al. 2013; Baio et al. 2018). To mimic this etiologic factor, the valproic acid model (VPA) has been used to induce ASD-like phenotypes in rodents by injecting pregnant dams with high doses of VPA during gestation (Ingram et al. 2000; Kim et al. 2011). Mechanisms of VPA exposure that likely induce ASD-like phenotypes in offspring include oxidative stress, histone deacetylase (HDAC) inhibition, changes in excitatory and inhibitory balance, and hyperserotonemia (Mabunga et al. 2015; Tanaka et al. 2018; Tartaglione et al. 2019; Chaliha et al. 2020). VPA-exposed offspring display impaired sociability and increased repetitive behaviors (Schneider and Przewlocki 2005; Schneider et al. 2007; Mehta et al. 2011; Banerjee et al. 2014).
The cerebellum and cognition in the VPA model
Previous research utilizing the VPA model has identified changes in Purkinje cells in the cerebellum such as reduced spine density (Ingram et al. 2000), smaller Purkinje cell size (Main and Kulesza 2017), and decreased androgen receptor expression across development (Perez-Pouchoulen et al. 2016). There are subtypes of Purkinje cells that are still being categorized, but a subset of cerebellar Purkinje cells express parvalbumin (PV +) (Hull and Regehr 2022). PV is a small calcium binding protein that regulates intracellular calcium processing in inhibitory neurons, including some Purkinje neurons (Hull and Regehr 2022). PV mRNA expression is reduced in Purkinje cells of those with ASD (Soghomonian et al. 2017), and it has been hypothesized that dysregulation of Purkinje cell function contributes to various deficits in ASD due to its role in calcium regulation in inhibitory neurons (Soghomonian et al. 2017). Loss of Purkinje neurons in VPA animals has also been associated with motor deficits (Martin et al. 2010). Lobules VI and VII, contribute to cognition, meaning that their dysregulation may also partially underlie cognitive deficits often observed in ASD (Stoodley 2016; Van Overwalle et al. 2020; Kawabata et al. 2022; Hodgdon et al. 2024).
This study quantified PV + expression to mark a subpopulation of Purkinje cells in lobules VI and VII of the right cerebellum. Lobules VI and VII were selected because they are connected to frontal cortex regions (Kelly and Strick 2003; Bostan et al. 2013). Thus, these cerebellar regions may play a role in modulating functions that require cognitive flexibility, which is frequently impaired in ASD (D’Angelo and Casali 2013; Shipman and Green 2019; Clark et al. 2021; Zhao et al. 2023). Indeed, lobules VI and VII contribute to ASD symptomatology (D’Mello et al. 2015), and lobule VI overgrowth has previously been associated with impaired set-shifting performance in VPA animals (Payne et al. 2021). Therefore, it was predicted that an increase in PV + neurons in the Purkinje cell layers of lobule VI would lead to impaired performance on set-shifting. This would support the hypothesis that altered cerebellar function, specifically Purkinje cell function, may underlie some cognitive symptoms of ASD.
Cognitive flexibility and set-shifting in the VPA model
Cognitive flexibility is a subcomponent of executive functioning that requires the ability to switch attention to changing reward contingencies in order to learn new ones. In those with ASD, multiple domains of executive function are disrupted, and impairments in tasks that require attention shifts are consistently observed (Leung and Zakzanis 2014). The set-shifting task (SST) is analogous to the Wisconsin Card Sorting task, which is used to assess cognitive flexibility in humans (Birrell and Brown 2000; Brown and Tait 2016). The SST relies on the frontal cortex (Bissonette et al. 2013), which is connected to and modulated by the cerebellar lobules VI and VII (Kelly and Strick 2003; Coffman et al. 2011; Suzuki et al. 2012). Posterior lobules of the cerebellum are important for regulating networks critical to cognition (Kawabata et al. 2022) and the cerebellum has been implicated in cognitive flexibility deficits in humans (Zhao et al. 2023). In the SST, animals learn to find food rewards based on odor and media cues. The individual stimuli change for each phase, but in intra-dimensional (ID) shifts, the rewarded dimension (i.e., odor or media) stays the same, whereas the rewarded dimension switches in extradimensional (ED) shifts (i.e., odor-to-media or media-to-odor). Individuals with ASD are impaired on tasks that have similar shifts of attention (Yerys et al. 2009; Yasuda et al. 2014; Westwood et al. 2016; Kiep and Spek 2017; Wang et al. 2018). Likewise, VPA animals also display impairments on the SST (Payne et al. 2021; McKinnell et al. 2021; Mali et al. 2023).
Excitatory/Inhibitory imbalance in ASD/VPA
Similar to its presence in the cerebellum, PV is also found in cortical interneurons where it regulates intracellular calcium signaling in inhibitory neurons (Arif 2009). Imbalance of excitatory and inhibitory neurons is commonly observed in many neurodevelopmental disorders (Woodward and Coutellier 2021), and individuals with ASD specifically have reduced PV + expression in the frontal cortex (Zikopoulos and Barbas 2013; Hashemi et al. 2018). Additionally, changes in excitatory and inhibitory balance are linked to changes in sensory perception and intellectual ability in ASD (Foss-Feig et al. 2017). In line with this, mice with PV knocked out exhibit ASD-like symptomatology (Wöhr et al. 2015), and studies in various animal models of ASD have found that this imbalance contributes to cognitive deficits (Lee et al. 2017). Therefore, PV + interneurons were also quantified in the medial prefrontal (mPFC) to better understand if VPA-exposed animals also exhibit signs of inhibitory imbalance, especially since the mPFC is critical for set-shifting (Birrell and Brown 2000; Ng et al. 2007; Chase et al. 2012).
Excitatory/inhibitory balance in the frontal cortex is also important for mediating anxiety-related behaviors (Kenwood et al. 2022), which are often disrupted in ASD. Therefore, rats were also tested on the elevated plus maze (EPM) to assess anxiety-like behavior. Stressors such as maternal separation, juvenile social isolation, and induced hypoxic stress decreases the number of inhibitory (PV +) cells in the mPFC (Liang et al. 2016; Gildawie et al. 2021). Individuals with ASD also have reduced PV + neuron counts in the frontal cortex (Hashemi et al. 2018). Although VPA exposure is a teratogenic stressor, it likely causes oxidative stress (Mabunga et al. 2015) and may thus lead to decreases in the number of PV + neurons in the mPFC (Liang et al. 2016). It was predicted that VPA animals would have fewer PV + cells in the mPFC. The raw SST results were reported previously (McKinnell et al. 2021), but the EPM data and examination of relationships between performance and cell counts are novel. See summary Table 1 for the data reported previously.
