ABSTRACT
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infection in infants and young children, placing substantial burden on patients, their families, and health systems. This observational, cross-sectional, web-based, survey study in the United States (during October – November 2023) assessed physicians’ perceptions of RSV disease and new immunization strategies, including their preferences for monoclonal antibodies (mAbs) and maternal immunizations as RSV preventive measures. Immunization preferences were quantified through discrete choice experiment (DCE). Physicians aged ≥ 18 years, who spent at least 60% of their time in direct patient care and worked in a practice providing immunization to patients aged ≤ 2 years were recruited through online panels. Eighty pediatricians and 20 family practitioners participated. Mean (SD) age of physicians was 52.3 (12.7) years; majority were male (64.0%). Most physicians strongly agreed with supporting all types of recommended childhood immunizations (77.0%) and were aware of new RSV immunization strategies under development or recently approved (91.0%). A majority moderately/strongly agreed that maternal immunization and mAbs provide protection to the baby (77.0% and 87.0%, respectively). In DCE, physicians chose RSV immunization 96.1% of the time vs no immunization (3.9%). The most important attributes that drove physicians’ preferences were: increasing durability of protection from 90 to 180 days (24.9%), increasing efficacy against RSV hospitalization from 57% to 80% (20.9%), and increasing efficacy against medically-attended RSV from 51% to 80% (20.2%). Understanding physicians’ attitudes and preferences regarding RSV immunization strategies is important as new RSV prevention methods become available and are introduced into clinical practice.
KEYWORDS: Attitudes, discrete choice experiment, immunization, knowledge, maternal vaccine, monoclonal antibodies, physician’s awareness, physician’s perception, preferences, respiratory syncytial virus
Introduction
Respiratory syncytial virus (RSV) is the leading cause of acute respiratory infections in infants and young children.1,2 Approximately 70% of infants experience at least one RSV infection within their first year of life, and, by the age of two, nearly all children will have a second RSV infection.3–5 In the United States (US), RSV infections result in a substantial burden of illness, leading to over 57,000 hospitalizations, 500,000 emergency department visits, and 1.5 million outpatient clinic visits annually among children under the age of five.6
While preterm infants and those with underlying medical conditions are at a higher risk of severe RSV infection, a significant proportion of infants who are hospitalized with RSV infection are otherwise healthy and do not possess any known risk factors.4 In response to the significant morbidity associated with RSV, the US Food and Drug Administration (FDA) approved two new prevention strategies in 2023 for infants and young children in the US. These strategies involve the use of a monoclonal antibody (mAb) called nirsevimab (Beyfortus™) and a maternal immunization known as bivalent prefusion RSVpreF vaccine (Abrysvo™).7,8
The current recommendations from the US Centers for Disease Control and Prevention (CDC) advise either maternal RSV vaccination or infant immunization with RSV mAbs. Maternal RSV vaccination is recommended for pregnant women between weeks 32 and 36 of pregnancy,9 while the RSV mAb is advised for infants aged 8 months and younger who are born during or entering their first RSV season. Additionally, for infants and children aged 8 to 19 months at an increased risk of severe RSV disease and entering their second RSV season, a single dose of the mAb is recommended.10
Considering that guidelines recommend that most infants receive immunization from either the maternal RSV vaccine or the RSV mAb, but not both, a comprehensive understanding of physicians’ awareness and preferences regarding these new RSV immunization strategies is timely and informative.11 Physicians’ knowledge plays a pivotal role in recommending vaccines to their patients, ultimately influencing patients’ acceptance of such preventive measures.12 Furthermore, the distinct clinical profiles of maternal RSV vaccination and RSV mAbs9,10 are expected to elicit independent preferences and concerns among healthcare professionals, influenced by their perceptions of the disease.13 Thus, the objective of this study is to assess physicians’ perceptions of RSV disease and the new RSV immunizations, as well as to explore their preferences for mAbs and maternal immunizations as preventive measures for RSV.
