Abstract
Summary
Investigating vertebral fractures and brain structure, we found significant gray matter volume reductions in the right hippocampus, amygdala, and parahippocampal gyrus, especially in males. These findings emphasize the importance of integrating skeletal and neural health in osteoporosis management.
Purpose
Vertebral fractures (VF) due to osteoporosis impact morbidity and quality of life in the elderly. The relationship between VF and changes in brain structure remains underexplored. This study aimed to investigate the association between VF and gray matter volume (GMV) reductions in specific brain regions and to explore potential sex differences.
Methods
Data from 1,751 participants (571 males, 1,180 females; mean age 64.9, range 18–97) in the fourth survey of the population-based Research on Osteoarthritis/Osteoporosis Against Disability study (2015–2016) were used. Participants were classified into those with and without VF (VF + and VF − groups) based on Genant’s semiquantitative method, assessed by spine radiographs. Voxel-based morphometry was applied to MRI images to measure GMV, and a general linear model analysis was performed to compare GMV between groups, adjusting for age, sex, total brain volume, and Mini-Mental State Examination scores as covariates. Additionally, a two-way analysis of variance was conducted on the significant GMV cluster, with sex and VF presence as independent variables, to explore interaction effects.
Results
The VF+ group consisted of 113 participants, while the VF− group included 1,638 participants. The analysis identified a significant cluster with reduced GMV in the VF + group compared to the VF − group. This cluster included the right hippocampus, right amygdala, and right parahippocampal gyrus. Further analysis revealed that males in the VF + group exhibited more pronounced GMV reductions in the significant cluster compared to females.
Conclusion
These findings suggest that VF is associated with significant reductions in brain regions critical for memory, emotional processing, and visuospatial memory, with more severe effects observed in males.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-025-07403-6.
Keywords: Brain volume, Gray matter volume, Magnetic resonance imaging, Vertebral fracture, Voxel-based morphometry
Introduction
Vertebral fractures (VF) are a prevalent consequence of osteoporosis, contributing significantly to morbidity and reduced quality of life among the elderly [1–5]. The global increase in life expectancy has led to a rising number of individuals at risk for osteoporosis and associated complications, including VF [6, 7]. These fractures not only cause chronic pain and disability but also increase the likelihood of subsequent fractures, posing a substantial burden on healthcare systems worldwide [1, 8–10]. Compared to other osteoporotic fractures, such as those in the distal radius or proximal femur, vertebral fractures are more likely to go undiagnosed and untreated, which exacerbates their impact on patients’ health outcomes [11].
Emerging research suggests a possible link between skeletal health and brain structure, particularly in the context of aging. Studies have shown that reduced bone mineral density (BMD) is associated with decreased brain volume and poorer cognitive performance [12, 13]. Despite these findings, the specific relationship between VF and changes in brain structure remains underexplored. Understanding this connection is crucial, as it may reveal important insights into the broader impacts of osteoporosis on overall health, including cognitive and emotional functions.
The Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study, initiated between 2005 and 2007, is a prospective cohort study aimed at exploring the genetic and environmental factors influencing bone and joint diseases [14–16]. It examines various risk factors, including clinical features, laboratory and radiographic findings, bone mass and geometry, lifestyle, nutrition, anthropometric measures, and fall propensity. The baseline survey involved 3,040 participants from urban, mountainous, and coastal communities. Subsequently, six follow-up surveys were conducted 3, 7, 10, 13, and 17 years after the baseline survey. In the third follow-up survey, conducted in 2012–2013, brain magnetic resonance imaging (MRI) was introduced in one region of the ROAD study protocol, followed by its introduction in two different regions (mountainous and coastal regions) in the fourth study. This inclusion marked a pivotal step in the investigation of age-related cognitive disorders. Notably, acquiring brain MRI data from the general population, rather than solely from patients, is an invaluable asset in neuroimaging research.
In the present study, we utilized the MRI data obtained from the fourth survey of the population-based ROAD study to investigate the mean brain volume among community residents and the factors influencing it. The primary objective of this study is to investigate the association between VF and gray matter volume (GMV) reductions in the brain and find the clusters which are significantly different due to the presence of VF. Furthermore, we aim to determine whether the presence of VF correlates with significant volume changes in these clusters, and to explore potential sex differences in these associations.
To achieve these objectives, we conducted a cross-sectional study using voxel-based morphometry (VBM) to measure GMV in a cohort of participants with and without VF. We performed a general linear model (GLM) analysis adjusted for potential confounders, including age, sex, TBV, and cognitive function, using mini-mental state examination (MMSE) scores as nuisance regressors. For the clusters identified as significantly different in the GLM analysis, we calculated the cluster volumes for each participant and performed a two-way analysis of variance (ANOVA) with sex and the presence of VF as independent factors to examine the interaction effect between sex and the presence of VF.
