Abstract
Background
Recent studies have revealed that CD8+ T cells can be activated via genetic upregulation of HIF-1α, thereby augmenting antitumor effector functions. HIF-1α upregulation can be attained by inhibiting HIF-prolyl hydroxylase (HIF-PH) under normoxic conditions, termed pseudohypoxia. This study investigated whether pseudohypoxia induced by HIF-PH inhibitors suppresses Microsatellite stable (MSS) colorectal cancer (CRC) by affecting tumor immune response.
Methods
The HIF-PH inhibitors Roxadustat and Vadadustat were utilized in this study. In vitro, we assessed the effects of HIF-PH inhibitors on human and murine colon cancer cell lines (SW480, HT29, Colon26) and murine T cells. In vivo experiments were performed with mice bearing Colon26 tumors to evaluate the effect of these inhibitors on tumor immune responses. Tumor and spleen samples were analyzed using immunohistochemistry, RT-qPCR, and flow cytometry to elucidate potential mechanisms.
Results
HIF-PH inhibitors demonstrated antitumor effects in vivo but not in vitro. These inhibitors enhanced the tumor immune response by increasing the infiltration of CD8+ and CD4+ tumor-infiltrating lymphocytes (TILs). HIF-PH inhibitors induced IL-2 production in splenic and intratumoral CD4+ T cells, promoting T cell proliferation, differentiation, and immune responses. Roxadustat synergistically enhanced the efficacy of anti-PD-1 antibody for MSS cancer by increasing the recruitment of TILs and augmenting effector-like CD8+ T cells.
Conclusion
Pseudohypoxia induced by HIF-PH inhibitors activates antitumor immune responses, at least in part, through the induction of IL-2 secretion from CD4+ T cells in the spleen and tumor microenvironment, thereby enhancing immune efficacy against MSS CRC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-025-04067-3.
Keywords: Colorectal cancer, Microsatellite stable, Hypoxia-inducible factor, Immune checkpoint inhibitors
Introduction
Colorectal cancer (CRC) stands as a prevalent ailment and ranks as the second leading cause of cancer-related mortality worldwide [1]. Immunotherapy emerges as a significant advancement in CRC treatment [2]. Mismatch repair (MMR) deficient tumors accrue numerous somatic mutations, including indels in microsatellite repeats, resulting in a distinct molecular phenotype termed microsatellite instability (MSI). While patients with MSI-high CRC exhibit favorable responses to immunotherapy, including immune checkpoint inhibitors (ICIs), those with microsatellite stable (MSS) CRC, constituting the majority, are unresponsive to ICIs [3]. Therefore, there is a pressing need for novel therapeutic approaches to enhance immunotherapy for MSS CRC patients.
Hypoxia-inducible factor (HIF) serves as a vital transcription factor, playing a crucial role in the body's adaptation to low oxygen levels, known as hypoxia. HIF upregulation promotes angiogenesis to counteract ischemic conditions [4]. In the oncologic setting, hypoxia is a defining feature of the tumor microenvironment (TME) and a critical driver of cancer hallmarks. Hypoxia modulates both immune and non-immune components within the TME, facilitating immune evasion and therapy resistance [5]. Severe hypoxia is a principal contributor to resistance against PD-1/PD-L1 inhibitor therapies. HIF-1 under hypoxic condition induces a state of terminal exhaustion in CD8+ T cells, characterized by the expression of CD39, a marker of this exhausted phenotype. Furthermore, hypoxia stimulates the secretion of vascular endothelial growth factor (VEGF) from terminally exhausted CD8+ T cells, promoting their differentiation into a fully exhausted state [5]. CD8+ T cells exposed to 1% oxygen (hypoxia) have been shown to exhibit enhanced maturation, survival, and cytotoxicity against cancer cells [6]. However, the expression dynamics of HIF-1α under normoxia remain elusive. Recent studies have demonstrated that upregulating HIF-1α in CD8+ T cells through VHL knockdown enhances cytotoxic function and suppresses tumor growth [7]. HIF expression is tightly regulated through hydroxylation by HIF-prolyl hydroxylase (HIF-PH) in response to hypoxia, with iron serving as a catalyst [8]. Inhibition of HIF-PH activity by iron chelators are expected to boost tumor immune response by upregulating HIF in CD8+ T cells, akin to VHL knockdown. However, HIF-PH inhibitors carry the risk of increasing HIF expression in cancer cells, thereby promoting tumor growth with enhancing angiogenesis [9]. It remains uncertain whether HIF-PH inhibitors with iron-chelating activity suppress tumor growth by activating tumor immune responses.
HIF-PH inhibitors such as Roxadustat and Vadadustat, used in the treatment of anemia related to chronic kidney disease (CKD) by enhancing HIF expression targeting the EPO gene under normoxia [10, 11]. Under conditions of HIF-PH inhibition, HIF expression increases even in the presence of normal oxygen levels, a state referred to as pseudohypoxia. This study investigates whether pseudohypoxia induced by HIF-PH inhibitors suppresses tumor growth by activating tumor immune responses and elucidates the underlying mechanisms.
Materials and methods
All experiments involving animals were conducted according to the ethical policies and procedures approved by The Animal Care and Use Committee of Okayama University (Approved No. OKU-2021900, OKU- 2022723, OKU- 2022879, OKU- 2023535). Details about materials and methods are provided in Supplemental Information.
