Abstract
The Echinococcus multilocularis protein Em18 is one of the most promising antigens for use in serodiagnosis of alveolar echinococcosis in human patients. Here we identify an antigenic relationship between Em18 and a 65-kDa immunodominant E. multilocularis surface protein previously identified as either EM10 or EmII/3. The NH2-terminal sequence of native Em18 was determined, revealing it to be a fragment of EM10. Experiments were undertaken to investigate the effect of proteinase inhibitors on the degradation of EM10 in crude extracts of E. multilocularis protoscoleces. Em18 was found to be the product of degradation of EM10 by cysteine proteinase. A recombinant Em18 (RecEm18, derived from 349K to 508K of EM10) was successfully expressed by using Escherichia coli expression system and then evaluated for use in serodiagnosis of alveolar echinococcosis. RecEm18 was recognized by 27 (87.1%) and 28 (90.3%) of 31 serum samples from clinically and/or pathologically confirmed alveolar echinococcosis patients by enzyme-linked immunosorbent assay and immunoblotting, respectively. Of 33 serum samples from cystic echinococcosis patients, 1 was recorded as having a weak positive reaction to RecEm18; however, none of the serum samples which were tested from neurocysticercosis patients (n = 10) or healthy people (n = 15) showed positive reactions. RecEm18 has the potential for use in the differential serodiagnosis of alveolar echinococcosis.
Alveolar echinococcosis (AE), caused by the larval stage of Echinococcus multilocularis, is a serious parasitic disease of humans in Northern hemisphere countries in the higher latitudes. Humans are infected with E. multilocularis by accidental ingestion of eggs excreted with the feces of carnivores harboring the adult tapeworm of this species. The eggs hatch in the small intestine of the human host releasing the oncosphere which migrates via the portal system into various organs, mainly the liver, and differentiates into the metacestode stage. The metacestodes propagate asexually like a tumor, leading to organ dysfunction. Since clinical symptoms usually do not become evident until 10 or more years after initial parasite infection, early diagnosis and treatment are important for the reduction of morbidity and mortality (1, 9). At present, diagnosis of AE is primarily based on imaging techniques, including echography, computed tomography, and magnetic resonance imaging. These imaging techniques are sometimes limited by the small size of visualized lesions and atypical images, which are difficult to distinguish from abscesses or neoplasms. Therefore, efforts have been directed toward identification and characterization of specific antigens of E. multilocularis metacestodes for development of immunodiagnostic test that can detect specific antibodies (8, 9, 10, 16-18, 20-22, 25, 27, 33-35, 37).
Using molecular and immunological techniques many researchers have attempted to identify E. multilocularis-specific antigens and showed the usefulness of recombinant antigens for serodiagnosis (3, 5, 10, 11, 30, 41). Vogel et al. (41) identified a cDNA clone from an E. multilocularis protoscolex library by screening with a pool of sera from AE patients. The clone, designated II/3, comprised an incomplete copy of the associated mRNA, and the expressed protein was shown to have potential for use in the serodiagnosis of AE. Muller et al. (30) subcloned a fragment of this cDNA, referred to as II/3-10, which retained the diagnostic epitopes but was more suited to use in immunoassays. Neither publication included DNA or protein sequence data. Subsequently, Frosch et al. (5) characterized a full-length mRNA from E. multilocularis protoscoleces, including the DNA sequence, and showed that the expressed antigen, designated EM10, had potential for use in the diagnosis of AE. Contemporaneously, Hemmings and McManus (11) characterized a partial cDNA, designated EM4, encoding an antigen which they also found to be potentially useful for serodiagnosis of AE. The II/3 and EM4 proteins were subsequently confirmed as being fragments of EM10. This full-length recombinant antigen and its associated full-length native antigen are hereafter referred to as EM10, whereas the designations EmII/3 or EmII/3-10 are retained for the fragments of EM10 described by Vogel et al. (41) and Muller et al. (30), respectively.