Methods
Participants
The ROAD study is a prospective cohort study initiated in 2005 in three communities in Japan: an urban region in Itabashi, Tokyo, a mountainous region in Hidakagawa, Wakayama, and a coastal region in Taiji, Wakayama. Details of this study have been previously described [15, 16]. This cross-sectional observational study was performed using data from the mountainous and coastal regions of the fourth survey of the ROAD study.
The fourth survey of the ROAD study was conducted in 2015–2016. Invitation letters for the fourth survey were distributed to residents whose names had been listed in the previous three ROAD study surveys. This survey included people who were (1) able to walk to the clinic where the survey was conducted, (2) able to provide self-reported data, and (3) able to provide their written informed consent. No exclusion criteria were specified.
Questionnaire, interview, and anthropometric measurements
This study was approved by the Ethics Committees of the University of Tokyo (nos. 1264 and 1326) and the Tokyo Metropolitan Institute of Gerontology (no. 5). Written informed consent was obtained from all participants.
Participants completed a 400-item interviewer-administered questionnaire that included various lifestyle characteristics, such as occupation, smoking habits, alcohol consumption, family history, medical history, physical activity, reproductive variables, and health-related quality of life. Current smokers were defined as those who smoked regardless of the number of pack-years, whereas never-smokers and former smokers were classified as nonsmokers. Current habitual alcohol consumption was defined as habitual alcohol consumption at least once a week regardless of quantity, whereas never-drinkers and former drinkers were classified as nondrinkers. Anthropometric measurements included height and weight, and the body mass index (BMI) was calculated using the following formula: [weight (kg)/height2 (m2)]. Medical information regarding the participants’ systemic, local, and mental statuses was obtained by experienced orthopedists.
Cognitive functioning was measured using the MMSE [17] – a 30-item cognitive screening test that measures orientation, registration, short-term memory, attention, concentration, language, and constructional capacity. The MMSE was primarily administered to participants aged 60 and older. Summary scores from the MMSE were used to measure cognitive functioning. Mild cognitive impairment (MCI) was defined as MMSE scores of 24–27, and dementia was defined as scores ≤ 23 [18, 19].
Radiographic assessment
Stand-up lateral radiographs of the whole spine were obtained for each participant by licensed radiography technicians using a 40-inch film. All radiographs were evaluated for the presence and severity of VF by a spine surgeon (C.H.) using Genant’s semiquantitative (SQ) method [2, 4, 10, 20–22]. The intra-rater and inter-rater reliability were detailed in the previous report [21, 22]. All visible vertebrae from T4 to the most caudal vertebra were assessed for SQ, where each vertebra was graded from 0 to 3 (0, normal; 1, mildly deformed; 2, moderately deformed; 3, severely deformed). Vertebrae with poor visibility or image quality were not graded and were excluded from the analysis. Participants with at least one vertebra with an SQ grade of 2 or higher were defined as VF + , while those with all vertebrae with an SQ grade of 1 or lower were defined as VF − .
Brain MRI acquisition
MRI data were acquired using a Philips Achieva 1.5 T-MRI scanner (Philips Medical Systems, Best, the Netherlands) in a mobile MRI implementation vehicle. T1-weighted structural images with contiguous sagittal slices were obtained using a protocol of magnetization-prepared rapid acquisition with gradient echo (Repetition Time = 9.3 ms, Echo Time = 4.6 ms, flip angle = 10°, field of view = 240 × 240 × 180 mm3, resolution = 0.94 × 0.94 × 1.0 mm3).
Processing and analysis of the brain MRI data
Image processing was performed using Statistical Parametric Mapping 12 (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm) via MATLAB (version 2021b, MathWorks, Sherborn, MA, USA) using the technique of VBM (Fig. 1). The structural images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid images. These images were spatially normalized to the Montreal Neurological Institute (MNI) standard space using diffeomorphic anatomical registration through the exponentiated lie algebra algorithm [23]. They were modulated to correct voxel signal intensity for volume displacement during normalization and reflect brain volume. Modulation ensures that spatially normalized images preserve the total amount of signal from each region. Expanded areas during warping are correspondingly reduced in intensity, while contracted areas are increased in intensity. This adjustment allows the total volume of each tissue type to be conserved across the normalization process, providing a more accurate comparison of local brain volume differences between groups. Moreover, the images were smoothed using an 8-mm full-width half maximum Gaussian kernel. The voxel size in the normalized images was 1.5 × 1.5 × 1.5 mm3. The total brain volume (TBV) was calculated as the sum of the absolute volume of the GM (GMV) and volume of the WM (WMV). Participants were excluded from the study if at least one of their TBV, GMV, or WMV was out of the range [median – 2.0 × interquartile value, median + 2.0 × interquartile value].