Results
HIF-PH inhibitors exhibited anti-tumor effects in vivo, but not in vitro
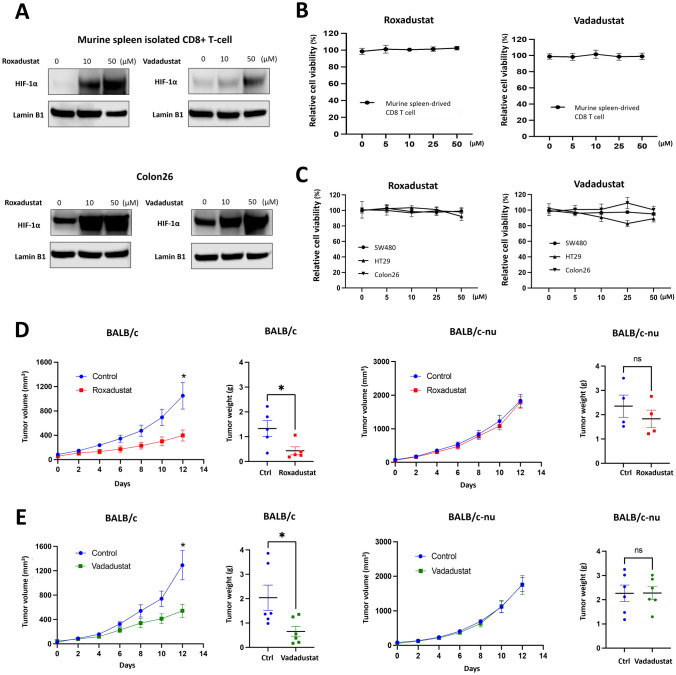
To verify the HIF-1α upregulation by HIF-PH inhibitors, the expression of HIF-1α in CD8+ T cells and colon cancer cells was evaluated following treatment with Roxadustat and Vadadustat, both of which possess iron-chelating properties. Roxadustat exhibited stronger iron chelating ability (Fig. S1A-B) and induced greater HIF-1α expression compared to Vadadustat (Fig. 1A and S1C). Importantly, neither Roxadustat nor Vadadustat affected the proliferation of CD8 + T cells or MSS colon cancer model cells, including human SW480 and HT29 cells and the murine Colon26 cells (Fig. 1 B-C and S1D) [12, 13]. To examine the in vivo effects of HIF-PH inhibitors, we employed the subcutaneous Colon26 tumor transplantation model. Roxadustat notably suppressed the tumor growth in immunocompetent BALB/c mice, but this effect was abrogated in BALB/c nude mice (Fig. 1D). The BALB/c nude mice, characterized by a pivotal mutation in the Foxn1 gene, exhibit defective thymus development and a consequent lack of mature T cells [14]. Vadadustat yielded similar outcome (Fig. 1E). Since HIF-PH inhibitors do not directly affect cancer cell proliferation, these findings imply that observed tumor growth suppression is mediated by immune responses.
Fig. 1.
HIF-PH inhibitors activate immune system to suppress the tumor. A Murine spleen-derived CD8+ T cells and Colon26 were treated with different HIF-PH inhibitors for 48 h. HIF-1α was measured using western blotting. B, C Murine spleen-derived CD8+ T cells and MSS colon cancer cell lines (SW480, HT29, Colon26) were treated with different concentrations of HIF-PH inhibitors for 48 h. Cell viability was assessed by XTT assay. D, E 7 days post Colon26 injection, BALB/c and BALB/c-nude mice were randomized into two groups: (i) Control; (ii) Roxadustat/Vadadustat; PBS and Roxadustat/Vadadustat were administered via oral route (Roxadustat: 50 mg/kg. every other day. Vadadustat: 150 mg/kg. every day.). Tumor volumes and weights of BALB/c and BALB/c-nude mice were measured at the indicated day under HIF-PH inhibitors treatment (Roxadustat’s n = 5, Vadadustat’s n = 6). The results are presented as the means ± S.E.M. of a representative experiment performed in triplicate. Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
HIF-PH inhibitors enhanced tumor immune response by increasing TIL infiltration
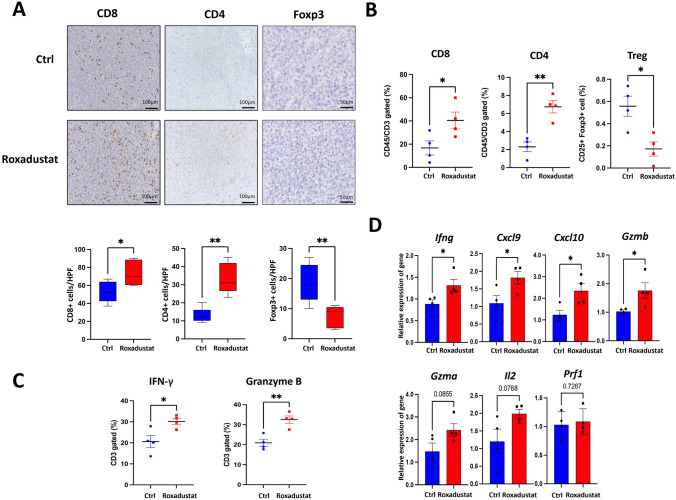
To analyze immune cell populations in the TME, collected tumors were analyzed by immunohistochemistry (IHC) and flow cytometry. Roxadustat treatment significantly increased both the number and percentage of CD8+ and CD4+ tumor-infiltrated lymphocytes (TILs) (Fig. 2A-B). The treatment also decreased the number and percentage of Foxp3+ regulatory T cells (Tregs). A previous study suggested HIF-1α could attenuate Treg cell differentiation by binding to and accelerating the degradation of Foxp3 protein, and our results are consistent with the report [15]. Similar outcomes were observed with Vadadustat (Fig. S2A). The number of Foxp3+ cells in the control group determined by IHC was slightly higher than that of CD4+cells, likely due to the heterogeneous distribution of Foxp3⁺ cells within the tumor tissue [16, 17]. Additionally, the number of T-bet-positive cells, a characteristic marker of CD4⁺ Th1 cells, was increased in the Roxadustat group, suggesting that Roxadustat also promotes the expansion of immune-enhancing CD4⁺ T cell subsets (Fig. S2B). Furthermore, flow cytometry revealed increased percentages of IFN-γ+ and Granzyme B+ CD3+ T cells in the Roxadustat group (Fig. 2C). Reverse transcription quantitative PCR (RT-qPCR) analysis of tumor tissues unveiled a significant upregulation of several immune-related genes, including Ifng, Gzmb, Cxcl9 and Cxcl10 (Fig. 2D). Furthermore, peripheral blood analysis of mice showed an increased number of white blood cells (WBC), supporting the enhanced TILs infiltration (Table S3). These results suggest that HIF-PH inhibitors promote the recruitment and activation of TILs through increased production of chemokine and cytokine, thereby enhancing antitumor immune responses.
Fig. 2.