Table 1.
Trials to criterion by task phase
| SD | CD | ID | R1 | ED | R2 | ||
|---|---|---|---|---|---|---|---|
| Control Females | 12.52 | 9.48 | 10.29 | 16.57 | 10.67 | 10.62 | Mean |
| 1.73 | 1.02 | 0.74 | 2.21 | 1.54 | 1.38 | SE | |
| VPA Females | 9.88 | 9.19 | 12.69 | 13.56 | 10.88 | 9.56 | Mean |
| 1.56 | 1.09 | 1.36 | 2.30 | 1.32 | 0.71 | SE | |
| Control Males | 9.18 | 9.71 | 9.12 | 14.35 | 10.18 | 13.47 | Mean |
| 0.90 | 1.63 | 1.47 | 2.23 | 1.25 | 1.46 | SE | |
| VPA Males | 8.56 | 10.25 | 9.69 | 11.88 | 10.19 | 12.56 | Mean |
| 0.88 | 1.36 | 1.02 | 1.42 | 1.36 | 1.70 | SE |
VPA females were impaired on the ID phase of the task. See McKinnell et al. 2021 for other behavioral details
SD simple discrimination, CD compound discrimination, ID intra-dimensional shift, ED extra-dimensional, R reversal
Materials and methods
Subjects
Sixteen pregnant rat dams (Long-Evans) from Charles River arrived at the facility on gestational day 6. On gestational day 12, dams were injected intraperitoneally with a single dose of saline (5 dams) or VPA (11 dams) (sodium valproate (Sigma), 250 mg/ml, mixed in saline, 600 mg/kg). More VPA dams were included because prior work has demonstrated that VPA litters are often smaller than control litters (Favre et al. 2013). Dams were briefly anesthetized on isoflurane gas to administer a less stressful injection. The isoflurane administration (required by the IACUC) was very brief, lasting only a few minutes, and should not have adversely affected the pups, as longer exposure is required to see adverse effects in offspring (Andropoulos 2018). All procedures were conducted in accordance with the Kansas State University Institutional Animal Control and Use Committee (IACUC) and the NIH guide for the care and use of laboratory animals. Rats were given free access to food except for when undergoing preparation for the set-shifting task. Lights were on from 7:00 a.m. to 7:00 p.m. Rats were reared with littermates until weaning and were then pair-housed with a same-sex littermate.
Timeline of events
Rats were run on the elevated plus maze task once between the postnatal days (P) 46–51 and then on set-shifting in adulthood, with training starting around P70.
Elevated plus maze (EPM)
Rats were placed in a pretest habituation arena (60 × 60 × 35 cm) for five minutes. A 40-W lightbulb was hung 60 cm above the maze. Then, animals were placed on the maze, in the same location and facing the same direction each time, and animals were allowed to freely explore the open and closed arms of the maze for five minutes. Time spent in the open and closed arms were recorded with Limelight software (Actimetrics, IL), and the apparatus was cleaned with Peroxigard™ spray and dried between each animal to eliminate scent cues from previous animals.
Set-shifting task procedures
Apparatus and stimuli
The SST procedure follows previous studies (Payne et al. 2021; McKinnell et al. 2021), and these were originally adapted from (Ng et al. 2007; Tait et al. 2014). A Plexiglass box (50 × 37.5 × 25 cm) with a black divider was used as the testing arena. The sliding door allowed access to the flowerpots, which were held to the floor of the chamber with Velcro to prevent tipping. All media stimuli were mixed and recycled between rats to allow any odor cues to be disseminated. Crushed Honey Nut Cheerio (General Mills, MN) powder was applied to all digging media to prevent reward odor from serving as a digging cue. Media were shredded manila folders, aspen shavings, foam rubber, felt, burlap ribbon, and silk ribbon. Odors were rum, vanilla, lemon, almond, cinnamon, and anise. A pilot study demonstrated that rats could discriminate these odors.
Training
Rats were trained on a set-shifting task with six stages presented in the following order: simple discrimination (SD), compound discrimination (CD), intra-dimensional (ID) shift, reversal 1 (R1), extra-dimensional (ED) shift, and reversal 2 (R2) to test cognitive flexibility. For a detailed description of each phase, see Supplemental Table 1. Before training, rats underwent food restriction for between 7 and 10 days and reached around 90% of their body weight. Honey Nut Cheerios (General Mills, MN) and a flowerpot were introduced into the home cage the night before training began for habituation. Basic training consisted of shaping digging behavior in the testing arena. Rats were allowed to obtain a small 1/3 piece of cereal on top of the bedding of the flowerpots. Over successive trials the cereal was buried progressively deeper until the rat was consistently and successfully digging to retrieve it at about 3.5 cm deep. Day two of training consisted of an odor discrimination (tea tree oil vs lavender) with plain pine bedding, and a media discrimination (shredded paper vs pine shavings) with unscented pots. Pots themselves were not scented, but rather blotting paper was taped to the inner lip of the ceramic pot and scented with a pipettor (5 μl). The paper was re-scented before every training session but, once a pot had been scented with the blotting paper, it was only ever scented with the same scent. In all sessions, the first four trials were considered discovery trials where the rat could sample both pots regardless of initial digging choice. These trials were not scored. After the discovery trials, the unchosen pot was lifted out of the arena immediately after the rat initiated a dig, which was defined as vigorously moving the medium with the nose or paws, to prevent digging in the other flowerpot. This was done to ensure that animals could not return to the other pot once a choice was made. Rats were removed from the task if they became inactive and were given a 10-min break. If the rat continued not to dig for three trials in a row after two ten-minute breaks, it was removed from the task and its data were excluded. The training criteria for basic training (day one) was ten consecutive digs with the reward fully buried whereas, for all other training phases, six consecutive correct trials were required to move to next phase of training or next phase of the task. All stimuli were counterbalanced with a Latin square design, and half of the rats were trained odor-to-medium shifts, and the other half medium-to-odor shifts to prevent bias.