Fig. 1.
Steps of image preprocessing. MNI; Montreal Neurological Institute
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 28.0 (IBM Corporation, Armonk, NY, USA). Demographic data were compared between the VF − and VF + groups. The Student’s t-test was used to compare continuous variables, whereas Pearson’s chi-square test was used to compare categorical variables. A general linear model (GLM) was used to explore brain regions in which GMVs in the VF + group were less than those in the VF − group using SPM12. To exclude the effects of individual brain size, age, and sex, we treated TBV, age, and sex as nuisance regressors in the GLM (p < 0.001 for cluster identification without correction, p < 0.05 for cluster level significance with family-wise error (FWE) correction). Moreover, MMSE scores were also treated as nuisance regressors in the GLM along with age, sex, and TBV to elucidate the effect of cognitive function. ‘Expected voxels per cluster’ calculated by SPM12 was used as the cluster size threshold in the GLM. We calculated the volume for each individual for the significant clusters from the GLM analysis. We conducted a two-way ANOVA with sex and the presence of VF as independent variables. The cluster volumes were normalized by dividing by each individual's TBV to account for the influence of brain size. We examined the main effects of sex and the presence of VF, as well as their interaction.
Results
Characteristics of the participants
Of the 2,146 participants in the fourth study (the baseline population), 1,762 had brain MRI data, and 7 of them were excluded due to abnormal TBV, GMV, or WMV. Of the 1,755 participants with brain MRIs, 4 did not have whole-spine radiographs. Thus, 1,751 participants (81.6% of baseline participants) (571 males and 1,180 females) were analyzed in this study. The VF+ group consisted of 113 participants, while the VF− group included 1,638 participants. The mean age of the VF + group was significantly higher than that of the VF − group (78.1 vs. 64.0 years, p < 0.001) (Table 1). Additionally, the VF + group had a higher proportion of females compared to the VF − group (83.2% vs. 66.3%, p < 0.001). The MMSE was conducted for 1,285 participants (73.4% of 1,751), of whom 1,171 (91.1%) were aged 60 years or older. The mean MMSE score (± standard deviation) was 29.1 ± 1.6 for the VF − group and 28.3 ± 2.1 for the VF + group, which was significantly different (p < 0.001). MCI was observed in 155 participants (13.4%) in the VF − group and 31 participants (28.7%) in the VF + group (p < 0.001). Dementia was identified in 15 participants (1.3%) in the VF − group and 4 participants (3.6%) in the VF + group (p = 0.055).
Table 1.
Baseline characteristics of the participants
| VF- | VF + | p | |||||
|---|---|---|---|---|---|---|---|
| N | 1638 | 113 | |||||
| Age strata (years) | |||||||
| –39 | 58 | 3.5% | 0 | 0.0% | |||
| 40–49 | 168 | 10.3% | 1 | 0.9% | |||
| 50–59 | 312 | 19.0% | 0 | 0.0% | |||
| 60–69 | 537 | 32.8% | 14 | 12.4% | |||
| 70–79 | 399 | 24.4% | 46 | 40.7% | |||
| 80– | 164 | 10.0% | 52 | 46.0% | |||
| Age | 64.0 | (12.5) | 78.1 | (8.4) | < 0.001 | *** | |
| Sex | |||||||
| Male | 552 | 33.7% | 19 | 16.8% | < 0.001 | *** | |
| Female | 1086 | 66.3% | 94 | 83.2% | |||
| Height (cm) | 157.8 | (9.1) | 149.2 | (8.8) | < 0.001 | *** | |
| Weight (kg) | 57.0 | (11.6) | 50.3 | (9.2) | < 0.001 | *** | |
| BMI | 22.8 | (3.5) | 22.6 | (3.4) | 0.562 | ||
| Smoking | 155 | 9.5% | 5 | 4.4% | 0.072 | ||
| Alcohol | 720 | 44.0% | 24 | 21.2% | < 0.001 | *** | |
| MMSE (N) | 1173 | 112 | |||||
| Mean score | 29.1 | (1.6) | 28.3 | (2.1) | < 0.001 | *** | |
| MCI | 155 | 13.4% | 31 | 28.7% | < 0.001 | *** | |
| Dementia | 15 | 1.3% | 4 | 3.6% | 0.055 | ||
The number in the parentheses indicates the standard deviation. *** p-value < 0.001
VF; vertebral fracture, BMI; body mass index, MMSE; mini-mental state examination, MCI; mild cognitive impairment. MCI defined as MMSE scores of 24–27, dementia as scores ≤ 23
VBM and GLM analysis
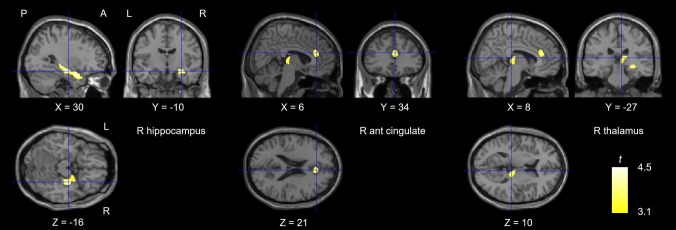