HIF-PH inhibitors enhance T cell efficacy, promote CD4+ and CD8+ T cell infiltration, and reduce regulatory T cell populations. A Representative images and positive cell number of CD8+, CD4+ and Foxp3+ cells stained from Colon26 tumor tissues of control and Roxadustat treatment group mice. The number of positive cells was manually counted from five selected high-power fields (HPFs) per tumor. (n = 5; scale bar,100 and 50 μm; quantitative data, blow). B, C Frequencies of the indicated cells (n = 4) for TME collected from tumor-bearing mice. D The mRNA levels of indicated markers were measured by RT-qPCR. Total mRNA was extracted from Colon26 tumor tissues (n = 4). The results are presented as the means ± S.E.M. of a representative experiment performed in triplicate. Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
HIF-PH inhibitors induced IL-2 production in splenic CD4+T cell, promoting T cell proliferation, differentiation, and immune response
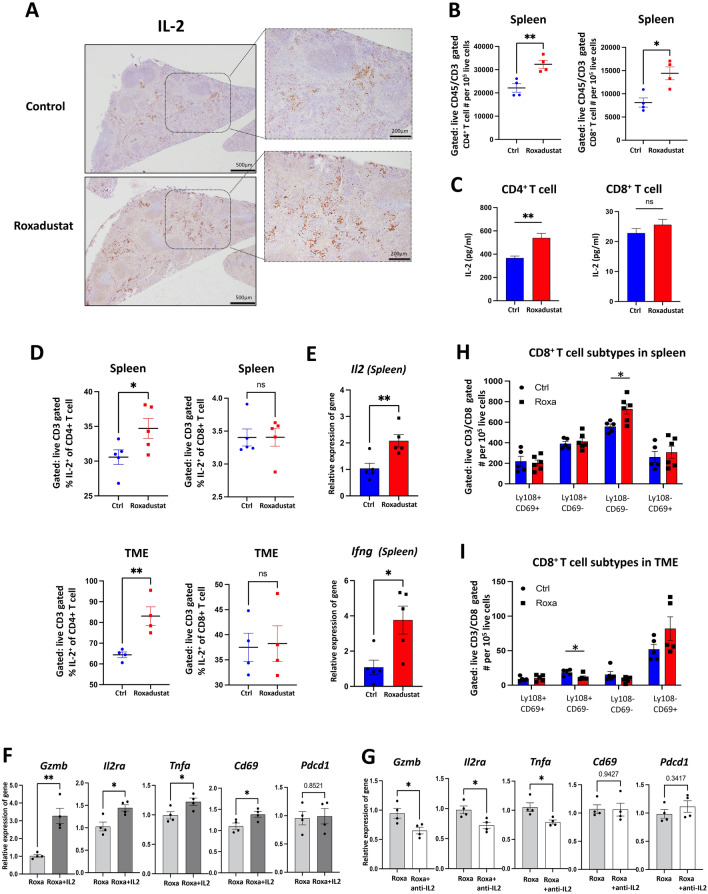
As described above, RT-qPCR analyses of tumor tissues and splenic CD4+ T cells revealed an upward trend in IL-2 expression, prompting further investigation into IL-2 expression within the lymphatic system (Fig. 2D and S3A). IL-2, a key T cell growth factor, promotes not only T cell proliferation but also the differentiation of CD8+ T cells into more potent cytotoxic subtypes [18]. Based on the RT-qPCR results, spleens were collected, and the expression of IL-2 was examined by IHC, RT-qPCR and flow cytometry. Roxadustat treatment enhanced IL-2 staining intensity and notably increased the number of CD4+ and CD8+ T cells in the spleens (Fig. 3A-B). To identify the cellular source of IL-2 in the spleen, CD4⁺ and CD8⁺ T cells isolated from murine spleens were analyzed for IL-2 secretion by ELISA in vitro. Both CD4+ and CD8+ T cells were examined due to reports that IL-2 can also be secreted by CD8+ T cells, although the primary source is CD4+ T cells [19]. ELISA results confirmed that Roxadustat significantly increased IL-2 secretion from isolated splenic CD4+ T cells (Fig. 3C). To validate these in vitro results, intracellular IL-2 expression in CD4⁺ and CD8⁺ T cells from the spleen and tumor was analyzed by flow cytometry. Roxadustat treatment increased the percentage of IL-2⁺ CD4⁺ T cells, but not CD8⁺ T cells, in both the spleen and TME (Fig. 3D). Consistently, RT-qPCR analysis of spleens revealed upregulated expression of Il2 and Ifng following Roxadustat treatment (Fig. 3E). Similar results were observed with Vadadustat (Fig. S3B-C). These findings suggest that HIF-PH inhibitors promote IL-2 production specifically in CD4⁺ T cells.
Fig. 3.
HIF-PH inhibitors elevate IL-2 levels in CD4+ T cell to boost T cell proliferation and CD8+ T cell activation. A Representative images of IL-2 protein in tumor-bearing mice spleen of control and Roxadustat group mice (scale bar, 500 μm; magnification scale bar, 200 μm). B Numbers of the indicated cells (n = 4) for spleen collected from tumor-bearing mice. C Concentrations of IL-2 from indicated murine spleen-derived cells stimulated with 5 μg/ml ConA and treated with 25 μM Roxadustat for 48 h. D IL-2 frequencies of the indicated cells (n = 4) for spleen and TME collected from tumor-bearing mice. E The mRNA levels of indicated markers were measured by RT-qPCR. Total mRNA was extracted from spleen of tumor-bearing mice (n = 5). F Isolated CD8⁺ T cells were stimulated with 5 μg/ml ConA and treated with 5 μM Roxadustat in the presence or absence of 50 ng/ml recombinant IL-2 for 12 h. mRNA expression levels were analyzed by RT-qPCR. G Isolated CD8⁺ T cells were cultured for 12 h in conditioned media (supernatants) collected from CD4⁺ T cells pre-treated with 5 μg/ml ConA and 5 μM Roxadustat, in the presence or absence of 2.5 μg/ml anti-IL-2 antibody. mRNA expression levels were analyzed by RT-qPCR. H, I Numbers of different CD8+ T cell subtypes (n = 5) for spleen and TME collected from tumor-bearing mice. The results are presented as the means ± S.E.M. of a representative experiment performed in triplicate. Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
We next explored the functional relevance of IL-2 in modulating CD8⁺ T cell activation through IL-2 supplementation and neutralization assays. RT-qPCR analyses revealed that IL-2 supplementation upregulated the expression of activation markers such as Cd69, Tnfa, and Il2ra (the IL-2 receptor alpha chain), along with an elevated level of the cytotoxic effector molecule granzyme B (Gzmb). In contrast, expression of Pdcd1 (encoding PD-1), an exhaustion marker, remained unchanged (Fig. 3F). These findings suggest that IL-2 derived from activated CD4+ T cells has the capacity to promote CD8+ T cell activation. Conversely, IL-2 blockade attenuated the expression of these activation-related genes, while Cd69 and Pdcd1 levels remained relatively unaffected (Fig. 3G). These findings suggest that IL-2 secreted by CD4+ T cells under HIF-PH treatment is a key driver of CD8+ T cell activation, although other activation signals may also contribute.