Immunohistochemistry
Rats were euthanized via overdose by intraperitoneally delivered pentobarbital (390 mg/ml) at 200 mg/kg. Once palpebral and withdrawal reflexes were no longer present, animals were transcardially perfused with 0.9% physiological saline, followed by 4% formaldehyde at pH of 7.2. Brains were extracted and placed in 4% formaldehyde for postfixing. After postfixing, brains were placed in a 30% sucrose solution for cryoprotection until they sank, and they were then frozen at − 80 °C until slicing. The frontal cortex was sliced coronally in 40-micron slices, and the cerebellum was cut off and the right side was sliced sagittally to view PV + cells in the Purkinje cell layer. Slices were washed with phosphate-buffered saline (PBS) and incubated in normal horse serum for 30 min. Primary antibody (anti-PV, Sigma P-3088, 1:40,000) was applied and incubated for 48 h at 4 °C. After washing again with PBS, secondary antibody was applied (2Ab anti-mouse, Vector Labs BA-2000, 1:200) and incubated for 2 h at room temperature. Tissue was then washed with PBS three times for 10 min each time. Finally, an ABC staining kit (Vectastain Elite ABC HRP kit) was used, followed by immersion in a diaminobenzidine (DAB) solution (25 mg DAB, in 100 ml 0.1 M Acetate Buffer, 200 mg D-glucose, 40 mg ammonium chloride, 2 g nickel sulfate) at pH 6.0 then washed with PBS buffer. Slices were then plated on slides and coverslipped.
Counting procedure
Pictures of the mPFC and cerebellar lobules VI and VII were taken at 2 × and 20 × with an Olympus brightfield microscope using blue, green, and red filters. Images from each filter were burned and stitched into one image of each region of interest. Cell counts were obtained by trained blind-to-condition researchers, similar to (Gibson et al. 2022) using ImageJ software to tag PV + cells. Only PV + cells were tagged in Image J with the cursor tool, and then total counts for each slice were exported for analysis. Right crus I and crus II (Atlas Figure numbers 172, 174, 176, and 178), and lobule VI a, b, and c (Atlas Figure numbers 162, 163, 164, and 165) were counted and averaged across the four slices (Fig. 1). For the mPFC, the average cell count for each animal was created by counting 3 separate sections aligning with Atlas Figure numbers 9, 11, and 13 (prelimbic and infralimbic areas) from Paxinos’ and Watson’s rat brain atlas (6th edition), Fig. 2.
Fig. 1.

A Control crus II from stitched together sagittal slice of cerebellum taken at 20x (left) and magnified inset with yellow arrow highlighting a PV + Purkinje cell (right). B VPA crus II from stitched together sagittal slice of cerebellum taken at 20x (left) and magnified inset with yellow arrow highlighting a PV + Purkinje cell
Fig. 2.

A Control mPFC stitched at 20x (left) and magnified inset with arrow pointing to a PV + neuron (right). B VPA mPFC stitched at 20x (left) and magnified inset with arrow pointing to a PV + neuron (right)
Data analysis
Linear mixed models were run for cell count data in JMP software with fixed effects of condition and sex. Litter was included in all models as a random effect to account for intra-litter likeness. Linear mixed models were also used to assess EPM data with a fixed effect of condition and litter as a random effect. Linear regression was used to examine cell count and behavioral data. N for mPFC cell counts: control female = 19; VPA female = 13; control male = 16; VPA male = 15. N for Cerebellum cell counts: control female = 20; VPA female = 15; control male = 18; VPA male = 16. Graphs were made in GraphPad Prism software.
Results
PV + Cell counts cerebellum and mPFC
There was a significant interaction between condition and sex (F1,59.06 = 14.99, p = 0.000) for PV + cell count in crus I (Fig. 3A). Tukey post hoc tests demonstrated that male VPA animals had more PV + cells than male controls (p = 0.001) and that VPA females had fewer PV + cells than VPA males (p = 0.004). For crus II, there was a significant interaction between condition and sex (F1,59.06 = 15.06, p = 0.000), and Tukey post hoc tests found that male VPA animals had more PV + cells than control males (p = 0.000), more than control females (p = 0.03), and more than VPA females (p = 0.001), (Fig. 3C). For the mPFC, there was a significant main effect of sex (F1,5.19 = 5.19, p = 0.02) and a significant interaction between condition and sex (F1,57.38 = 15.87, p = 0.002) (Fig. 3C). Tukey post hoc tests found that VPA males had fewer PV + cells than VPA females (p = 0.000) as well as fewer than control males (p = 0.006), Fig. 3C. For lobule VIa, there was a main effect of condition (F1,10.18 = 19.19, p = 0.001) where VPA animals had more PV + cells than control animals (Fig. 3D). There were no significant condition or sex differences for lobules VIb or VIc (Fig. 3E, F).
Fig. 3.
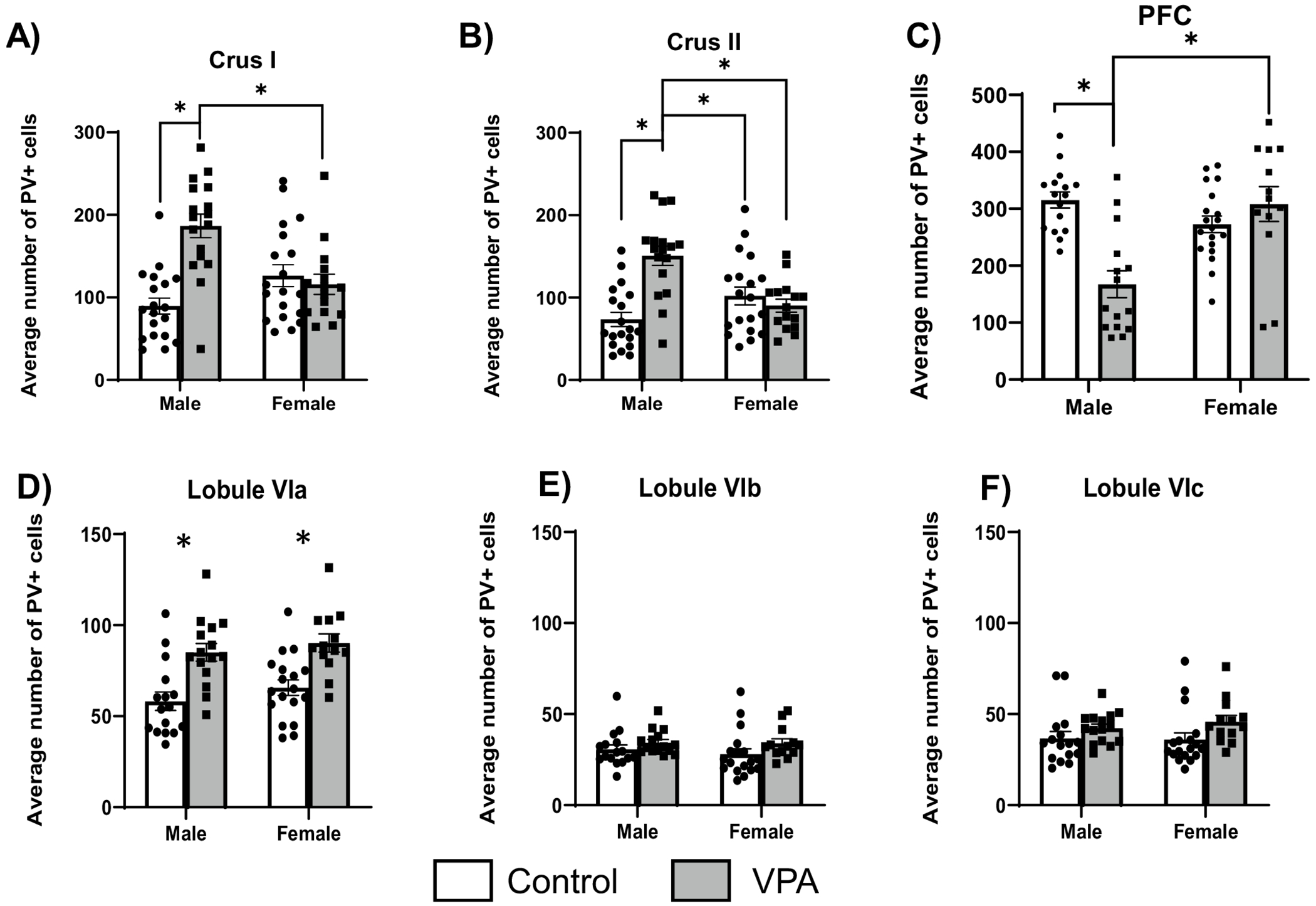
Effects of VPA on PV + cells according to brain region and sex. A In crus I, VPA male rats had increased PV + cell counts compared to control males and VPA females (*p < .05). B In crus II, VPA males had an increased PV + cell counts compared to all other groups (*p < .05). C In medial prefrontal cortex there were significantly fewer PV + neurons in male VPA animals compared to control males and female VPA animals (*p < .05). Each dot is the average cell count across 3 frontal sections for each individual animal counted. D In Lobule VIa, males and females had increased PV + cell counts (*p < .05). E and F No group differences were found in lobule VI b or c. Each dot is the average cell count across 4 sections of the cerebellar region