VBM was conducted using SPM12. The mean TBV was 1059.4 ± 119.4 cm3 for males and 965.4 ± 98.8 cm3 for females. The expected voxels per cluster was 105, which was used as the cluster size threshold. GLM analysis with TBV and sex as nuisance regressors revealed that GMVs in the VF + group were less than those in the VF − group in the right hippocampus (t = 4.99 at the peak voxel), left hippocampus (t = 4.08), right fusiform gyrus (t = 3.84), right inferior occipital gyrus (t = 3.81), right anterior cingulate (t = 3.77), and left amygdala (t = 3.56) (Table S1 and Fig. S1). We also conducted GLM analysis with MMSE scores as nuisance regressors along with TBV and sex. For participants who did not undergo MMSE, the total mean MMSE score of 29.0086 was used. This analysis revealed that GMVs in the VF + group were less than those in the VF − group in the right hippocampus (t = 4.43), right anterior cingulate (t = 3.89), and left amygdala (t = 3.70) (Table 2 and Fig. 2). The cluster with the right hippocampus showed significantly lower volumes in the VF + group compared to the VF − group (MNI coordinates: x = 30, y = − 10, and z = − 16; cluster size = 1821 voxels; p-FWE = 0.002). This cluster consisted of the right hippocampus (40%), right amygdala (18%), right parahippocampal gyrus (18%), and right superior temporal pole (16%) (Fig. 3).
Table 2.
Clusters with reduced GMV in the VF + group compared to the VF − group
| Region | MNI coordinates of peak | Peak t value | Cluster | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | Size | p-FWE | |||
| R hippocampus | 30 | −10 | −16 | 4.43 | 1821 | 0.002 | ** |
| R ant cingulate | 6 | 34 | 21 | 3.89 | 352 | 0.318 | |
| R thalamus | 8 | −27 | 10 | 3.70 | 440 | 0.220 | |
Nuisance regressors are age, sex, and mini-mental state examination score. Coordinates x, y, and z represent the local maximum in a cluster. Only regions with at least 105 voxels are listed. ** p-value < 0.01
GMV; gray matter volume, VF; vertebral fracture, MNI; Montreal Neurological Institute, p-FWE; p-value after family-wise error correction, R; right, ant; anterior
Fig. 2.
Brain area with reduced GMV in the VF + group compared to the VF − group. A significant cluster was observed in the right hippocampus. Nuisance regressors are age, sex, and mini-mental state examination score. GMV; gray matter volume, VF; vertebral fracture, P; posterior, A; anterior, L; left, R; right, ant; anterior
Fig. 3.

Brain regions included in the significant cluster involving the right hippocampus. The cluster mainly belongs to the limbic system. R; right, sup; superior
Two-way ANOVA for the significant cluster
The mean volume of the cluster was 2.75 ± 0.40 cm3 for males and 2.54 ± 0.33 cm3 for females. A two-way ANOVA was performed to analyze the effects of sex and the presence of VF on the cluster volume normalized by TBV. The analysis identified significant main effects for sex (F(1,1747) = 23.755, p < 0.001, η2 = 0.013), and the presence of VF (F(1,1747) = 94.664, p < 0.001, η2 = 0.051). Additionally, there was a significant interaction effect between sex and the presence of VF (F(1,1747) = 11.090, p < 0.001, η2 = 0.006). Figure 4 illustrates the interaction effect, showing that the reduction in cluster volume in the VF + group is greater in males than females.
Fig. 4.

Results of the two-way ANOVA for the significant cluster. The results showed that the reduction in cluster volume in the VF + group is greater in males than females. Sex and the presence of VF were used as independent variables. The cluster volumes for each participant were adjusted by dividing by the total brain volume (TBV). Error bars represent standard errors. M; male, F; female, VF; vertebral fracture, ANOVA; analysis of variance
To further validate these findings, a similar two-way ANOVA was conducted without normalizing the cluster volume by TBV, using TBV as a weighting variable in a weighted least squares analysis. The results were consistent with the previous analysis, identifying significant main effects for sex (F(1,1747) = 6.309, p < 0.012, η2 = 0.004), and the presence of VF (F(1,1747) = 103.806, p < 0.001, η2 = 0.056). The interaction effect between sex and the presence of VF also remained significant (F(1,1747) = 5.148, p = 0.023, η2 = 0.003) (Fig. S2).