Moreover, IL-2 secretion may support the enhancement of tumor immune responses. To assess CD8⁺ T cell phenotypes, flow cytometry was performed on cells from the spleen and tumor (Fig. 3H-I). CD8+ T cells negative for Ly108 and CD69, markers of effector-like transitory CD8+ T cells [20, 21], were significantly increased in spleen treated by Roxadustat (Fig. 3H). Additionally, the proportion of Ly108+ CD69− CD8⁺ T cells, markers of stem-like progenitor CD8+ T cells, was significantly decreased (Fig. 3I). This suggests that Roxadustat facilitates the accumulation of effector-like CD8+ T cells in the spleen and promotes the differentiation of stem-like CD8+ T cells, thereby potentially priming subsequent antitumor immune responses. Furthermore, the percentage of Ly108− and CD69+ T cells, which are characteristic of exhausted CD8⁺ T cells that still retain significant cytotoxic potential [20], was also high in the tumor. This phenotypic shift in both the spleen and TME indicates effective tumor targeting by cytotoxic CD8⁺ T cells, potentially supported by IL-2 secretion from CD4⁺ T cells in both compartments. Collectively, these results suggest that HIF-PH inhibitor induces IL-2 derived from CD4⁺ T cells to enhance antitumor immunity by promoting the functional activation and differentiation of cytotoxic CD8⁺ T cells.
Roxadustat enhances the efficacy of Anti-PD-1 antibody for MSS cancer with increasing TILs
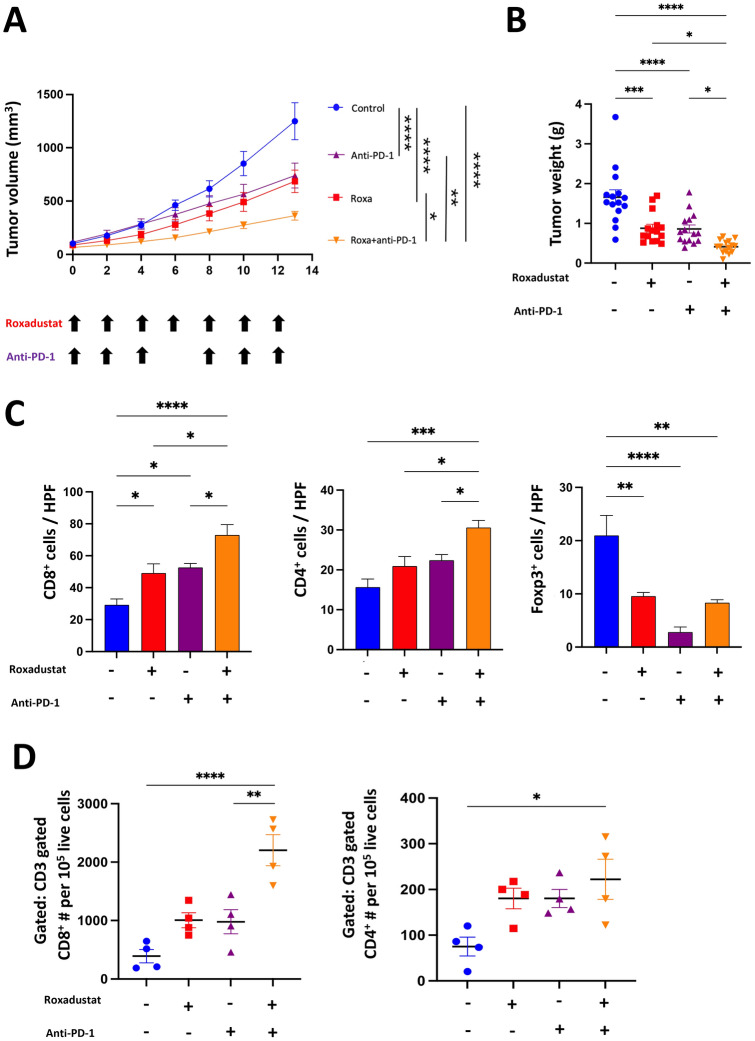
In clinical scenarios, MSS tumors are often characterized by a dearth of mutant neoantigens, resulting in insufficient immune responses. These tumors have demonstrated a diminished response to immune check point inhibitors (ICIs), as evidenced by shorter progression-free survival (PFS) and overall survival (OS) [22, 23]. Considering the results of our in vivo studies, HIF-PH inhibitors were expected to enhance antitumor immune responses and improve the efficacy of ICIs. We therefore evaluated the combination therapy of Roxadustat and anti-PD-1 antibody in a subcutaneous Colon26 tumor model. The combination therapy significantly suppressed the tumor growth compared with either monotherapy (Fig. 4A-B). The number of CD8+ and CD4+ TILs was significantly increased in the combination group (Fig. 4C-D and S4A). The number of Foxp3-positive cells in the combination group was similar to that in the Roxadustat monotherapy group (Fig. 4C). These results indicate that Roxadustat enhances the efficacy of anti-PD-1 therapy in MSS tumors by increasing the infiltration of TILs.
Fig. 4.