Cerebellum and cognitive performance
There was as significant finding where increased PV + cell count in lobule VIa predicted worse performance (more trials to criterion) on the ID phase of the SST for control males only (y = 0.1573*x + 0.6497, R2 = 0.26, p = 0.04) (Fig. 4). There was a similar relationship for lobule VIc where, for control males, increased PV + cell count associated with impaired performance on the ID phase of the task (Control males y = 0.2588*x + 0.3076, R2 = 0.38, p = 0.009). There was a near-significant trend in the same direction for VPA males (y = 0.2414*x—0.3510, R2 = 0.24, p = 0.07). No relationships between Purkinje layer PV + cell count and SST performance were found for females.
Fig. 4.

Performance on ID phase and PV + cells (Purkinje layer) in lobule VIa
Control males with fewer PV + cells lobule VIa completed the ID phase in fewer trials than those with more (left). There was similar near-significant trend in the same direction for VPA animals. There was no significant relationship for females (right).
mPFC and EPM
On the EPM, there were no significant condition or sex differences in time spent in closed or open arms. However, there was a significant relationship between the number of PV + cells in mPFC and time spent in closed arms where rats with fewer cells spent less time in the closed arms (Fig. 5, left), but this was only for control rats (y = 0.1279*x + 37.15, R2 = 0.21, p = 0.04). As would also be expected, the opposite relationship was found for time spent in open arms (Fig. 5, right) where control animals with more mPFC PV + cells spent less time in the open arms, (y = − 0.07787*x + 36.20, R2 = 0.22, p = 0.03).
Fig. 5.

Number of PV + cells and time spent in closed arms (left) and open arms (right) Control rats with more PV + (inhibitory) neurons spent more time in closed arms than animals with fewer PV + cells. Control rats with more PV + neurons spent less time in open arms
Discussion
This study identifies male-specific alterations in PV + cell populations in the mPFC and cerebellar lobules VI and VII of VPA animals. Since PV + neurons are inhibitory in nature, the findings here are consistent previous findings of altered excitatory/inhibitory balance in males with ASD. Male VPA animals also had increases in PV + Purkinje cell numbers in lobule VI, and these were associated with impaired SST performance. PV + Purkinje cell counts were also elevated in the crus I and II regions of lobule VII in VPA males.
Cerebellar PV + Neurons and Behavior
The increase in PV + Purkinje cell counts in the cerebellum is consistent with prior findings where male VPA rats displayed overgrowth in lobule VI (Payne et al. 2021). Past studies have found decreases in Purkinje cells using different markers (e.g., calbindin) to tag neurons at different developmental timepoints (Cho et al. 2016; Perez-Pouchoulen et al. 2016; Roux et al. 2019). There are subtypes of Purkinje cells that are still being categorized (Hull and Regehr 2022), and therefore these results may represent only a subpopulation of Purkinje cells. This could account for the discrepancies between studies. Other studies have focused on the reduced size of Purkinje cells that have fewer dendritic branches (Main and Kulesza 2017), which are also smaller in people with ASD (Fatemi et al. 2002); however, cell size was not quantified in this study. There are also developmental time-dependent changes mediated by sex across cerebellar lobules. In VPA females, lobule VI Purkinje cell counts were reduced at P14, but increased in lobule IX (Perez-Pouchoulen et al. 2016). The brains used in this project were collected after P70, which is young adulthood. Therefore, sex- and time-dependent changes may also explain differences in findings between studies or the general lack of differences in females.
With regard to sex differences, prior work found that VPA females are more impaired on the SST than VPA males (McKinnell et al. 2021). This study extends those findings by demonstrating that task performance is in part impacted by the number of PV + cells in lobule VI of the cerebellum in males. This study provides evidence that PV + cells in the Purkinje layer of lobule VI modulate set-shifting performance on the ID phase in males. Elevated PV + cell counts were associated with impaired performance on the set-shifting task, which is consistent with past findings of enlarged lobule VI volumes in VPA males that were associated with impaired SST performance (Payne et al. 2021). It is possible that there is an overproduction or impaired pruning of PV + cells in lobule VI in male VPA rats, and this may partially underlie the volumetric change. Repeated VPA exposure in males can cause cerebellar hypoplasia (Main and Kulesza 2017); however, rats with only one VPA exposure in utero do not have decreased cerebellar volumes of this lobule as measured by MRI (Payne et al. 2021). Impairments in VPA females may be arising from dysregulation of other brain areas, such as the anterior cingulate cortex. Future studies could use other techniques such as in vivo electrophysiology to examine these differences.