Methods
Survey design and eligibility criteria
This was an observational, cross-sectional, web-based survey study to evaluate knowledge, attitudes, and preferences about RSV immunizations among physicians in the US between October and November 2023. Knowledge and attitudes were captured through 22 survey questions and immunization strategy preferences were quantified through a discrete choice experiment (DCE). DCE is a well-established preference elicitation method to quantify which attributes drive preferences between and analyze the trade-offs that various stakeholders (e.g., health care providers [HCPs]) are willing to make.14
Physicians (comprising both pediatricians and family practitioners) were recruited for the study through local online panels owned by AllGlobal and their affiliated partners. Prospective participants, preidentified as pediatricians or general practitioners in the online panels, were randomly selected and received an e-mail containing a link to the online survey. The survey included an online eligibility screener, an informed consent statement, and the questionnaire, all integrated within the online platform. Physicians who expressed interest in participating were screened for eligibility until the target sample size was reached. The study recruitment strategy aimed to have broad representation of physicians across the US and to enroll 80% pediatricians and 20% family practitioners. Only physicians who completed the survey in its entirety received compensation based on fair market value.
Physicians were eligible if they 1) provided direct patient care to patients aged 2 years or younger (minimum threshold was 100 pediatric visits in a typical month for pediatricians and 50 for general practitioners), 2) worked in a practice that offers vaccinations for children aged 2 years or younger, 3) spent at least 60% of their time in direct patient care, 4) had at least 3 years of clinical experience, 5) were board-certified, 6) practiced within the United States, 7) were aged 18 years or older, and 8) could read and understand English.
Survey development and measures
The quantitative survey questionnaire was developed based on a targeted literature review and further refined based on results from qualitative research and cognitive interviews. Qualitative research identified aspects of mAb and maternal immunization for RSV that could influence physicians’ motivations and obstacles to prescribing mAb or maternal immunization. Interviews were conducted via telephone in the US with 6 pediatricians and 2 family practitioners. These interviews helped to elucidate what mattered most to physicians when considering the choice of an RSV immunization strategy and, thus, informed a list of immunization strategy attributes for testing in the DCE.
After qualitative research, 8 web/telephone cognitive pretests were conducted to ensure that the quantitative questionnaire was understood by respondents. The respondents reviewed the questionnaire with a trained moderator who asked pretest questions; respondents were given the option to verbally share comments and provide feedback. Any potential issues with respect to relevance, clarity, interpretation, and appropriateness of the survey content were then identified and corrected in the final quantitative survey.
The following data were collected: Physicians’ demographics and clinical practice characteristics (e.g., age, specialty, years of practice); attitudes and behaviors toward immunization (e.g., supporting all types of recommended childhood immunizations); clinical experience with RSV (e.g., number of RSV cases that physicians provided care for in the past 12 months); perception of RSV risk and severity (physicians were asked to state their degree of agreement or disagreement with a series of statements regarding RSV [e.g., RSV can be serious for premature infants, younger patients, or those with underlying disease]); awareness of new RSV immunization strategies (RSV mAbs and maternal immunization); and perception of new RSV immunization strategies (e.g., perceived characteristics of mAb and maternal immunizations such as safety and durability).
In the DCE, physicians were asked to consider two hypothetical immunization profiles and a fixed “no immunization” choice shown side-by-side and choose the option that was preferable to them (Supplementary Table S1). Each hypothetical immunization profile consisted of combinations of 8 ‘attributes’ (e.g., efficacy against RSV hospitalization) and 2–3 ‘attribute levels’ (e.g., 57% less likely, 68% less likely) (Supplementary Table S2). Attributes were selected based on the previous qualitative research, and levels were selected based on clinical trials of new RSV immunizations: nirsevimab and a RSV prefusion F vaccine.15–18 The hypothetical immunization strategies had their attribute levels shuffled according to an experimental design. No actual strategy was named in the survey.
Statistical analysis
The study was descriptive in nature and had no initial hypothesis to test. The target sample size of 100 respondents was calculated such that at least a medium effect size (Cohen’s d of at least 0.5) could be detected with an alpha of 0.05 and 80% power in a subgroup analysis. Furthermore, based on this target sample size and the DCE design, it was calculated that 14 choice tasks answerable by respondents, with an estimated opt-out rate of 10%, would lead to an error margin of 5.4% which was an acceptable trade-off between robustness and burden of the survey.
All data collected in the quantitative survey were reported descriptively, using frequencies, means, and standard deviations (SDs). Subgroups (pediatricians and family practitioners) were compared using Wilcoxon rank sum tests for continuous data and Fisher’s exact tests for categorical data.
