Abstract
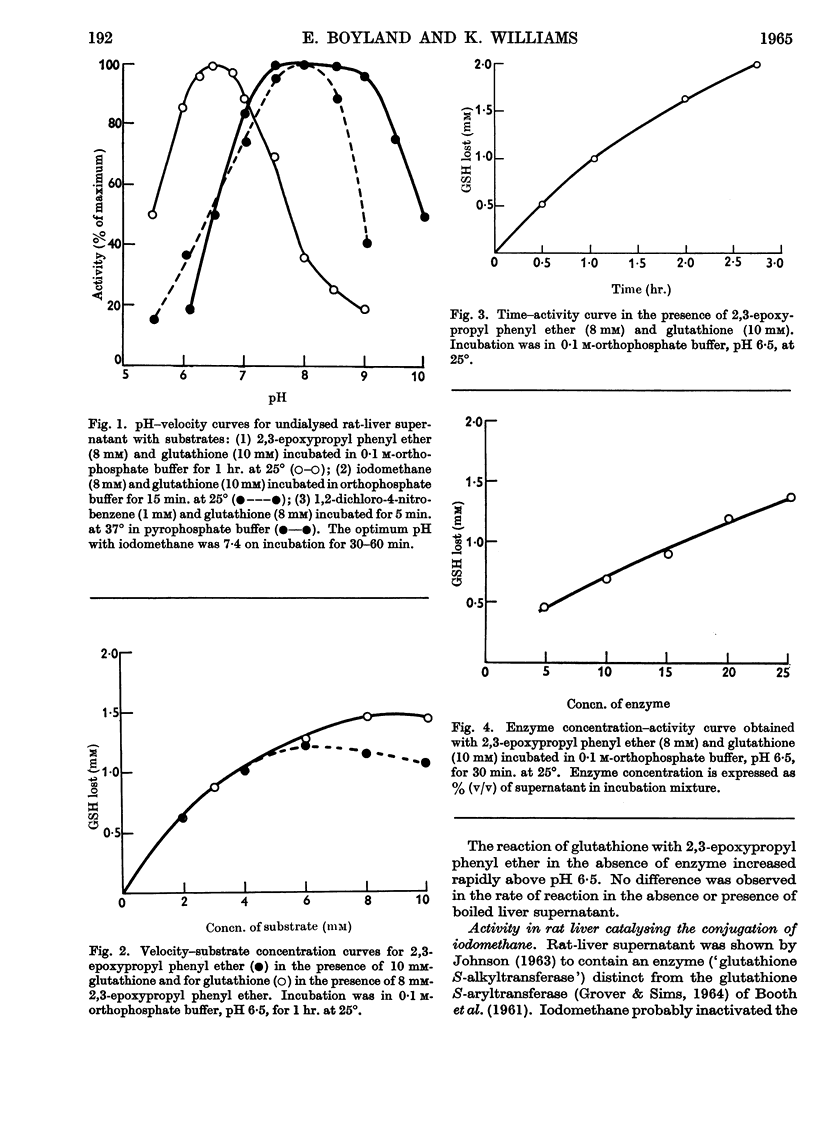
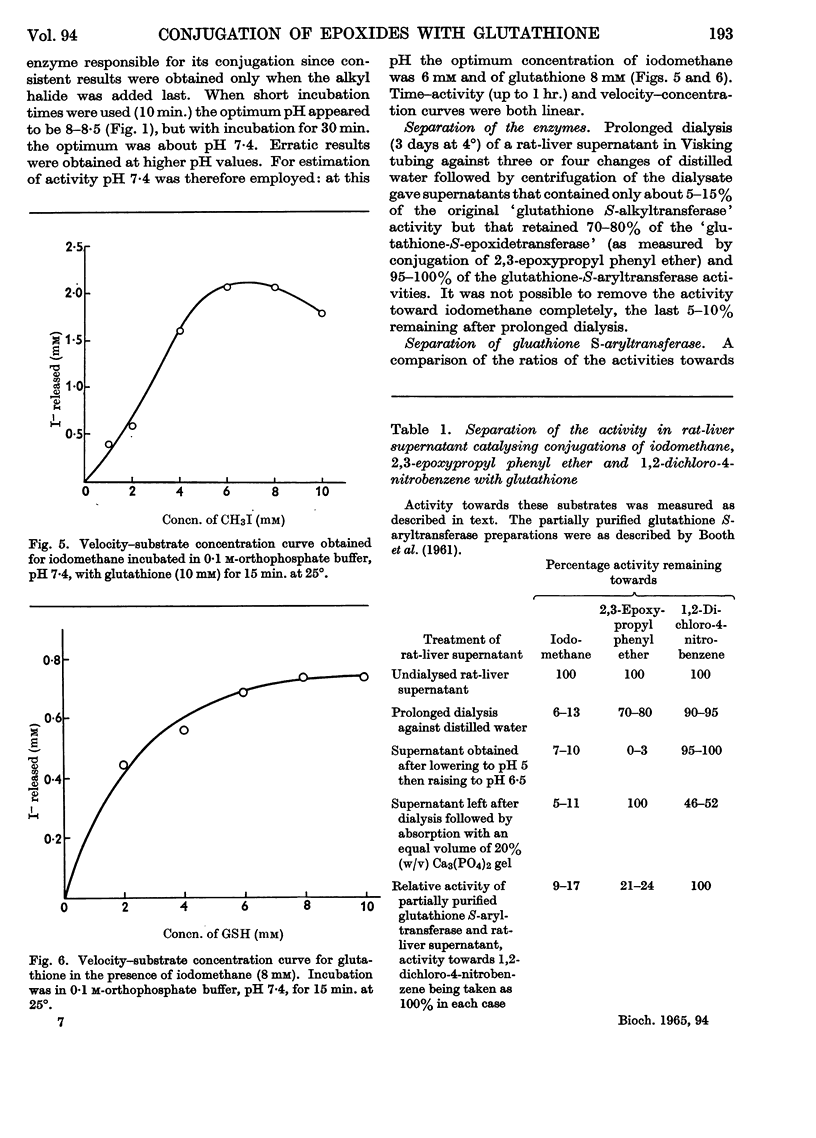
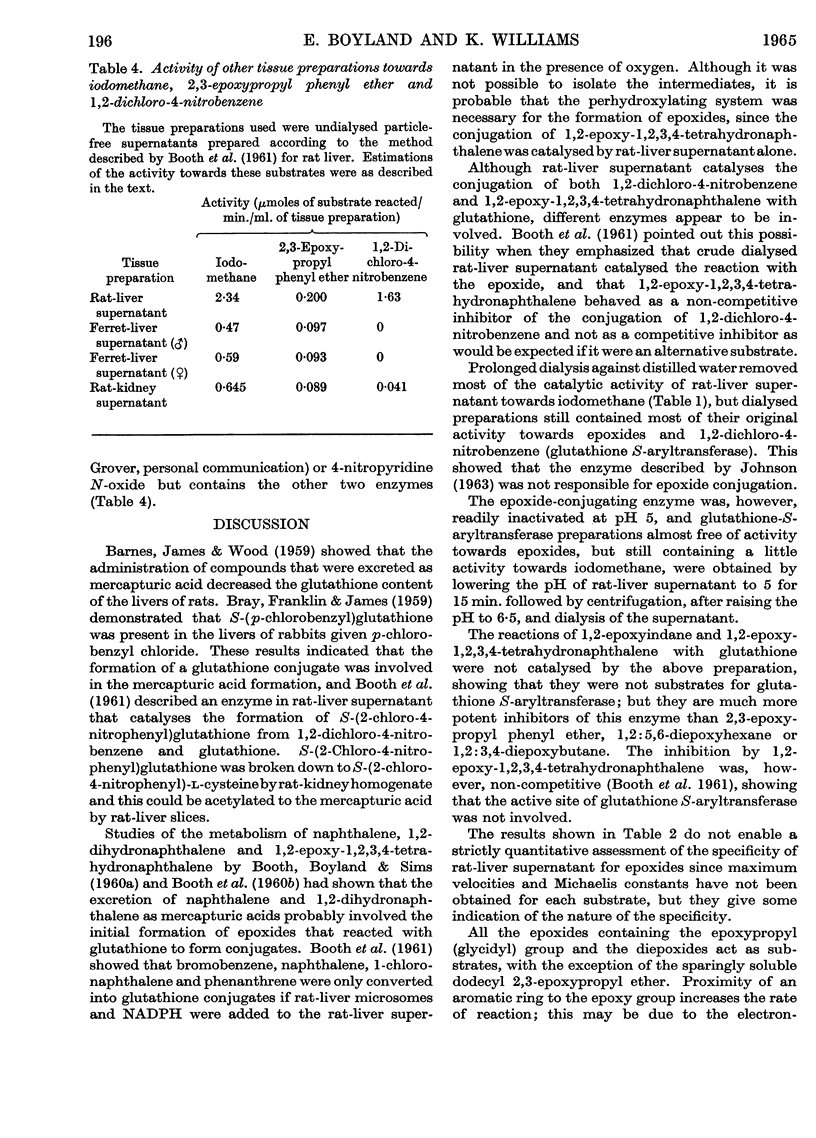
1. Liver supernatant preparations from rats and ferrets catalyse the conjugation of some epoxides with glutathione. The enzyme involved might be called `glutathione S-epoxidetransferase', as it is different from glutathione S-aryltransferase, the enzyme catalysing the conjugation of 1,2-dichloro-4-nitrobenzene, 4-nitro-pyridine N-oxide and other cyclic compounds with glutathione and from the enzyme catalysing the conjugation of iodomethane and glutathione. 2. The enzyme does not catalyse the reaction with cysteine. It is not inactivated by dialysis but is unstable at pH 5·0. 3. The role of the enzyme in metabolism of foreign compounds is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AL-KASSAB S., BOYLAND E., WILLIAMS K. An enzyme from rat liver catalysing conjugations with glutathione. 