Discussion
Our study found that the VF + group exhibited significantly lower GMV in a specific brain cluster compared to the VF − group. This cluster included the right hippocampus, right amygdala, right parahippocampal gyrus, and right superior temporal pole. Additionally, a significant interaction effect between sex and the presence of VF was observed for the reduction in GMV in this specific cluster.
Few studies have suggested a link between VF and brain atrophy [24]. Bae et al. investigated the relationship between osteoporotic vertebral compression fractures (OVCFs) and brain volume using MRI. They included 246 osteoporotic patients and analyzed their brain volume using semi-automated tools. They found a significant association between OVCFs and reduced brain parenchyma volume, alongside increased lateral ventricle volume. In line with their findings, our study also identified significant reductions in GMV in a specific brain cluster, including the right hippocampus, right amygdala, right parahippocampal gyrus, and right superior temporal pole in the VF + group. Both studies highlight the impact of vertebral fractures on brain structure, suggesting that these fractures may contribute to neurodegeneration. While Bae et al. focused on the overall brain parenchyma and lateral ventricles, our study provides a more detailed regional analysis, pinpointing specific areas of gray matter reduction. This complementary evidence reinforces the notion that vertebral fractures are not only a skeletal concern but also have significant implications for brain health, particularly in regions critical for cognitive and emotional processing.
There were some reports about the relationship between brain structure and BMD [12, 13]. Loskutova et al. examined BMD in early Alzheimer's disease (AD) and its relationship to brain structure and cognition [12]. They found that BMD was lower in patients with early AD compared to non-demented controls and that lower BMD was associated with reduced whole brain volume and poorer cognitive performance, particularly in memory tasks. Zhang et al. conducted a mediation analysis investigating the associations among BMD, brain atrophy, and gait variability (the stride-to-stride fluctuations in walking) [13]. They found that lower BMD, particularly in the lumbar spine, was associated with higher gait variability, and that brain atrophy in regions such as the primary motor cortex and sensorimotor cortex mediated this relationship. These studies highlight the systemic nature of bone loss and its association with global brain atrophy. However, VF differ from BMD loss alone, as they may reflect specific factors beyond systemic bone metabolism.
In our study, the observed GMV reductions in the VF + group were localized to the hippocampus, amygdala, and parahippocampal gyrus, regions known to be functionally specialized for memory, emotional processing, and visuospatial cognition. Unlike systemic BMD loss, which has been hypothesized to be associated with global brain volume reductions, the observed localization of GMV loss aligns with the concept that brain regions exhibit functional specialization. This specific association suggests that vertebral fractures may reflect not only systemic metabolic bone loss but also meaningful processes such as chronic pain, reduced physical activity, or functional mobility limitations caused by the fractures. These physical and functional consequences could be related to localized brain structure changes, highlighting the distinct nature of VF beyond being a simple surrogate marker for BMD.These studies indicate a broader connection between bone health and brain structure, extending beyond specific conditions like VF. The integration of these findings highlights the importance of considering both skeletal and neural health in managing conditions such as osteoporosis and neurodegenerative diseases. In our study, we found a significant association between the reduction in specific brain clusters and the presence of VF, even after adjusting for MMSE scores. This suggests that the reduction in brain regions responsible for cognition may be associated with VF; however, due to the cross-sectional design of this study, we cannot determine causality. It is equally possible that VF could lead to reduced mobility and physical activity, which in turn impacts brain structure by decreasing environmental interactions and stimulation.
The cluster identified in our study includes brain regions such as the right hippocampus, right amygdala, and right parahippocampal gyrus, which are crucial for memory and emotional processing. The limbic system, comprising the hippocampus and amygdala, is integral to cognitive and emotional functions [25, 26]. The hippocampus is essential for memory formation and spatial navigation [27, 28]. Age-related changes in the hippocampus are linked to declines in memory and increased susceptibility to psychosocial stress and mental health issues [29]. The amygdala is involved in emotional processing and memory, with age-related changes affecting emotional regulation [30, 31]. The parahippocampal gyrus, part of the medial temporal lobe memory system, is crucial in maintaining and updating memories [32]. It is involved in visuospatial processing, particularly in extracting scene layout information [33], and is essential for visuospatial memory functions and contextual associations [34–36]. Bohbot et al. showed that patients with lesions that included the right parahippocampal cortex were severely impaired on a task that required learning the spatial configuration of objects on a computer screen; these patients, however, were not impaired at learning the identity of objects [35]. The association between VF and reductions in the parahippocampal gyrus volume may suggest a bidirectional relationship. VF can lead to decreased mobility and physical activity, potentially contributing to decreased visuospatial memory function due to reduced environmental interaction. Conversely, impaired visuospatial memory function might increase the risk of falls and subsequent VF. Although this remains a hypothesis, the relationship suggests that interventions aimed at improving both physical mobility and cognitive functions, particularly visuospatial memory, could benefit individuals at risk of VF.