Physicians’ attribute-level preference weights and the relative importance of immunization strategy attributes were generated from the DCE. In the DCE, the preference weights for each attribute level were estimated using a hierarchical Bayesian model fitted to the choice data,19 with the underlying choice-probability model being conditional logit. The relative importance was calculated at the respondent level by dividing the range of each attribute (utility of highest level minus utility of lowest level) by the sum of ranges of all attributes and then multiplying by 100. For each respondent, the relative importance estimates across attributes summed to 100%. All survey analyses were conducted using RStudio Version 2023.12.1+402 and Sawtooth Software Lighthouse Studio Version 9.12.1 or higher for DCE analysis.
The study was reviewed by the Sterling Institutional Review Board (IRB) and was granted exemption on March 07, 2023.
Results
A total of 259 physicians entered the survey, of which 159 physicians were excluded (18 did not complete the survey; 95 did not meet eligibility criteria, including 65 who did not meet the threshold for the number of treating patients under 2 years old; 43 entered after the specialty-specific quota was met; and 3 had a data quality issue). Ultimately, 100 participants were included in the final analysis (Supplementary Figure S1).
Physicians’ demographics and clinical practice characteristics
According to our pre-defined quota sampling, 80 physicians were specialized in pediatrics and 20 were family practitioners. Overall, the mean (SD) age of physicians was 52.3 (12.7) years, and most were male (64.0%) (Table 1).
Table 1.
Demographics and clinical experience of physicians.
| Characteristics | Overall, N = 100 |
Family practitioners, n = 20 |
Pediatricians, n = 80 |
p-value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | .082 | |||
| N | 96 | 19 | 77 | |
| Mean (SD) | 52.3 (12.7) | 48.2 (13.1) | 53.3 (12.5) | |
| Gender#, n (%) | .14 | |||
| Male | 64 (64.0) | 13 (65.0) | 51 (63.8) | |
| Female | 33 (33.0) | 5 (25.0) | 28 (35.0) | |
| Region, n (%) | .2 | |||
| Midwest | 20 (20.0) | 4 (20.0) | 16 (20.0) | |
| Northeast | 31 (31.0) | 3 (15.0) | 28 (35.0) | |
| South | 29 (29.0) | 6 (30.0) | 23 (28.8) | |
| West | 20 (20.0) | 7 (35.0) | 13 (16.3) | |
| Clinical practice | ||||
| Post-training number of years of clinical experience, mean (SD) | 20.0 (10.9) | 16.0 (10.5) | 21.0 (10.9) | .049 |
| Time spent in direct patient care, mean% (SD%) | 96.2 (5.9) | 96.2 (5.8) | 96.2 (6.0) | .6 |
| Number of patients aged ≤2 years treated in a month, mean (SD) | 145.3 (67.9) | 94.4 (72.2) | 158.0 (60.8) | <.001 |
| RSV experience | ||||
| Approximate number of RSV cases in children aged ≤2 years in the past 12 months, mean (SD) [range] | 86.3 (89.2) [0.0–400.0] | 36.0 (28.2) [0.0–100.0] | 98.9 (94.7) [0.0–400.0] | .002 |
| RSV patients aged ≤2 years having mild symptoms, mean% (SD%) [range] | 59.9 (26.6) [0.0–100.0] | 64.9 (25.7) [20.0–100.0] | 58.7 (26.8) [0.0–100.0] | .4 |
| RSV patients aged ≤2 years having moderate symptoms requiring pharmacotherapy, mean% (SD) [range] | 28.3 (21.9) [0.0–85.0] | 26.1 (20.7) [0.0–65.0] | 28.9 (22.3) [0.0–85.0] | .7 |
| RSV patients aged ≤2 years having severe symptoms requiring ED visits or hospitalization, mean% (SD) [range] | 11.8 (14.8) [0.0–100.0] | 9.1 (11.0) [0.0–35.0] | 12.5 (15.6) [0.0–100.0] | .10 |
RSV, respiratory syncytial virus; SD, standard deviation.
*P-values for comparison of family practitioners vs pediatricians are reported; Wilcoxon rank sum test for continuous data and Fisher’s exact test for categorical data. p-value < .05 was considered to be significant.
