Abstract
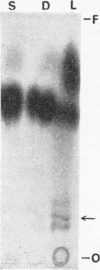
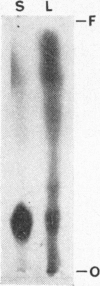
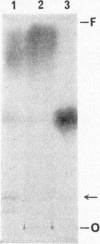
1. When dilute aqueous emulsions of radioactive cholesterol are exposed to daylight, extensive photolysis may take place. This results chiefly in the formation of substances more polar than cholesterol, some of which are probably acidic. Substances less polar than cholesterol are formed to a smaller extent. Photolysis is greatest when the emulsions are strongly acidic or strongly alkaline. 2. Photolysis takes place rapidly if radioactive cholesterol stored in the dry state, either on glass or on filter paper, is exposed to daylight. 3. If precautions are taken to minimize exposure to daylight during analysis and storage of the samples, the amount of non-enzymic alteration of cholesterol in biological experiments may be negligible.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FIESER L. F. Some aspects of the chemistry and biochemistry of cholesterol. Science. 1954 May 21;119(3099):710–716. doi: 10.1126/science.119.3099.710. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S. The conversion of cholesterol-4-C14 to acids and other products by liver mitochondria. J Biol Chem. 1956 Sep;222(1):109–120. [PubMed] [Google Scholar]
- KODICEK E., ASHBY D. R. Paper chromatography of vitamin D and other sterols. Biochem J. 1954 Mar 20;57(ANNUAL):xii–xiii. [PubMed] [Google Scholar]
- WHITEHOUSE M. W., STAPLE E., GURIN S. Catabolism in vitro of cholesterol. I. Oxidation of the terminal methyl groups of cholesterol to carbon dioxide by rat liver preparations. J Biol Chem. 1959 Feb;234(2):276–281. [PubMed] [Google Scholar]