Our study also revealed a significant interaction between sex and VF status, showing that males in the VF + group exhibited more pronounced reductions in the specific brain cluster compared to females in the VF + group. This novel finding suggests that while postmenopausal women may experience more significant bone density loss relative to brain volume reduction due to the effects of osteoporosis, men may experience concurrent reductions in both brain volume and bone density. This indicates that the neurodegenerative and osteoporotic processes might occur simultaneously in men. Therefore, the integrated approach to patient care should consider these sex-specific differences. For females, particularly postmenopausal women, interventions might need to prioritize bone health to mitigate extensive bone density loss, whereas for males, strategies should focus on both maintaining bone health and preventing brain atrophy. This personalized approach could potentially improve patient outcomes by addressing both skeletal and neural aspects of health.
One of the strengths of our study is the use of VBM, which allows for precise measurement of gray matter volumes across the entire brain. Additionally, the large sample size of 1,751 participants enhances the generalizability of our findings. The inclusion of both male and female participants and the examination of sex-specific effects add further depth to our analysis. Furthermore, the adjustment for cognitive function using MMSE scores strengthens the validity of our findings.
However, there are several limitations in the present study. First, the participants were recruited from only two regions (coastal and mountainous), which may limit the generalizability of our findings to the broader population. However, we compared anthropometric measurements and the prevalence of smoking and alcohol consumption between our participants and the general Japanese population and found minimal significant differences [15, 37]. This suggests that our study sample may be somewhat representative of the general population. Second, the cross-sectional design of this study makes it challenging to establish causal relationships between vertebral fractures and brain volume reductions. Longitudinal studies are generally required to confirm causality. Given the long-term follow-ups involved in our study, we plan to analyze the results from longitudinal surveys in future research to better understand these relationships. Third, this study focused on simple brain volume comparisons and did not include advanced imaging techniques such as diffusion-weighted tractography or functional MRI analyses. Given that MRI scans were performed as part of health check-ups for 1,755 local residents, it was impractical to conduct more detailed MRI investigations due to cost and human resource constraints. Fourth, when we limited the analysis to participants aged 60 years and older (n = 1,212), statistical significance in some results was lost, likely due to the reduced sample size in this subgroup. Even with age included as a nuisance regressor in the analysis, the results may still have been influenced by the presence of younger participants in the full dataset. Fifth, only 1,285 participants (66.9%) completed the MMSE, primarily because the MMSE was administered mainly to participants aged 60 and over, which limits the comprehensiveness of our cognitive assessments. Sixth, there were several bone-related limitations: we lacked bone density information, which could provide further insights into the relationship between bone health and brain structure; we did not have information on whether the vertebral fractures were new or old, or whether they were traumatic or non-traumatic (pathological fractures); and information on the use of osteoporosis medications (such as antiresorptive agents, anabolic agents, vitamin D supplements, etc.) was also not available. This lack of detailed fracture and treatment information might affect the interpretation of our results. Despite these limitations, our study provides important insights into the relationship between vertebral fractures and brain structure, highlighting the need for integrated approaches to managing skeletal and neural health.
Conclusion
In conclusion, our study demonstrates a significant association between VF and reductions in specific brain regions, particularly in the right hippocampus, right amygdala, and right parahippocampal gyrus. These findings suggest that VF may have broader implications for brain health, affecting areas crucial for memory and emotional processing. The observed interaction between sex and VF status, with more pronounced brain volume reductions in males, indicates that neurodegenerative and osteoporotic processes may occur simultaneously in men. These results underscore the importance of early detection and intervention targeting both skeletal and neural health to mitigate cognitive decline and reduce the risk of VF. Future research should continue to explore these relationships through longitudinal studies and advanced imaging techniques to develop comprehensive strategies for managing osteoporosis and associated neurodegenerative conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Dr. Naoki Hirabayashi of Kawakami Clinic, Hidakagawa Town; Mrs. Tomoko Takijiri, and other members of the public office in Hidakagawa Town; and Mrs. Tamako Tsutsumi, Mr. Soichiro Yoshimura, Mrs. Kanami Maeda, and other members of the public office in Taiji Town for their assistance in locating and scheduling the participants for examinations. We would also like to thank Mrs. Kyoko Hattori, Mrs. Saeko Sahara, and Mr. Noriyuki Oe for their assistance with data reduction and administration.