#Non-binary: family practitioners, n = 1; overall, n = 1. Prefer not to answer: family practitioners, n = 1; pediatricians, n = 1; overall n = 2.
The average percentage of time spent by physicians in various clinical settings was: 57.7% (48.7%) in group practice, followed by 16.9% (36.9%) in a hospital setting, and 13.3% (33.7%) in solo practice (data not shown). Physicians spent most of their time in direct patient care (96.2% [5.9%]); no statistically significant difference was observed between pediatricians and family practitioners (p = .6) (Table 1). On average, physicians had 20.0 (10.9) years of post-training clinical experience; years of experience were significantly higher for pediatricians than family practitioners (21.0 [10.9] vs 16.0 [10.5]; p = .049). On average, in a typical month, physicians treated 145.3 (67.9) patients aged ≤2 years, which was significantly higher for pediatricians than family practitioners (158.0 [60.8] vs 94.4 [72.2]; p < .001).
Physicians’ attitudes and behaviors toward immunization
Most physicians strongly agreed with supporting all types of recommended childhood immunizations (77.0%); 89.0% of physicians moderately or strongly agreed that they tried their best to persuade parents to get all recommended immunizations for their newborns or infants (Supplementary Table S3). No statistically significant differences were observed between pediatricians and family practitioners (p > .05).
Overall, 58.0% of physicians offered pre-natal visits and raised the topic of newborn immunization; the percentage was significantly higher for pediatricians than family practitioners (63.8% vs 35.0%, respectively; p = .020).
Physicians’ clinical experience with RSV
Over the 12-month period preceding the study, physicians reported providing direct care for, on average, 86.3 (89.2) cases of RSV in children aged ≤2 years; pediatricians cared for a significantly higher number of cases compared with family practitioners (98.9 [94.7] vs 36.0 [28.2]; p = .002) (Table 1). Sixty percent of RSV patients aged ≤2 years seen by physicians had mild symptoms not requiring any treatment (59.9% [26.6%]), nearly a third had moderate symptoms requiring pharmacotherapy (28.3% [21.9%]), and the remaining had severe symptoms requiring ED visits or hospitalization (11.8% [14.8%]).
Physicians’ perception of RSV risk and severity
Almost all physicians moderately or strongly agreed (99.0%) that RSV can be serious for premature infants, younger patients, or those with underlying disease (Figure 1), with a higher percentage of pediatricians strongly agreeing with the statement than family practitioners (93.8% vs 75.0%, p = .025) (Supplementary Table S4). Most physicians moderately or strongly agreed that RSV is very common among children <2 years of age (94.0%). Most physicians (91.0%) also moderately or strongly agreed that RSV is usually mild but can lead to hospitalization. Additionally, a majority moderately or strongly agreed that RSV is unpredictable (82.0%), and that RSV evokes fear among parents (75.0%). Two-thirds of physicians moderately or strongly agreed that there is a lack of good treatment options for RSV (66.0%); a higher percentage of pediatricians (vs family practitioners) moderately or strongly agreed with the statement (72.5% vs 40.0%, p = .021).
Figure 1.

Physicians’ perception of RSV risk and severity.
RSV, respiratory syncytial virus.
Physicians’ awareness of new RSV immunization strategies
Most physicians were aware of new RSV immunization strategies under development or recently approved (91.0%, n = 91) (data not shown). Among these 91 physicians who were aware, 82 (90.1%) were aware of RSV mAbs under development or recently approved, and 84 (92.3%) were aware of RSV maternal immunization. Pediatricians were more likely than family practitioners to be aware of RSV mAbs (93.3% vs 75.0%, respectively; p = .048) and maternal immunization (96.0% vs 75.0%, respectively; p = .017).
Physicians’ perception of new RSV immunization strategies: maternal immunization and monoclonal antibodies
Maternal immunization: The majority of physicians moderately or strongly agreed that maternal immunization is safe during pregnancy (80.0%) and that it provides immediate protection to the baby after birth (77.0%). Further, a majority of physicians moderately or strongly agreed that the efficacy and safety of maternal immunization depend on gestational age (72.0%) and that babies might still need additional immunization after birth (70.0%). Two-thirds moderately or strongly agreed that maternal immunization provides protection during the entire RSV season (63.0%). Forty-two percent of physicians moderately or strongly agreed that placental transfer of antibodies from maternal immunization is unpredictable (Figure 2A). No statistically significant differences were observed between pediatricians and family practitioners (Supplementary Table S5).