Roxadustat and anti-PD-1 combination therapy enhances MSS tumor suppression and increase TILs. A–D 7 days after colon 26 inoculation, BALB/c mice were randomized into four groups: (i) Control; (ii) Roxadustat; (iii) aPD1; (iv) Roxadustat + aPD-1. Roxadustat (50 mg/kg) was administered orally, and aPD-1(7 mg/kg) intraperitoneally. (A-B) Tumor growth and weight of Colon26 tumors in BALB/c mice treated with PBS, Roxadustat, aPD-1, or Roxadustat + aPD-1. (n = 15/ group pooled from three experiments). C IHC was used to analyze the staining of CD8+, CD4+ and Foxp3+ cells for TME collected from tumor-bearing mice (n = 5). The number of positive cells was counted manually. D Numbers of CD4+ and CD8+ T cells (n = 5) for TME collected from tumor-bearing mice were measured by flow cytometry. The results are presented as the means ± S.E.M. of a representative experiment performed in triplicate. ANOVA followed by Tukey’s multiple comparison test was applied. *P < 0.05, **P < 0.01, ***P < 0.001
Synergistic increase of effector-like CD8+T cell population by Roxadustat combined with anti-PD-1 therapy
To elucidate the mechanism of enhanced antitumor effects obtained by the combination therapy, detailed T cell phenotype in the tumor and spleen was analyzed. The number of CD8+ T cells expressing Ly108, indicative of stem-like CD8+ T cells [21], was significantly reduced in the Roxadustat and combination group (Fig. 5A). Additionally, the number of CD8+ T cells expressing Tim3 as well as Ly108− PD1low and Tim3+ PD1low, a representative marker of effector-like CD8+ T cells [21, 24], was markedly increased in the combination group (Fig. 5B and S5A-B). Furthermore, the mean fluorescence intensity (MFI) of the exhaustion marker PD-1 on CD8⁺ T cells was reduced in all treatment groups, with the most pronounced decrease observed in the combination group, suggesting enhanced cytotoxic potential of CD8⁺ T cells following combination therapy (Fig. 5C). These findings suggested that the combination therapy induced both the proliferation and phenotypic transition of CD8+ T cells from a stem-like to an effector-like state within the TME. To verify the underlying mechanism, increased IL-2 staining intensity was confirmed by IHC in the combination therapy group compared to anti-PD-1 monotherapy group (Fig. 5D and S5C). In parallel, the numbers of CD8+ and CD4+ T cells were also increased in the spleen (Fig. 5E).
Fig. 5.
Synergistic increase of effector-like CD8+ T-cell population by Roxadustat combined with anti-PD-1 therapy. 14 days post treatment initiation, the indicated tissues from tumor-bearing BALB/c mice were examined by flow cytometry (n = 4/group). A, B Numbers of the indicated subtypes for CD8+ T cells collected from TME of tumor-bearing mice. C PD-1 MFI of CD8+ T cells collected from TME of tumor-bearing mice. D Representative images of IL-2 protein in tumor-bearing mice spleen of control and Roxadustat group mice (scale bar, 500 μm; magnification scale bar, 200 μm). E Numbers of the indicated T cells collected from spleen of tumor-bearing mice. F Kaplan–Meier shows progression-free survival of patients with MSS colon cancer classified by stable MSI and the level of HIF-1 expression: high (red) and low (black), measured with Affymetrix arrays (IDs: 220946_s_at). Data were from GSE143985 in GEO database. The results are presented as the means ± S.E.M. of a representative experiment performed in triplicate. ANOVA followed by Tukey’s multiple comparison test was applied. *P < 0.05, **P < 0.01, ***P < 0.001
In a clinical context, database analysis revealed that high HIF-1 expression in MSS cancer patients correlated with improved prognosis compared to low expression, further corroborating our findings (Fig. 5F). Together, these results suggest that the combination therapy of Roxadustat and anti-PD-1 antibody promotes the induction of effector-like CD8+ T cells within the tumor, a process supported by Roxadustat-induced increased IL-2 production. This outcome is attributed to both the induction of effector-like CD8+ T cells by anti-PD-1 antibody and the differentiation of CD8+ T cells facilitated by Roxadustat.
Discussion
Our discussion highlights that pseudohypoxia, induced by HIF-PH inhibitors, leads to an increase in HIF-1α levels in both immune and tumor cells under normoxic conditions. Although concerns were raised that elevated HIF-1α levels might facilitate tumor growth via VEGF upregulation, the activation of the tumor immune response mitigated this risk, resulting in tumor growth inhibition. HIF-1α is recognized as a promoter of tumor progression, and substantial efforts have been directed towards developing HIF inhibitors as therapeutic agents due to their upstream regulation of VEGF. However, most of these attempts have been unsuccessful [25]. There is compelling evidence supporting our hypothesis in both immune and cancer cells. The expression of HIF-1α is regulated by Von Hippel-Lindau (VHL) and factor inhibiting HIF (FIH). Both VHL and FIH modulate cellular responses to oxygen levels, adapting to hypoxia: their knockdown increases HIF-1α expression. CD8+ T cells with high HIF-1α expression due to VHLKO or FIHKO were reported to enhance the effector response to persistent antigen [26, 27]. Additionally, FIHKO in cancer cells, particularly lung cancer, was shown to suppress tumor growth in an orthotopic lung tumor model by increasing angiomotin (Amot), a key component of the Hippo tumor suppressor pathway [28]. Conversely, HIF-1α depletion was reported to disrupt T cell receptor activation and antitumor T cell function, providing opposing evidence [29, 30].
In the spleen, we observed a significant increase in IL-2 level. IL-2 is a well-established immune-activating cytokine, particularly in T cell-mediated immunity [31]. IL-2 promotes the proliferation and differentiation of T cells, crucial for an effective immune response [32, 33]. However, IL-2 supplemental therapies, such as IL-2 analogues, were reported to increase Treg populations [34, 35]. Interestingly, HIF-1α impedes Treg differentiation by binding to and hastening the degradation of the Foxp3 protein, which contribute to increased TILs [15]. Indeed, IL-2 induction by HIF-PH inhibitors appears superior in reducing Treg level compared to other IL-2 therapies. These findings support the notion that HIF-1α upregulation therapy, including HIF-PH inhibitors, exerts significant antitumor effects, corroborating our observations. While previous studies focused on HIF regulation through genetic manipulation in immune or cancer cells, our study demonstrate that HIF-PH inhibitors can enhance antitumor responses without such manipulation.