Funding
Open Access funding provided by The University of Tokyo. This work was supported by a Grant-in-Aid funding from the Ministry of Health, Labour and Welfare: H25-Choujyu-007 (Director, Noriko Yoshimura), and H25-Nanchitou (Men)-005 (Director, Sakae Tanaka), 19FA1401 (Director, Sakae Tanaka), 19FA0701 (Director, Hiroyuki Oka), 19FA1901 (Director, Estuo Chosa), 24FA1003 (Director, Sakae Tanaka), and 24FA0601 (Director, Noriko Yoshimura). The study was also supported by Scientific Research grants B19H03895, and B26293139, and Challenging Exploratory Research grants 21K19631, and 18K18447 to Noriko Yoshimura; Challenging Exploratory Research grants 21K18291 to Kanae Mure, Scientific Research grants B26293331, and Challenging Exploratory Research grants 26670307 to Shigeyuki Muraki; Scientific Research grants B26293329, and Challenging Exploratory Research grant 25670293 to Toru Akune; Scientific Research grant 19H05654 to Sakae Tanaka; and by Collaborating Research with NSF from the Ministry of Education, Culture, Sports, Science and Technology in Japan 08033011–00262 (Director, Noriko Yoshimura). The study was partly supported by grants from the Japan Agency for Medical Research and Development (17dk0110028h0001, Director, Noriko Yoshimura; 17gk0210007h0003, and 19gk0210018h0002, 22gk0210034h0001, 23gk0210034h0002, 24gk0210034h003, Director, Sakae Tanaka; 22dk0110047h0001, 23dk0110047h0002, 24dk0110047h0003, Director, Kanae Mure; 22dk0110048h0001, 23dk0110048h0002, 24dk0110048y0003, Director, Hiroyuki Oka). Further, the study was partly supported by grants from the Japan Osteoporosis Society (Noriko Yoshimura, Shigeyuki Muraki, Hiroyuki Oka, and Toru Akune) and Japan Osteoporosis Foundation (2015, Noriko Yoshimura) and research aids from the Japanese Orthopaedic Association (JOA-Subsidized Science Project Research 2014–1, Director, Kozo Nakamura), the Japanese Society for Musculoskeletal Medicine (2015, Director, Shigeyuki Muraki; and 2017, Director, Noriko Yoshimura), Mitsui Sumitomo Insurance Welfare Foundation (2016, Director, Noriko Yoshimura; 2024, Director, Toshiko Iidaka), Japan Dairy Association (2017, Director, Noriko Yoshimura), and Suzuken Memorial Foundation (2023, Director, Noriko Yoshimura).
Data availability
Data will be made available upon reasonable request.
Declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papaioannou A, Watts NB, Kendler DL, Yuen CK, Adachi JD, Ferko N (2002) Diagnosis and management of vertebral fractures in elderly adults. Am J Med 113:220–228 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen HT, Nguyen BT, Thai THN, Tran AV, Nguyen TT, Vo T, Mai LD, Tran TS, Nguyen TV, Ho-Pham LT (2024) Prevalence, incidence of and risk factors for vertebral fracture in the community: the Vietnam Osteoporosis Study. Sci Rep 14:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson WA, Carlson BC, Poppendeck H et al (2020) Osteoporosis-related Vertebral Fragility Fractures: A Review and Analysis of the American Orthopaedic Association’s Own the Bone Database. Spine (Phila Pa 1976) 45:E430–E438 [DOI] [PubMed] [Google Scholar]
- 4.Rostom S, Allali F, Bennani L, Abouqal R, Hajjaj-Hassouni N (2012) The prevalence of vertebral fractures and health-related quality of life in postmenopausal women. Rheumatol Int 32:971–980 [DOI] [PubMed] [Google Scholar]
- 5.Old JL, Calvert M (2004) Vertebral compression fractures in the elderly. Am Fam Physician 69:111–116 [PubMed] [Google Scholar]
- 6.Salari N, Darvishi N, Bartina Y, Larti M, Kiaei A, Hemmati M, Shohaimi S, Mohammadi M (2021) Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg Res 16:669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayer SR, Mears SC, Pangle AK, Mendiratta P, Wei JY, Azhar G (2021) Does Superior Bone Health Promote a Longer Lifespan? Geriatr Orthop Surg Rehabil 12:21514593211036230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Huang X, Wu J et al (2022) The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front Endocrinol (Lausanne) 13:882241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohar A, Getzler I, Behrbalk E (2023) Higher Mortality Rate in Patients with Vertebral Compression Fractures is due to Deteriorated Medical Status Prior to the Fracture Event. Geriatr Orthop Surg Rehabil 14:21514593231153104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes JB, Fung LK, Cha CC, Gabriel GM, Takayama L, Figueiredo CP, Pereira RM (2012) The impact of asymptomatic vertebral fractures on quality of life in older community-dwelling women: the Sao Paulo Ageing & Health Study. Clinics (Sao Paulo) 67:1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller PD (2016) Underdiagnosis and Undertreatment of Osteoporosis: The Battle to Be Won. J Clin Endocrinol Metab 101:852–859 [DOI] [PubMed] [Google Scholar]