Figure 2.

Physicians’ perception of new RSV immunization strategies.
RSV, respiratory syncytial virus.
Monoclonal antibodies: The majority of physicians moderately or strongly agreed that RSV mAbs offer immediate and direct protection (87.0%) (Figure 2B). A significant difference was observed between family practitioners and pediatricians (p = .014); a higher percentage of pediatricians (vs family practitioners) moderately or strongly agreed with the statement (90.0% vs 75.0%) while a lower percentage moderately or strongly disagreed (0.0% vs 10.0%) (Supplementary Table S5). The majority of physicians moderately or strongly agreed that RSV mAbs are safe for infants (86.0%) and that RSV mAbs provide protection during the entire RSV season (82.0%). Overall, 81.0% of physicians moderately or strongly agreed that RSV mAbs can be administered during routine pediatric visits and 79.0% moderately or strongly agreed that RSV mAbs protect newborns, infants, and older children. Additionally, approximately two-thirds of physicians moderately or strongly agreed that they preferred a fixed dosing regimen over a weight-based dosing regimen (62.0%).
Discrete choice experiment
Physicians’ attribute-level preference weights
Overall, physicians chose RSV immunization 96.1% of the time vs no immunization (3.9%) across the DCE choice tasks (data not shown). With respect to immunization profiles, physicians preferred the RSV mAb over a maternal immunization (utility: 0.6; weight: 0.3 vs − 0.3; Supplementary Figure S2).
Based on the preference weights, example trade-offs that physicians were willing to make included a risk of preterm birth (0.7 − [ − 0.7] = 1.4) in exchange for an increase in efficacy against RSV hospitalization from 68% to 80% (1.6 − [0.0] = 1.6), and shorter immunization durability from 180 to 150 days (1.6 − [0.8] = 0.8) in exchange for an increase in efficacy against medically attended RSV from 70% to 80% (1.2 − [0.5] = 0.7).
Physicians’ relative importance of immunization strategy attributes
We identified three key attributes that were most important for physicians’ preferences. The attribute that ranked the highest in terms of numerical importance was an increase in the durability of protection provided from 90 to 180 days (24.9%). This was followed by an increase in efficacy of the immunization against RSV hospitalization from 57% to 80% (20.9%), and an increase in efficacy against medically attended RSV from 51% to 80% (20.2%) (Figure 3). Extending the time since the immunization became available from less than 1 year to 5 years (10.7%), risk of pre-term birth (from no to yes, 9.6%), type of immunization and administration (from maternal immunization to mAb, 8.2%), and type of dosing regimen (from adjusted dose to fixed dose, 4.5%) followed in importance. Common immunization side-effects was the least important attribute (yes vs no, 0.9%) (Figure 3). The relative importance of attributes varied by physician specialty, though the three key attributes of durability, efficacy against RSV hospitalization, and efficacy against medically attended RSV remained the most important across both specialties. Notably, efficacy against medically attended RSV had a significantly (p < .05) higher importance for family practitioners versus pediatricians (data not shown).
Figure 3.
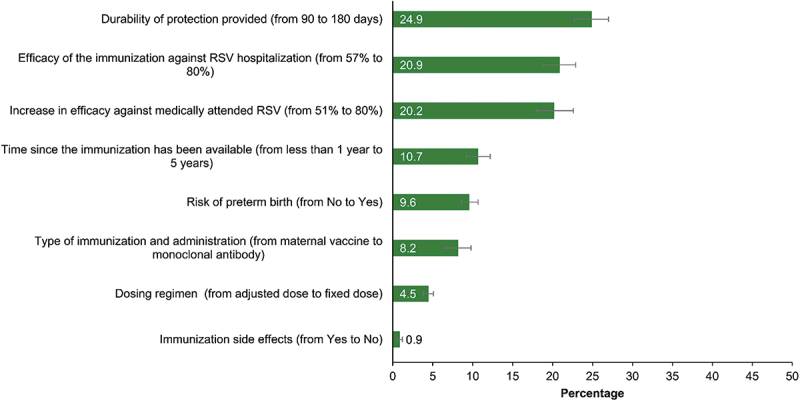
Physicians’ relative importance of RSV immunization strategy attributes.