Our findings revealed that CD8+ T cell activation is mediated, at least in part, by CD4⁺ T cell-derived IL-2. Anti-PD-1 therapy was reported to provide a significant proliferation of stem-like CD8+T cells [36]. Additionally, our results showed a notable increase in effector-like CD8+ T cells in the spleen and decrease of stem-like CD8+ T cells in the tumor, which indicates HIF-PH inhibitors could promote CD8+ T cell differentiation. This shift indicates that activated CD8+ T cells effectively attacked cancer cells in the TME, which was supported by an increased number and activation of CD4+ and CD8+ T cells in the spleen. Collectively, pseudohypoxia induced by HIF-PH inhibitors enhances the tumor immune response via IL-2 induction by CD4+ T cells in the spleen and TME. Moreover, recent studies have provided additional insights into the functional characteristics of these subsets. Ly108⁻ TIM3⁺ terminal CD8⁺ T cells produce significantly higher levels of IFN-γ, TNF, and Granzyme B compared to Ly108⁺ TIM3⁻ stem-like progenitor CD8⁺ T cells [24]. Similarly, TIM3⁺Ly108⁻ terminally dysfunctional CD8⁺ T cells also exhibit high Granzyme B expression [37]. Based on the insights, a dual-marker analysis revealed a significant rise in effector-like transitory CD8+ T cells, characterized as PD-1low and TIM3+ or PD-1low and Ly108− within the combination therapy group (Fig. S5A-B).
Iron chelation is pivotal in increasing HIF-1α expression for antitumor immune responses as shown here and also by inhibiting the progression of cancer cell malignancy [38]. Iron is essential for both normal and cancer cell proliferation. Iron chelators have been reported to suppress the proliferation and function of various cancer cells, particularly cancer stem cells (CSCs) [39, 40]. While iron chelation or depletion generally does not enhance cancer cell proliferation and function, its effect on tumor immune responses remains unclear. This study provides new insights indicating that pseudohypoxia induced by iron chelators can activate the tumor immune response, suggesting potential pharmaceutical targets. Our new evidence is expected to contribute to establishing pseudohypoxia induced by iron chelation as a novel approach to cancer immunotherapy.
Regarding drug selection, dosage, and clinical applicability, careful consideration is required. Although the dosages administered to mice were selected based on prior studies, the clinically relevant dosage in humans is substantially lower [41–45]. Despite efforts to reduce the dosage, the outcomes were insufficient (Fig. S6A). The approved dosages of Roxadustat and Vadadustat for humans are significantly lower compared to those used in mice [46, 47]. The safety of HIF-PH inhibitors for MSS CRC patients remains unclear, despite Roxadustat and Vadadustat being clinically available for renal anemia patients. Moreover, the duration for which HIF-1α expression is sustained within the tumor remains unclear. Western blot and IHC analyses indicated that HIF-1α expression was markedly elevated in the once-daily Vadadustat group but not significantly in the once-every-two-days Roxadustat group (Fig. S6B-C). This difference may be attributed to the intrinsic instability and rapid degradation of HIF-1α, suggesting the possibility that long-acting iron chelators may robustly induce HIF-1α in tumors, thereby exerting a tumor-suppressive effect. Furthermore, in our animal experiments conducted at the tested concentrations, no adverse mortality cases were observed. Thus, this study suggests a possibility that HIF-PH inhibitors have the potential, in principle, to activate antitumor immunity.
The TME comprises various cell types and substances, and there are significant considerations regarding hypoxia and pseudohypoxia. For instance, hypoxic extracellular vesicles (EVs) play a central role in cancer progression by driving M2 macrophage polarization, expanding myeloid-derived suppressor cells (MDSCs), inducing epithelial-to-mesenchymal transition (EMT), facilitating angiogenesis, and enhancing cancer cell proliferation [5]. Hypoxic EVs are reported to impede antitumor immunity, thereby fostering cancer progression [48]. Tumor-derived microvesicles promote the expansion of regulatory T cells and induce apoptosis in tumor-reactive activated CD8+ T cells, and is a key promoter of distant metastasis [49, 50]. EVs are well-suited for targeted drug delivery, which may affect ICI responses, and loading them with therapeutic agents can enhance immunotherapy efficacy [51]. Additionally, this study did not assess the effects of IL-2 or its receptor blockade, nor did it examine CD4+ T cell depletion using in vivo tumor models. Future studies are required to evaluate the systemic and local effects of HIF-PH inhibitors and IL-2 induction on tumor-associated immune cells, including macrophages and fibroblasts [52, 53].
In conclusion, our study demonstrates that HIF-PH inhibitors can be useful to activate the antitumor immune response, especially in combination with ICIs, for the treatment of MSS CRC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Shiho Komaki and Haruyuki Watanabe for their kind technical assistance with the pathological experiments.
Abbreviations
- CKD
Chronic kidney disease
- CRC
Colorectal cancer
- CSCs
Cancer stem cells
- FIH
Factor inhibiting HIF
- GZMB
Granzyme B
- HIF
Hypoxia-inducible factor
- HIF-PH
HIF-prolyl hydroxylase
- HPF
High-power field
- ICIs
Immune checkpoint inhibitors
- IFN-γ
Interferon-gamma
- MFI
Mean fluorescence intensity
- MMR
Mismatch repair
- MSI
Microsatellite instability
- MSS
Microsatellite stable
- OS
Overall survival
- PFS
Progression-free survival
- TILs
Tumor-infiltrating lymphocytes
- TME
Tumor microenvironment
- VHL
Von Hippel-Lindau
- WBC
White blood cell
Author contributions
Conceptualization: YC, TO. Data generation and analysis: YC, TO, YH. Writing/reviewing: YC, TO, YY, AM. Technical or material support: MT, YH, YW. Supervision: TO, HT, KN, MF, YY, AM. Funding acquisition: YC, TO. TO and AM are responsible for the overall content as the guarantors.
Funding
Open Access funding provided by Okayama University. This work was supported by JSPS KAKENHI Grant Numbers JP22K08712 (to TO), The Sanyo Broadcasting Foundation (to TO) and China Scholarship Council (to YC).