The regression analysis for lobule VIa suggests that, in control animals, volume and PV + neuron count may play significant roles in regulating cognition. Animals with higher PV + cell counts in lobule VI were impaired on the ID phase of the SST, and this trend was nearly significant for VPA animals. This finding is supported by a past study which also found that excess volume in lobule VI is associated with poorer ID phase performance (Payne et al. 2021). Lobule VI sends connections to the anterior cingulate cortex (ACC) (Suzuki et al. 2012), which is one of the critical structures for ID shifting (Ng et al. 2007; Bubb et al. 2020). Future studies should manipulate cells within lobule VI and assess neural activity in the ACC, especially as relates to ID performance. It is also possible that, when networks are wired differently across development, altered patterns of connectivity persist into adulthood in ASD. There is also evidence of cerebellar hyperplasia in humans with ASD (Courchesne et al. 1994b; Palmen et al. 2005; Stoodley 2014), suggesting that delayed or slowed pruning may persist into adulthood for certain lobules.
PV + Neurons and the mPFC
Decreased PV + cell counts were found in the mPFC for male VPA animals compared to age-matched controls. There were no differences between female VPA and control animals in the mPFC, which is an interesting result suggesting that sex differences may be present in VPA animals. In humans, VPA exposure during pregnancy skews the sex ratio in those diagnosed to closer to 1:1, rather than the 3:1 ratio seen in the general population (Tartaglione et al. 2019). This makes the VPA model a uniquely suitable tool for understanding sex differences in ASD, and understanding differences between male and female VPA animals could provide insights into biological differences that impact unique symptom profiles found in females with ASD (Mandy et al. 2018). A prior VPA study found no difference in PV + neurons in the mPFC, but at a much younger age than this study (Lauber et al. 2016). Here, the reduction in PV + neuron counts in the mPFC is a novel finding, which suggests that adult males may have specific excitatory/inhibitory imbalances in this region in early adulthood, which may explain sex-mediated differences in symptom profile for males and females with ASD. The sex differences in PV + neuronal expression are not well understood in young adulthood but impact a wide array of behaviors including extinction learning and novelty seeking (Woodward and Coutellier 2021) and are also impacted by sex hormones (Delevich et al. 2021). Previous studies have found that, in the mPFCs of control animals, the numbers of PV + neurons encased by perineuronal nets level off in young adulthood in both sexes (Delevich et al. 2021), but this could be altered by VPA exposure. Here, there was not a clear relationship for PV + cells and performance on phases of the task. This could be due to several factors as PV + number is only one metric of PFC function. Overall volume of mPFC, which includes multiple neuron types, is linked to behavioral performance in female VPA animals (Mali et al. 2023). Total inactivation or lesion of mPFC does impair ED performance (Birrell and Brown 2000; Ng et al. 2007), but only counting PV + neurons may not be sensitive enough to detect that relationship.
Anxiety and PV + Neurons
VPA rats did not show higher behavioral markers of anxiety as measured by the EPM. However, in control rats, the relationship between PV + cells and behavior suggests that increases in PV + neurons, which are inhibitory, inhibit exploratory behavior in open spaces. This aligns with hypotheses where extra inhibition in the frontal cortex is linked to higher anxiety (Page and Coutellier 2019). VPA animals did not demonstrate this relationship, as they did not exhibit additional anxiety-like behaviors compared to controls. This may be because the EPM was conducted in adolescence, but all rats were euthanized after set-shifting, which was completed in adulthood. Therefore, the results here do not necessarily discount the link between PV + neurons and anxiety behavior in VPA animals, especially as PV density changes from adolescence to adulthood (Gildawie et al. 2020) and there are complex relationships between GABAergic and glutamatergic synapses in frontal cortex that may be influenced by VPA exposure (Fukuchi et al. 2009; Kim et al. 2014, 2016). Tentatively, the current data suggests that PV + neurons may not be contributing to the regulation of anxiety-like behaviors in VPA animals in the same way as in controls. A lack of PV + neurons could also contribute to other symptoms of ASD such as dysregulation of emotional responses (Ferguson and Gao 2018). Indeed, PV + neuron dysfunction has been linked to ASD phenotypes in mice (Shin et al. 2021). This study suggests that differences in PV + cells in the mPFC, even in control animals, may be linked to behaviors in anxiety-provoking environments, implicating them in this type of behavior.
Female ASD phenotypes differ from males
One hypothesis of how females with ASD differ from the general population is that their brain has been more “masculinized” than the general female population (Deng and Wang 2021). Females with ASD also have different phenotypic patterns; for instance, they may show fewer outward signs of repetitive behaviors but are more likely to have an intellectual disability (James and Grech 2020). Females with ASD also tend to camouflage symptoms more often than males (Green et al. 2019). In the current study, PV + neurons counts were decreased in the mPFC in male VPA animals, but not for female VPA animals. The drop in cell count for VPA males has been found for other markers of inhibitory cells across other brain regions, such as a decrease in cal-bindin (CB) expression across cerebellar lobules (Main and Kulesza 2017), as well as reduced CB + puncta in vestibular nuclei (Mansour et al. 2022), and fewer CB + cells in auditory cortex (Kosmer and Kulesza 2024). In addition, post-synaptic proteins (e.g., PSD-95) are upregulated more in male VPA animals which may contribute to differences in cell function (Kim et al. 2013). The lack of decrease found in VPA females supports the prior hypothesis, where VPA female cell counts are more similar to control levels. Additionally, females with ASD have regional differences in brain volume that change along a different trajectory than males with ASD (Walsh et al. 2021) and this is also found in VPA rats (King et al. 2024). Decreased PV + neurons in the mPFC can change the balance between excitation and inhibition, which may disrupt attentional processing (Ferguson and Gao 2018). Future studies should examine other behaviors that could be impacted by these cell differences in male and female VPA rats.
Limitations
Limitations of this study include that cells were counted manually instead of by automated software due to batch-to-batch variability in staining intensity. Additionally, only about 60% of Purkinje neurons are intensely positive for PV, the marker used here (Brandenburg et al. 2021), so these findings may only be generalizable to a subpopulation of Purkinje neurons. Cell counts in the cortex were across multiple cortical layers, which has been found to be differentially impacted by VPA in other cortical regions (Kosmer and Kulesza 2024). Future studies could use double labeling to get a better understanding of which cell types are altered by VPA exposure. A relatively small number of litters was also used for this project, but this should be largely controlled by the mixed-effects analysis with litter as a random effect.