RSV, respiratory syncytial virus.
Error bars indicate 95% confidence interval.
Discussion
This observational, cross-sectional, web-based survey in the US evaluated physicians’ perceptions about RSV and new RSV immunization strategies, including their knowledge, attitudes, and immunization preferences for mAbs and maternal immunizations. Very few published studies have evaluated HCPs’ attitudes toward RSV2,20 and preferences for RSV immunization strategies.2,21 Considering the recent introduction of novel immunization strategies for RSV infections,7,8 this study adds insights on the importance of a comprehensive set of attributes from efficacy (e.g., efficacy against medically attended RSV and against hospitalization) and safety (e.g., risk of preterm birth) to implementation aspects (e.g., dosing regimen), which were not measured in previous studies conducted in the US.
Physicians reported strong support for recommended childhood immunizations and reported actively engaging with parents of young children to discuss and advocate the importance of immunizations. The well-established high burden of RSV22,23 aligns with physicians’ extensive awareness of the disease. Along with the awareness of the specific risks associated with RSV, the majority of physicians were aware of new RSV immunization strategies under development or those recently approved. In our DCE, the very low preference weight for ‘no immunization’ could be interpreted as a very high acceptability of RSV immunizations by physicians.
The findings from our DCE with respect to the acceptability of any RSV prevention strategy, and the importance of efficacy and durability, are consistent with a recent study of physician preferences by Beusterien et al. They also reported that HCPs strongly preferred a RSV prevention option over no option, and that the effectiveness and duration of protection were the most important attributes for a RSV preventive strategy.21 Given the high burden of RSV disease in infants and the acceptable efficacy and durability demonstrated against RSV endpoints mAb and preF vaccine clinical trials,15,18,24 it is not surprising that the new immunization strategies are widely accepted and recommended by physicians. Furthermore, several real-world studies have demonstrated the effectiveness of nirsevimab25–28 shortly after launch.
In our DCE, however, HCPs preferred the RSV mAb over the maternal immunization, which contrasts with the findings of Beusterien et al., where HCPs preferred maternal immunizations over mAb.21 These differing results could be explained by the types of HCPs included in each study. In our study, obstetrician-gynecologists (OB/GYNs) were not included, while pediatrics was the predominant specialty. Beusterien et al. included an almost even distribution of pediatricians, OB/GYNs, and family medicine doctors. Nevertheless, the type of immunization (RSV mAb vs maternal immunization) was not as important an attribute for HCPs overall, compared to efficacy and durability in both studies. Moreover, in Beusterien et al. study, the type of immunization was not a significantly important attribute even when analyzed by type of patient population treated (that is, HCPs who treat pregnant people only, infants only, or both).
In the current study, physicians were willing to administer a mAb, a newer class of immunization, during routine visits. They more frequently perceived RSV mAbs as providing protection during the entire RSV season compared to the maternal immunization. Additionally, physicians preferred a fixed dosing regimen over a weight-based dosing regimen. The addition of an RSV immunization strategy with robust clinical profiles and lower implementation barriers can be useful in maximizing protection for infants from severe RSV disease.
Our study results collectively suggest a readiness to adopt innovative strategies for RSV prevention, potentially leading to high RSV immunization coverage.
Strengths and limitations
There are several strengths related to our study design. First, our study comprised a geographically diverse sample of physicians recruited from across the US. To ensure construct validity in the survey instrument, qualitative interviews were utilized to identify the motivations and obstacles to prescribing mAb as an immunization strategy for RSV, which informed the development of a quantitative questionnaire and selection of immunization strategy attributes for the DCE.
Our study has certain limitations. The use of convenience sampling methods, a relative small sample size of online physician panels may affect the generalizability of results. Additionally, the 18 physicians who entered the survey but did not complete it, and thus were not captured in our study, could have represented different viewpoints against immunization. While both pediatricians and family practitioners were included in the study, preferences were not elicited from prescribers of maternal immunizations (OB/GYN specialists). While we found that the perceptions between specialty types are generally consistent, the small sample of family practitioners may limit our ability to detect a wide spectrum of their perspectives. Since the study required participants to self-report attitudes and behaviors, results are subject to potential response biases such as social desirability, recall bias, or false reporting (intentional or unintentional).29 The hypothetical immunization strategy profiles and attributes used in the DCE may not reflect all aspects of an immunization strategy which can influence preferences. Lastly, results may not be applicable to other regions with significantly different immunization practices and healthcare systems. Further research, ideally with a larger sample size for better representativeness, will be necessary to confirm and refine our findings.