Data availability
Figure 5F Kaplan–Meier shows progression-free survival of patients with MSS colon cancer classified by stable MSI and the level of HIF-1 expression: high (red) and low (black), measured with Affymetrix arrays (IDs: 220946_s_at). Data were from GSE143985 in GEO database.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experiments involving animals were conducted according to the ethical policies and procedures approved by The Animal Care and Use Committee of Okayama University (Approved No. OKU-2021900, OKU- 2022723, OKU- 2022879, OKU- 2023535).
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Toshiaki Ohara and Akihiro Matsukawa have contributed equally to this work.
Contributor Information
Toshiaki Ohara, Email: t_ohara@cc.okayama-u.ac.jp.
Akihiro Matsukawa, Email: amatsu@md.okayama-u.ac.jp.
References
- 1.Tirendi S, Marengo B, Domenicotti C, Bassi AM, Almonti V, Vernazza S (2023) Colorectal cancer and therapy response: a focus on the main mechanisms involved. Front Oncol 13:1208140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke AJ, Skelton WP, Starr JS, Parekh H, Lee JJ, Overman MJ, Allegra C, George TJ (2019) Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J Natl Cancer Inst 111(11):1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu I, Dakwar A, Takabe K (2023) Immunotherapy: recent advances and its future as a neoadjuvant, adjuvant, and primary treatment in colorectal cancer. Cells 12(2):258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuda T, Azuma T, Ohtani M, Masaki R, Ito Y, Yamazaki Y, Ito S, Kuriyama M (2005) Hypoxia-inducible factor 1 alpha and vascular endothelial growth factor overexpression in ischemic colitis. World J Gastroenterol 11(10):1535–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortezaee K, Majidpoor J, Kharazinejad E (2023) The impact of hypoxia on tumor-mediated bypassing anti-PD-(L)1 therapy. Biomed Pharmacother 162:114646 [DOI] [PubMed] [Google Scholar]
- 6.Gropper Y, Feferman T, Shalit T, Salame TM, Porat Z, Shakhar G (2017) Culturing CTLs under hypoxic conditions enhances their cytolysis and improves their anti-tumor function. Cell Rep 20(11):2547–2555 [DOI] [PubMed] [Google Scholar]
- 7.Liikanen I, Lauhan C, Quon S, Omilusik K, Phan AT, Bartroli LB, Ferry A, Goulding J, Chen J, Scott-Browne JP, Yustein JT, Scharping NE, Witherden DA, Goldrath AW (2021) Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells. J Clin Invest. 131(7) [DOI] [PMC free article] [PubMed]
- 8.Ogawa C, Tsuchiya K, Maeda K (2023) Hypoxia-inducible factor prolyl hydroxylase inhibitors and iron metabolism. Int J Mol Sci 24(3):3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20(21):4368–4380 [DOI] [PubMed] [Google Scholar]
- 10.Barratt J, Andric B, Tataradze A, Schomig M, Reusch M, Valluri U, Mariat C (2022) Erratum to: Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomised, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant 37(4):805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP (2019) Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 381(11):1001–1010 [DOI] [PubMed] [Google Scholar]
- 12.Carethers JM, Pham TT (2000) Mutations of transforming growth factor beta 1 type II receptor, BAX, and insulin-like growth factor II receptor genes in microsatellite unstable cell lines. In Vivo 14(1):13–20 [PubMed] [Google Scholar]
- 13.Sato Y, Fu Y, Liu H, Lee MY, Shaw MH (2021) Tumor-immune profiling of CT-26 and Colon 26 syngeneic mouse models reveals mechanism of anti-PD-1 response. BMC Cancer 21(1):1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Liao S, Xiao Z, Pan Q, Wang X, Shen K, Wang S, Yang L, Guo F, Liu HF, Pan Q (2022) The development and improvement of immunodeficient mice and humanized immune system mouse models. Front Immunol 13:1007579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F (2011) Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146(5):772–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz Perez N, Pensabene JM, Galbo PM Jr, Sadeghipour N, Xiu J, Moziak K, Yazejian RM, Welch RL, Bell WR, Sengupta S, Aulakh S, Eberhart CG, Loeb DM, Eskandar E, Zheng D, Zang X, Martin AM (2024) VISTA emerges as a promising target against immune evasion mechanisms in medulloblastoma. Cancers (Basel). 16(15):2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T (2015) Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43(3):579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190 [DOI] [PubMed] [Google Scholar]
- 19.Kahan SM, Bakshi RK, Ingram JT, Hendrickson RC, Lefkowitz EJ, Crossman DK, Harrington LE, Weaver CT, Zajac AJ (2022) Intrinsic IL-2 production by effector CD8 T cells affects IL-2 signaling and promotes fate decisions, stemness, and protection. Sci Immunol. 7(68):eabl6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, Yan P, Nzingha K, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Huang AC, Wherry EJ (2020) Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52(5):825–841828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S (2021) CD8(+) T cell exhaustion in cancer. Front Immunol 12:715234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusrat M (2020) Response to anti-PD-1 in microsatellite-stable colorectal cancer: a STAT need. Clin Cancer Res 26(22):5775–5777 [DOI] [PubMed] [Google Scholar]
- 23.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S, Comstock DE, Weiss SA, Brown FD, Panda A, Zimmer MD, Manguso RT, Hodi FS, Rodig SJ, Sharpe AH, Haining WN (2019) Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20(3):326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codony VL, Tavassoli M (2021) Hypoxia-induced therapy resistance: Available hypoxia-targeting strategies and current advances in head and neck cancer. Transl Oncol 14(3):101017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bargiela D, Cunha PP, Velica P, Krause LCM, Brice M, Barbieri L, Gojkovic M, Foskolou IP, Rundqvist H, Johnson RS (2024) The factor inhibiting HIF regulates T cell differentiation and anti-tumour efficacy. Front Immunol 15:1293723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Del Rio A, Prieto-Fernandez E, Egia-Mendikute L, Antonana-Vildosola A, Jimenez-Lasheras B, Lee SY, Barreira-Manrique A, Zanetti SR, de Blas A, Velasco-Beltran P, Bosch A, Aransay AM, Palazon A (2023) Factor-inhibiting HIF (FIH) promotes lung cancer progression. JCI Insight. 8(20):e167396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, Hatschek T, Lovrot J, Foukakis T, Goldrath AW, Bergh J, Johnson RS (2017) An HIF-1alpha/VEGF-A axis in cytotoxic t cells regulates tumor progression. Cancer Cell 32(5):669-683e665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH, Freedman BD, Turka LA (2005) Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci U S A 102(47):17071–17076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg SA (2014) IL-2: the first effective immunotherapy for human cancer. J Immunol 192(12):5451–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkhi MY, Ma Q, Ahmad S, Junghans RP (2015) T cell exhaustion and Interleukin 2 downregulation. Cytokine 71(2):339–347 [DOI] [PubMed] [Google Scholar]