Conclusion
The current results provide evidence of sex differences in PV + neurons in the frontal cortex and cerebellum between VPA males and females. These differences could contribute to different phenotypes, for instance VPA males with more PV + neurons in Lobule VIa had impaired ID phase performance. Future studies could use optogenetics or other approaches to tag specific cell types and inactivate them to measure the impact on cognitive performance, anxiety and social behaviors. Lastly, data from the EPM task suggest that PV + neuron count in the mPFC may be a predictor of anxiety-like behaviors.
Supplementary Material
Funding
This work was supported by the National Institute of General Medical Science GM113109 which supports the Cognitive and Neurobiological Approaches to Plasticity (CNAP) Center, which awarded a Pilot grant to BP and provided core lab support. Start-up funds from Kanas State University and a USRG from KSU to BP.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00221-024-06902-y.
Conflict of interest None.
Ethical approval and consent to participate Not applicable.
Consent for publication Not applicable.
Data Availability Statement
Available upon request.
References
- Allen G, Muller RA, Courchesne E (2004) Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol Psychiatry 56:269–279. 10.1016/j.biopsych.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Andropoulos DB (2018) Effect of anesthesia on the developing brain: infant and fetus. Fetal diagnosis and therapy 77030:1–11. 10.1159/000475928 [DOI] [PubMed] [Google Scholar]
- Arif SH (2009) A Ca(2+)-binding protein with numerous roles and uses: parvalbumin in molecular biology and physiology. BioEssays 31:410–421. 10.1002/bies.200800170 [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A et al. (1998) A clinicopathological study of autism. Brain 121(5):889–905. 10.1093/brain/121.5.889 [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL et al. (2018) Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites United States, 2014. Morb Mortal Wkly Rep Surveill Summ 67:1–24. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Engineer CT, Sauls BL et al. (2014) Abnormal emotional learning in a rat model of autism exposed to valproic acid in utero. Front Behav Neurosci 8:387. 10.3389/fnbeh.2014.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ (2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4325. 10.1523/JNEUROSCI.20-11-04320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR (2013) Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res 250:91–102. 10.1016/j.bbr.2013.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241–254. 10.1016/j.tics.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg C, Smith LA, Kilander MBC et al. (2021) Parvalbumin subtypes of cerebellar Purkinje cells contribute to differential intrinsic firing properties. Mol Cell Neurosci 115:103650. 10.1016/j.mcn.2021.103650 [DOI] [PubMed] [Google Scholar]
- Brown VJ, Tait DS (2016) Attentional set-shifting across species. Curr Top Behav Neurosci 28:363–396. 10.1007/7854_2015_5002 [DOI] [PubMed] [Google Scholar]
- Bubb EJ, Aggleton JP, Mara SMO, Nelson AJD (2020) Original article chemogenetics reveal an anterior cingulate-thalamic pathway for attending to task-relevant information. Cereb Cortex. 10.1093/cercor/bhaa353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Geda E, Sacco K et al. (2011) Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry 82:1304–1313. 10.1136/jnnp.2010.239111 [DOI] [PubMed] [Google Scholar]
- Chaliha D, Albrecht M, Vaccarezza M et al. (2020) A systematic review of the valproic-acid-induced rodent model of autism. Dev Neurosci 42:12–48. 10.1159/000509109 [DOI] [PubMed] [Google Scholar]
- Chase EA, Tait DS, Brown VJ (2012) Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci 36:2368–2376. 10.1111/j.1460-9568.2012.08141.x [DOI] [PubMed] [Google Scholar]
- Cho HS, Kim TW, Ji ES et al. (2016) Treadmill exercise ameliorates motor dysfunction through inhibition of Purkinje cell loss in cerebellum of valproic acid-induced autistic rats. J Exerc Rehabil 12:293–299. 10.12965/jer.1632696.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Gronborg TK, Sorensen MJ et al. (2013) Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309:1696–1704. 10.1001/jama.2013.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SV, Semmel ES, Aleksonis HA et al. (2021) Cerebellar-subcortical-cortical systems as modulators of cognitive functions. Neuropsychol Rev 31:422–446. 10.1007/s11065-020-09465-1 [DOI] [PubMed] [Google Scholar]
- Coffman KA, Dum RP, Strick PL (2011) Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A 108:16068–16073. 10.1073/pnas.1107904108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA et al. (1988) Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 318:1349–1354. 10.1056/NEJM198805263182102 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R et al. (1994) Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: Identification of hypoplastic and hyperplastic subgroups with MR imaging. Am J Roentgenol 162:123–130. 10.2214/ajr.162.1.8273650 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R et al. (1994) Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. Ajramerican J Roentgenol 162:123–131. 10.2214/ajr.162.1.8273650 [DOI] [PubMed] [Google Scholar]
- Crucitti J, Hyde C, Enticott PG, Stokes MA (2020) Are vermal lobules VI-VII smaller in autism spectrum disorder? Cerebellum 19:617–628. 10.1007/s12311-020-01143-5 [DOI] [PubMed] [Google Scholar]
- D’Angelo E, Casali S (2013) Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 10.3389/fncir.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Klinger M, Okada NJ, Wilbrecht L (2021) Coming of age in the frontal cortex: the role of puberty in cortical maturation. Semin Cell Dev Biol 118:64–72. 10.1016/j.semcdb.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Wang S (2021) Sex differentiation of brain structures in autism: findings from a gray matter asymmetry study. Autism Res 14:1115–1126. 10.1002/aur.2506 [DOI] [PubMed] [Google Scholar]
- D’Mello AM, Stoodley CJ (2015) Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 10.3389/fnins.2015.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ (2015) Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clin 7:631–639. 10.1016/j.nicl.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G et al. (2002) Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol 22:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre MR, Barkat TR, Lamendola D et al. (2013) General developmental health in the VPA-rat model of autism. Front Behav Neurosci 7:88. 10.3389/fnbeh.2013.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BR, Gao W-J (2018) PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits 12:37. 10.3389/fncir.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Adkinson BD, Ji JL et al. (2017) Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol Psychiatry 81:848–862. 10.1016/j.biopsych.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Nii T, Ishimaru N et al. (2009) Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res 65:35–44. 10.1016/j.neures.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Gibson JM, Howland CP, Ren C et al. (2022) A critical period for development of cerebellar-mediated autism-relevant social behavior. J Neurosci off J Soc Neurosci 42:2804–2823. 10.1523/JNEUROSCI.1230-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildawie KR, Honeycutt JA, Brenhouse HC (2020) Region-specific effects of maternal separation on perineuronal net and parvalbumin-expressing interneuron formation in male and female rats. Neuroscience 428:23–37. 10.1016/j.neuroscience.2019.12.010 [DOI] [PubMed] [Google Scholar]