Conclusion
Our study’s findings showed that physicians supported all types of recommended childhood immunizations, recognized the high clinical burden of RSV among children <2 years of age, and are aware of available and emerging immunization strategies. Physicians reported efficacy and durability to be the most important attributes, and common side-effects as the least important attribute when considering RSV immunization options. Understanding physicians’ attitudes and preferences regarding RSV and RSV immunization strategies is important as new RSV prevention methods become available and are introduced into clinical practice.
Supplementary Material
Acknowledgments
Writing, editorial, and formatting assistance was provided by Janet Oommen, PharmD, and Sudha Korwar, PhD, from Indegene Pvt. Ltd. which was contracted and funded by Oracle Life Sciences.
Biography
Yoonyoung Choi, BPharm, M.S., Ph.D. is a pharmacoepidemiologist and pharmacist in the Department of Outcomes Research at Merck since 2018. She earned her Ph.D. in pharmacoepidemiology from the University of Florida College of Pharmacy. Her research focuses on infectious diseases and vaccines, including natural history of disease, effectiveness analyses, and surveillance studies.
Funding Statement
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure statement
BV, BD, and XG are employed by Oracle Life Sciences, which received funding support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. EB was employed by Oracle Life Sciences at the time of the study.
Author contributions
YC, Conception, Critical review & editing; EB, Conception, Critical review & editing; BV, Conception; BD, Conception, Critical review & editing; BC, Conception, Critical review & editing; TP, Conception, Critical review & editing; XG, Conception, Critical review & editing. All authors have approved the draft and agree to be accountable for all aspects of the work.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645515.2025.2498264
References
- 1.Openshaw PJM, Chiu C, Culley FJ, Johansson C.. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35(1):501–8. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 2.Congedo G, Lombardi GS, Zjalic D, Di Russo M, La Gatta E, Regazzi L, Indolfi G, Staiano A, Cadeddu C. Knowledge, attitudes and behaviours of a sample of Italian paediatricians towards RSV and its preventive strategies: a cross-sectional study. Ital J Pediatr. 2024;50(1):35. doi: 10.1186/s13052-024-01593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 4.Hall C, Weinberg G, Iwane M, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, Bottomley C, Nokes DJ. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176(9):794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Respiratory Syncytial Virus-Associated Mortality (RSV-Associated Mortality). National Notifiable Diseases Surveillance System (NNDSS). [accessed 2024 May 7]. https://ndc.services.cdc.gov/case-definitions/respiratory-syncytial-virus-associated-mortality-2019/.
- 7.FDA Approves New Drug to Prevent RSV in Babies and Toddlers. [accessed 2024 May 13]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers.
- 8.FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. [accessed 2024 Jul 22]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants.
- 9.Healthcare providers: RSV vaccination for pregnant people. CDC Vaccines & immunizations. [accessed 2024 May 7]. https://www.cdc.gov/vaccines/vpd/rsv/hcp/pregnant-people.html.
- 10.Healthcare providers: RSV immunization for infants and young children. CDC Vaccines & immunizations. [accessed 2024 May 13]. https://www.cdc.gov/vaccines/vpd/rsv/hcp/child.html.