- 33.Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA (2018) Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 3(25):eaat1482 [DOI] [PubMed] [Google Scholar]
- 34.Ghelani A, Bates D, Conner K, Wu MZ, Lu J, Hu YL, Li CM, Chaudhry A, Sohn SJ (2020) Defining the threshold IL-2 signal required for induction of selective treg cell responses using engineered IL-2 muteins. Front Immunol 11:1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez R, Poder J, LaPorte KM, Malek TR (2022) Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol 22(10):614–628 [DOI] [PubMed] [Google Scholar]
- 36.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537(7620):417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto M, Araki K, Cardenas MA, Li P, Jadhav RR, Kissick HT, Hudson WH, McGuire DJ, Obeng RC, Wieland A, Lee J, McManus DT, Ross JL, Im SJ, Lee J, Lin JX, Hu B, West EE, Scharer CD, Freeman GJ, Sharpe AH, Ramalingam SS, Pellerin A, Teichgraber V, Greenleaf WJ, Klein C, Goronzy JJ, Umana P, Leonard WJ, Smith KA, Ahmed R (2022) PD-1 combination therapy with IL-2 modifies CD8(+) T cell exhaustion program. Nature 610(7930):173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishitani S, Noma K, Ohara T, Tomono Y, Watanabe S, Tazawa H, Shirakawa Y, Fujiwara T (2016) Iron depletion-induced downregulation of N-cadherin expression inhibits invasive malignant phenotypes in human esophageal cancer. Int J Oncol 49(4):1351–1359 [DOI] [PubMed] [Google Scholar]
- 39.Szymonik J, Wala K, Gornicki T, Saczko J, Pencakowski B, Kulbacka J (2021) The impact of iron chelators on the biology of cancer stem cells. Int J Mol Sci 23(1):89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Tabu K, Aimaitijiang A, Taga T (2022) Therapy-resistant nature of cancer stem cells in view of iron metabolism. Inflamm Regen 42(1):34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii K, Fujishima Y, Kita S, Kawada K, Fukuoka K, Sakaue TA, Okita T, Kawada-Horitani E, Nagao H, Fukuda S, Maeda N, Nishizawa H, Shimomura I (2024) Pharmacological HIF-1 activation upregulates extracellular vesicle production synergistically with adiponectin through transcriptional induction and protein stabilization of T-cadherin. Sci Rep 14(1):3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madai S, Kilic P, Schmidt RM, Bas-Orth C, Korff T, Buttner M, Klinke G, Poschet G, Marti HH, Kunze R (2024) Activation of the hypoxia-inducible factor pathway protects against acute ischemic stroke by reprogramming central carbon metabolism. Theranostics 14(7):2856–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Cheng Y, Zhang P, Huang D, Zhai X, Feng Z, Fang D, Liu C, Du J, Cai J (2024) FG-4592 protected haematopoietic system from ionising radiation in mice. Immunology 172(4):614–626 [DOI] [PubMed] [Google Scholar]
- 44.Zuk A, Si Z, Loi S, Bommegowda S, Hoivik D, Danthi S, Molnar G, Csizmadia V, Rabinowitz M (2022) Preclinical characterization of vadadustat (AKB-6548), an oral small molecule hypoxia-inducible factor prolyl-4-hydroxylase inhibitor, for the potential treatment of renal anemia. J Pharmacol Exp Ther 383(1):11–24 [DOI] [PubMed] [Google Scholar]
- 45.Nakai T, Saigusa D, Kato K, Fukuuchi T, Koshiba S, Yamamoto M, Suzuki N (2024) The drug-specific properties of hypoxia-inducible factor-prolyl hydroxylase inhibitors in mice reveal a significant contribution of the kidney compared to the liver to erythropoietin induction. Life Sci 346:122641 [DOI] [PubMed] [Google Scholar]
- 46.Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, Block GA, Burke S, Castillo FP, Jardine AG, Khawaja Z, Koury MJ, Lewis EF, Lin T, Luo W, Maroni BJ, Matsushita K, McCullough PA, Parfrey PS, Roy-Chaudhury P, Sarnak MJ, Sharma A, Spinowitz B, Tseng C, Tumlin J, Vargo DL, Walters KA, Winkelmayer WC, Wittes J, Eckardt KU, Group PTS (2021) Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med 384(17):1589–1600 [DOI] [PubMed] [Google Scholar]
- 47.Czock D, Keller F (2022) Clinical pharmacokinetics and pharmacodynamics of roxadustat. Clin Pharmacokinet 61(3):347–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, Chouaib S (2016) Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology 5(4):e1062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL (2009) Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol 183(6):3720–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H, Akiyoshi K, Shiku H (2018) Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun 9(1):435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Najafi S, Majidpoor J, Mortezaee K (2023) Extracellular vesicle-based drug delivery in cancer immunotherapy. Drug Deliv Transl Res 13(11):2790–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang Z, Meng Q, Xu J, Wang W, Zhang B, Liu J, Liang C, Hua J, Zhao Y, Yu X, Shi S (2023) Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun (Lond) 43(1):3–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu JY, Huang TW, Hsieh YT, Wang YF, Yen CC, Lee GL, Yeh CC, Peng YJ, Kuo YY, Wen HT, Lin HC, Hsiao CW, Wu KK, Kung HJ, Hsu YJ, Kuo CC (2020) Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell 77(2):213-227e215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figure 5F Kaplan–Meier shows progression-free survival of patients with MSS colon cancer classified by stable MSI and the level of HIF-1 expression: high (red) and low (black), measured with Affymetrix arrays (IDs: 220946_s_at). Data were from GSE143985 in GEO database.