- Gildawie KR, Ryll LM, Hexter JC et al. (2021) A two-hit adversity model in developing rats reveals sex-specific impacts on prefrontal cortex structure and behavior. Dev Cogn Neurosci 48:100924. 10.1016/j.dcn.2021.100924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JS, Sillitoe RV (2019) Functional outcomes of cerebellar malformations. Front Cell Neurosci 13:1–20. 10.3389/fncel.2019.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Travers AM, Howe Y, McDougle CJ (2019) Women and autism spectrum disorder: diagnosis and implications for treatment of adolescents and adults. Curr Psychiatry Rep 21:22. 10.1007/s11920-019-1006-3 [DOI] [PubMed] [Google Scholar]
- Hashemi E, Ariza J, Rogers H et al. (2018) The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cereb Cortex 28:690. 10.1093/cercor/bhx063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgdon EA, Anderson R, Al AH et al. (2024) MRI morphometry of the anterior and posterior cerebellar vermis and its relationship to sensorimotor and cognitive functions in children. Dev Cogn Neurosci 67:101385. 10.1016/j.dcn.2024.101385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Regehr WG (2022) The cerebellar cortex. Annu Rev Neurosci 45:151–175. 10.1146/annurev-neuro-091421-125115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM (2000) Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol 22:319–325. 10.1016/S0892-0362(99)00083-5 [DOI] [PubMed] [Google Scholar]
- James WH, Grech V (2020) Potential explanations of behavioural and other differences and similarities between males and females with autism spectrum disorder. Early Hum Dev 140:104863. 10.1016/j.earlhumdev.2019.104863 [DOI] [PubMed] [Google Scholar]
- Kawabata K, Bagarinao E, Watanabe H et al. (2022) Functional connector hubs in the cerebellum. Neuroimage 257:119263. 10.1016/j.neuroimage.2022.119263 [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8445. 10.1523/JNEUROSCI.23-23-08432.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenwood MM, Kalin NH, Barbas H (2022) The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacol off Publ Am Coll Neuropsychopharmacol 47:260–275. 10.1038/s41386-021-01109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK (2003) Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev 25:377–383. 10.1016/S0387-7604(03)00056-1 [DOI] [PubMed] [Google Scholar]
- Kiep M, Spek AA (2017) Executive functioning in men and women with an autism spectrum disorder. Autism Res 10:940–948. 10.1002/aur.1721 [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim P, Go HS et al. (2011) The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett 201:137–143. 10.1016/j.toxlet.2010.12.018 [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim P, Go HS et al. (2013) Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J Neurochem 124:832–843. 10.1111/jnc.12147 [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee D-K, Go HS et al. (2014) Pax6-dependent cortical gluta-matergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol 49:512–528. 10.1007/s12035-013-8535-2 [DOI] [PubMed] [Google Scholar]
- Kim KC, Choi CS, Kim JW et al. (2016) MeCP2 modulates sex differences in the postsynaptic development of the valproate animal model of autism. Mol Neurobiol 53:40–57. 10.1007/s12035-014-8987-z [DOI] [PubMed] [Google Scholar]
- King C, Mali I, Strating H et al. (2024) Region-Specific Brain Volume Changes Emerge in Adolescence in the Valproic Acid Model of Autism and Parallel Human Findings. Dev Neurosci. 10.1159/000538932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmer K, Kulesza R (2024) Cortical dysmorphology and reduced cortico-collicular projections in an animal model of autism spectrum disorder. Cereb Cortex 34:146–160. 10.1093/cercor/bhad501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber E, Filice F, Schwaller B (2016) Prenatal valproate exposure differentially affects parvalbumin-expressing neurons and related circuits in the cortex and striatum of mice. Front Mol Neurosci 9:1–16. 10.3389/fnmol.2016.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lee J, Kim E (2017) Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry 81:838–848. 10.1016/j.biopsych.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Leung RC, Zakzanis KK (2014) Brief report: cognitive flexibility in autism spectrum disorders: a quantitative review. J Autism Dev Disord 44:2628–2645. 10.1007/s10803-014-2136-4 [DOI] [PubMed] [Google Scholar]
- Leung RC, Vogan VM, Powell TL et al. (2016) The role of executive functions in social impairment in autism spectrum disorder. Child Neuropsychol a J Norm Abnorm Dev Child Adolesc 22:336–344. 10.1080/09297049.2015.1005066 [DOI] [PubMed] [Google Scholar]
- Liang D, Li G, Liao X et al. (2016) Developmental loss of parvalbumin-positive cells in the prefrontal cortex and psychiatric anxiety after intermittent hypoxia exposures in neonatal rats might be mediated by NADPH oxidase-2. Behav Brain Res 296:134–140. 10.1016/j.bbr.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Liu J, Yao L, Zhang W et al. (2017) Gray matter abnormalities in pediatric autism spectrum disorder: a meta-analysis with signed differential mapping. Eur Child Adolesc Psychiatry 26:933–946. 10.1007/s00787-017-0964-4 [DOI] [PubMed] [Google Scholar]
- Mabunga DF, Gonzales EL, Kim JW et al. (2015) Exploring the Validity of Valproic Acid Animal Model of Autism. Exp Neurobiol 24:285–301. 10.5607/en.2015.24.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main SL, Kulesza RJ (2017) Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 340:34–48. 10.1016/j.neuroscience.2016.10.052 [DOI] [PubMed] [Google Scholar]
- Mali I, Payne M, King C et al. (2023) Adolescent female valproic acid rats have impaired extra-dimensional shifts of attention and enlarged anterior cingulate cortices. Brain Res 1800:148199. 10.1016/j.brainres.2022.148199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, Pellicano L, St Pourcain B et al. (2018) The development of autistic social traits across childhood and adolescence in males and females. J Child Psychol Psychiatry 59:1143–1151. 10.1111/jcpp.12913 [DOI] [PubMed] [Google Scholar]
- Mansour Y, Burchell A, Kulesza R (2022) Abnormal vestibular brain-stem structure and function in an animal model of autism spectrum disorder. Brain Res 1793:148056. 10.1016/j.brainres.2022.148056 [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G (2010) Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci 31:544–556. 10.1111/j.1460-9568.2009.07073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell ZE, Maze T, Ramos A et al. (2021) Valproic acid treated female Long-Evans rats are impaired on attentional set-shifting. Behav Brain Res 397:112966. 10.1016/j.bbr.2020.112966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WS, Kelly SE, Unruh KE et al. (2022) Cerebellar volumes and sensorimotor behavior in autism spectrum disorder. Front Integr Neurosci 16:821109. 10.3389/fnint.2022.821109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MV, Gandal MJ, Siegel SJ (2011) mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS One. 10.1371/journal.pone.0026077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Wang Z, Schmitt LM et al. (2015) The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci 9:296. 10.3389/fnins.2015.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS et al. (2007) Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci 27:12123–12132. 10.1523/JNEUROSCI.2745-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CE, Coutellier L (2019) Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev 105:39–51. 10.1016/j.neubiorev.2019.07.024 [DOI] [PubMed] [Google Scholar]