- 11.Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices — U.S. CDC. Morbidity and mortality weekly report (MMWR). [accessed 2025 Apr 11]. https://www.cdc.gov/mmwr/volumes/72/wr/mm7241e1.htm?s_cid=mm7241e1_w#print. [DOI] [PMC free article] [PubMed]
- 12.Pringle W, Greyson D, Graham JE, Dubé È, Mitchell H, Russell ML, MacDonald SE, Bettinger JA. “I try to take all the time needed, even if i do not have it!”: knowledge, attitudes, practices of perinatal care providers in Canada about vaccination. Vaccine X. 2024;18:100490. doi: 10.1016/j.jvacx.2024.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarro Alonso J, Bont LJ, Bozzola E, Herting E, Lega F, Mader S, Nunes MC, Ramilo O, Valiotis G, Olivier CW, et al. RSV: perspectives to strengthen the need for protection in all infants. Emerg Themes Epidemiol. 2021;18(1):15. doi: 10.1186/s12982-021-00104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark MD, Determann D, Petrou S, Moro D, De B-GE. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902. doi: 10.1007/s40273-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 15.Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–846. doi: 10.1056/nejmoa2110275. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–425. doi: 10.1056/nejmoa1913556. [DOI] [PubMed] [Google Scholar]
- 17.Simões EAF, Center KJ, Tita ATN, Swanson KA, Radley D, Houghton J, McGrory SB, Gomme E, Anderson M, Roberts JP, et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. N Engl J Med. 2022;386(17):1615–1626. doi: 10.1056/NEJMoa2106062. [DOI] [PubMed] [Google Scholar]
- 18.Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, Baker J, Pérez Marc G, Radley D, Shittu E, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–1464. doi: 10.1056/nejmoa2216480. [DOI] [PubMed] [Google Scholar]
- 19.Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JFP. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Heal. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Riccò M, Ferraro P, Peruzzi S, Zaniboni A, Ranzieri S. Respiratory syncytial virus: knowledge, attitudes and beliefs of general practitioners from North-Eastern Italy (2021). Pediatr Rep. 2022;14(2):147–165. doi: 10.3390/pediatric14020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beusterien KM, Law AW, Maculaitis MC, Will O, Kopenhafer L, Olsen P, Hauber B, Vietri JT, Cappelleri JC, Coulter JR, et al. Healthcare providers’ and pregnant people’s preferences for a preventive to protect infants from serious illness due to respiratory syncytial virus †. Nato Adv Sci Inst Se. 2024;12(5):560. doi: 10.3390/vaccines12050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C LJ, Weinberg G, Edwards K, Edwards KM, Staat MA, Prill MM, Gerber SI, Langley GE. Respiratory syncytial virus– associated outpatient visits among children younger than 24 months. J Pediatr Infect Dis Soc. 2019;8(3):284–286. doi: 10.1093/jpids/piz011. [DOI] [PubMed] [Google Scholar]
- 23.Curns AT, Rha B, Lively JY, Sahni LC, Englund JA, Weinberg GA, Halasa NB, Staat MA, Selvarangan R, Michaels M, et al. Respiratory syncytial virus-associated hospitalizations among children <5 years=”” old: =”” 2016=”” to=””>. Pediatrics. 2024;153(3):e2023062574. doi: 10.1542/peds.2023-062574. [DOI] [PubMed] [Google Scholar]
- 24.Muller W, Madhi S, Nuñez B, Baca Cots M, Bosheva M, Dagan R, Hammitt LL, Llapur CJ, Novoa JM, Saez Llorens X, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med. 2023;388(16):1533–1534. doi: 10.1056/NEJMc2214773. [DOI] [PubMed] [Google Scholar]
- 25.Ares-Gómez S, Mallah N, Santiago-Pérez MI, Pardo-Seco J, Pérez-Martínez O, Otero-Barrós M-T, Suárez-Gaiche N, Kramer R, Jin J, Platero-Alonso L, et al. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study. Lancet Infect Dis. 2024;24(8):817–828. doi: 10.1016/S1473-3099(24)00215-9. [DOI] [PubMed] [Google Scholar]
- 26.Assad Z, Romain AS, Aupiais C, Shum M, Schrimpf C, Lorrot M, Corvol H, Prevost B, Ferrandiz C, Giolito A, et al. Nirsevimab and hospitalization for RSV bronchiolitis. N Engl J Med. 2024;391(2):144–154. doi: 10.1056/nejmoa2314885. [DOI] [PubMed] [Google Scholar]
- 27.Ernst C, Bejko D, Gaasch L, Hannelas E, Kahn I, Pierron C, Del Lero N, Schalbar C, Do Carmo E, Kohnen M, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Eurosurveillance. 2024;29(4):1–5. doi: 10.2807/1560-7917.ES.2024.29.4.2400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moline HL, Tannis A, Toepfer AP, Williams JV, Boom JA, Englund JA, Halasa NB, Staat MA, Weinberg GA, Selvarangan R, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209–214. doi: 10.15585/mmwr.mm7309a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43(1):87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.