2. Replacement of nitro groups. Biochem J. 1963 Apr;87:4–9. doi: 10.1042/bj0870004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES M. M., JAMES S. P., WOOD P. B. The formation of mercapturic acids. 1. Formation of mercapturic acid and the levels of glutathione in tissues. Biochem J. 1959 Apr;71(4):680–690. doi: 10.1042/bj0710680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTH J., BOYLAND E., SIMS P. Metabolism of polycyclic compounds. 15. The conversion of naphthalene into a derivative of glutathione by rat-liver slices. Biochem J. 1960 Jan;74:117–122. doi: 10.1042/bj0740117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY H. G., FRANKLIN T. J., JAMES S. P. The formation of mercapturic acids. 2. The possible role of glutathionase. Biochem J. 1959 Apr;71(4):690–696. doi: 10.1042/bj0710690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS C. J., YOUNG L. Biochemical studies of toxic agents. 9. The metabolic conversion of indene into cis- and trans- indane-1: 2-diol. Biochem J. 1956 Jun;63(2):264–269. doi: 10.1042/bj0630264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sato T., Sims P. Metabolism of polycyclic compounds. 17. The reaction of 1:2-dihydronaphthalene and 1:2-epoxy-1:2:3:4-tetrahydronaphthalene with glutathione catalysed by tissue preparations. Biochem J. 1960 Oct;77(1):182–186. doi: 10.1042/bj0770182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R. Effects of a gene mutation in Neurospora crassa relating to glutamic dehydrogenase formation. J Gen Microbiol. 1954 Oct;11(2):236–246. doi: 10.1099/00221287-11-2-236. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Conjugations with glutathione. Distribution of glutathione S-aryltransferase in vertebrate species. Biochem J. 1964 Mar;90(3):603–606. doi: 10.1042/bj0900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. Improvements to the ultraviolet-absorption optical system of an ultracentrifuge. Biochem J. 1963 Apr;87(1):9–11. doi: 10.1042/bj0870009. [DOI] [PMC free article] [PubMed] [Google Scholar]