- Palmen SJMC, Hulshoff Pol HE, Kemner C et al. (2005) Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol Med 35:561–570. 10.1017/s0033291704003496 [DOI] [PubMed] [Google Scholar]
- Payne M, Mali I, McKinnell ZE et al. (2021) Increased volumes of lobule VI in a valproic acid model of autism are associated with worse set-shifting performance in male Long-Evan rats. Brain Res 1765:147495. 10.1016/j.brainres.2021.147495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pouchoulen M, Miquel M, Saft P et al. (2016) Prenatal exposure to sodium valproate alters androgen receptor expression in the developing cerebellum in a region and age specific manner in male and female rats. Int J Dev Neurosci 53:46–53. 10.1016/j.ijdevneu.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Roux S, Bailly Y, Bossu JL (2019) Regional and sex-dependent alterations in Purkinje cell density in the valproate mouse model of autism. NeuroReport 30:82–88. 10.1097/WNR.0000000000001164 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2019) The cerebellum and cognition. Neurosci Lett 688:62–75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30:80–90. 10.1038/sj.npp.1300518 [DOI] [PubMed] [Google Scholar]
- Schneider T, Ziolkowska B, Gieryk A et al. (2007) Prenatal exposure to valproic acid disturbs the enkephalinergic system functioning, basal hedonic tone, and emotional responses in an animal model of autism. Psychopharmacology 193:547–556. 10.1007/s00213-007-0795-y [DOI] [PubMed] [Google Scholar]
- Shapiro BL (2001) Developmental instability of the cerebellum and its relevance to Down syndrome. J Neural Transm Suppl. 10.1007/978-3-7091-6262-0_2 [DOI] [PubMed] [Google Scholar]
- Shin S, Santi A, Huang S (2021) Conditional Pten knockout in parvalbumin- or somatostatin-positive neurons sufficiently leads to autism-related behavioral phenotypes. Mol Brain 14:24. 10.1186/s13041-021-00731-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman ML, Green JT (2019) Cerebellum and cognition: does the rodent cerebellum participate in cognitive functions? Neurobiol Learn Mem 170:1–16 [DOI] [PubMed] [Google Scholar]
- Sivaswamy L, Kumar A, Rajan D et al. (2010) A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J Child Neurol 25:1223–1231. 10.1177/0883073809358765 [DOI] [PubMed] [Google Scholar]
- Soghomonian J-J, Zhang K, Reprakash S, Blatt GJ (2017) Decreased parvalbumin mRNA levels in cerebellar Purkinje cells in autism. Autism Res off J Int Soc Autism Res 10:1787–1796. 10.1002/aur.1835 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ (2014) Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci 8:1–17. 10.3389/fnsys.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2016) The Cerebellum and Neurodevelopmental Disorders. Cerebellum 15:34–37. 10.1007/s12311-015-0715-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki L, Coulon P, Sabel-Goedknegt EH, Ruigrok TJ (2012) Organization of cerebral projections to identified cerebellar zones in the posterior cerebellum of the rat. J Neurosci 32:10854–10870. 10.1523/JNEUROSCI.0857-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Chase EA, Brown VJ (2014) Attentional set-shifting in rodents: a review of behavioural methods and pharmacological results. Curr Pharm Des 20:5046–5059. 10.2174/1381612819666131216115802 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sato A, Kasai S et al. (2018) Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol Autism 9:60. 10.1186/s13229-018-0243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglione AM, Schiavi S, Calamandrei G, Trezza V (2019) Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 159:107477. 10.1016/j.neuropharm.2018.12.024 [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J et al. (1999) Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci off J Soc Neurosci 19:5632–5643. 10.1523/JNEUROSCI.19-13-05632.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden ME, Gill JS, Sillitoe RV (2021) Abnormal cerebellar development in autism spectrum disorders. Dev Neurosci 43:181–190. 10.1159/000515189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Manto M, Cattaneo Z et al. (2020) Consensus paper: cerebellum and social cognition. Cerebellum 19:833–868. 10.1007/s12311-020-01155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJM, Wallace GL, Gallegos SM, Braden BB (2021) Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. NeuroImage Clin 31:102719. 10.1016/j.nicl.2021.102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jing J, Igarashi K et al. (2018) Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res off J Int Soc Autism Res 11:1148–1156. 10.1002/aur.1967 [DOI] [PubMed] [Google Scholar]
- Westwood H, Stahl D, Mandy W, Tchanturia K (2016) The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychol Med 46:1809–1827. 10.1017/S0033291716000581 [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML et al. (2008) Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum 7:406–416. 10.1007/s12311-008-0043-y [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Rosene DL et al. (2009) Density of cerebellar basket and stellate cells in autism: evidence for a late developmental loss of Purkinje cells. J Neurosci Res 87:2245–2254. 10.1002/jnr.22056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Orduz D, Gregory P et al. (2015) Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry 5:e525. 10.1038/tp.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Swanson MR, Elison JT et al. (2017) Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism 8:8. 10.1186/s13229-017-0126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward EM, Coutellier L (2021) Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev 131:1228–1242. 10.1016/j.neubiorev.2021.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Hashimoto R, Ohi K et al. (2014) Cognitive inflexibility in Japanese adolescents and adults with autism spectrum disorders. World J Psychiatry 4:42–48. 10.5498/wjp.v4.i2.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Harrison B et al. (2009) Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional shift test correlate with repetitive behaviors. Autism 13:523–539. 10.1177/1362361309335716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Zhang H, Ma L et al. (2023) Reduced volume of the left cerebellar lobule VIIb and its increased connectivity within the cerebellum predict more general psychopathology one year later via worse cognitive flexibility in children. Dev Cogn Neurosci 63:101296. 10.1016/j.dcn.2023.101296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H (2013) Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci 7:609. 10.3389/fnhum.2013.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request.