- 12.Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM (2009) Bone density and brain atrophy in early Alzheimer’s disease. J Alzheimers Dis 18:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Lu H, Fan M et al (2024) Bidirectional mediation of bone mineral density and brain atrophy on their associations with gait variability. Sci Rep 14:8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura N, Muraki S, Nakamura K, Tanaka S (2017) Epidemiology of the locomotive syndrome: The research on osteoarthritis/osteoporosis against disability study 2005–2015. Mod Rheumatol 27:1–7 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura N, Muraki S, Oka H et al (2009) Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 27:620–628 [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T (2010) Cohort profile: research on Osteoarthritis/Osteoporosis Against Disability study. Int J Epidemiol 39:988–995 [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- 18.Rakusa M, Granda G, Kogoj A, Mlakar J, Vodusek DB (2006) Mini-Mental State Examination: standardization and validation for the elderly Slovenian population. Eur J Neurol 13:141–145 [DOI] [PubMed] [Google Scholar]
- 19.Ciesielska N, Sokolowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kedziora-Kornatowska K (2016) Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol 50:1039–1052 [DOI] [PubMed] [Google Scholar]
- 20.Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148 [DOI] [PubMed] [Google Scholar]
- 21.Horii C, Iidaka T, Muraki S et al (2022) The cumulative incidence of and risk factors for morphometric severe vertebral fractures in Japanese men and women: the ROAD study third and fourth surveys. Osteoporos Int 33:889–899 [DOI] [PubMed] [Google Scholar]
- 22.Horii C, Asai Y, Iidaka T et al (2019) Differences in prevalence and associated factors between mild and severe vertebral fractures in Japanese men and women: the third survey of the ROAD study. J Bone Miner Metab 37:844–853 [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113 [DOI] [PubMed] [Google Scholar]
- 24.Bae IS, Kim JM, Cheong JH, Han MH, Ryu JI (2019) Association between cerebral atrophy and osteoporotic vertebral compression fractures. PLoS ONE 14:e0224439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajmohan V, Mohandas E (2007) The limbic system. Indian J Psychiatry 49:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaacson RL (2013) A perspective for the interpretation of limbic system function. Physiol Psychol 8:183–188 [Google Scholar]
- 27.Bird CM, Burgess N (2008) The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–194 [DOI] [PubMed] [Google Scholar]
- 28.Voss JL, Bridge DJ, Cohen NJ, Walker JA (2017) A Closer Look at the Hippocampus and Memory. Trends Cogn Sci 21:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spindler M, Thiel CM (2023) Hypothalamic microstructure and function are related to body mass, but not mental or cognitive abilities across the adult lifespan. Geroscience 45:277–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao T, Zhang S, Lee LE, Chao HH, van Dyck C, Li CR (2018) Exploring Age-Related Changes in Resting State Functional Connectivity of the Amygdala: From Young to Middle Adulthood. Front Aging Neurosci 10:209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Jacques PL, Dolcos F, Cabeza R (2009) Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci 20:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger JI, Billig AJ, Sedley W, Kumar S, Griffiths TD, Gander PE (2024) What is the role of the hippocampus and parahippocampal gyrus in the persistence of tinnitus? Hum Brain Mapp 45:e26627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann O, Mattingley JB (2016) Functional Organization of the Parahippocampal Cortex: Dissociable Roles for Context Representations and the Perception of Visual Scenes. J Neurosci 36:2536–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Wang Z, Du Z et al (2020) Impaired Parahippocampal Gyrus-Orbitofrontal Cortex Circuit Associated with Visuospatial Memory Deficit as a Potential Biomarker and Interventional Approach for Alzheimer Disease. Neurosci Bull 36:831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohbot VD, Allen JJ, Dagher A, Dumoulin SO, Evans AC, Petrides M, Kalina M, Stepankova K, Nadel L (2015) Role of the parahippocampal cortex in memory for the configuration but not the identity of objects: converging evidence from patients with selective thermal lesions and fMRI. Front Hum Neurosci 9:431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aminoff EM, Kveraga K, Bar M (2013) The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17:379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura N, Iidaka T, Horii C et al (2022) Epidemiology of locomotive syndrome using updated clinical decision limits: 6-year follow-ups of the ROAD study. J Bone Miner Metab 40:623–635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.