Recently, we reported another novel antigen, termed Em18 (18-kDa protein under reducing condition), partially purified by preparative isoelectric-focusing electrophoresis (IEFE) from E. multilocularis protoscoleces and demonstrated its usefulness for highly sensitive and specific diagnosis of AE by either enzyme-linked immunosorbent assay (ELISA) or immunoblotting (16-18, 22). The sensitivity and specificity of Em18 for AE are very compatible to those of recombinant EM10 or truncated fragments thereof (18, 21), raising the question as to whether EM10 and Em18 are antigenically or otherwise related. We here describe a partial amino acid sequence of the native Em18 antigen, confirming that Em18 is a fragment of the EM10 protein, and demonstrate that recombinant Em18 is highly effective in immunoassays for serodiagnosis of AE.
MATERIALS AND METHODS
Preparation of parasite material.
E. multilocularis (Furano isolate, Hokkaido, Japan) metacestode material was obtained from laboratory reared Mongolian gerbils infected by intraperitoneal passage.
Crude E. multilocularis antigen extract was prepared from fresh whole cyst tissue materials. The parasite organism was lysed with three times volume of neutral lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]) or acidic lysis buffer (0.1 M citrate buffer [pH 5.0], 150 mM NaCl, 1% Triton X-100, 0.1% SDS). After one freeze-thaw cycle and centrifugation at 10,000 × g for 30 min at 4°C, the supernatant was recovered and kept at −80°C until use.
Patient serum samples.
A total of 31 serum samples of patients with AE confirmed by image analysis and/or serology of immunoblot analysis with the native Em18 purified by IEFE from protoscoleces of E. multilocularis were examined for this study. Seven and twenty-four serum samples were from patients in Alaska and Hokkaido, Japan, respectively. Serum samples from other parasitic diseases were also examined. Four, twenty, and nine cystic echinococcosis (CE) serum samples were from Japan (imported cases), Australia, and the United States, respectively, and ten serum samples obtained from patients with Taenia solium neurocysticercosis (NCC), clinically and serologically confirmed, were from the Centers for Disease Control (19-21, 29). Australian CE samples all were serologically confirmed in Melbourne by both ELISA and immunoelectrophoresis against Arc 5 (32). Healthy Japanese persons (n = 15) were used as negative controls.
SDS-PAGE and immunoblot analysis.
Crude E. multilocularis antigen extract was incubated at 37°C for 1 h in the presence or absence of proteinase inhibitors. The proteinase inhibitors used in this study were as follows: 10 mM AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], 5 μg of aprotinin/ml, 50 μM leupeptin, 1 μM pepstatin A, 5 μM trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane (E64), and 5 mM EDTA. All proteinase inhibitors were purchased from Sigma, St. Louis, Mo. Protein analysis by SDS-polyacrylamide gel electrophoresis (PAGE) was carried out by the method of Laemmli (24). Each crude E. multilocularis antigen extract was solubilized with SDS sample buffer (10 mM Tris-HCl [pH 6.8] containing 2% SDS, 5% 2-mercaptoethanol, and 10% glycerol) at 100°C for 5 min and separated electrophoretically in a 12.5% polyacrylamide gel. For immunoblot analysis, the separated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane sheet (Millipore, Tokyo, Japan) as described by Towbin et al. (39). The sheet was blocked with 3% skim milk (Morinaga, Tokyo, Japan) and probed with patients' or recombinant EmII/3-10-immunized rabbit sera (3), followed by treatment with peroxidase-conjugated anti-human or rabbit immunoglobulin G (IgG) antibodies (Cappel, West Chester, Pa.). 4-Chloro-1-naphthol was used for color development.
Preparation of monospecific antibodies to EmII/3.
Anti-EmII/3-10 monospecific antibodies were immunoaffinity purified from high-titer AE patient sera with recombinant EmII/3-10 (see below). Briefly, antibodies bound to recombinant EmII/3-10 on PVDF after SDS-PAGE separation were eluted with 0.2 M glycine buffer (pH 2.6). The monospecific antibody solution was quickly neutralized with 1.0 M Tris-HCl (pH 9.0)-150 mM NaCl and used for immunoblot analysis.
Purification of native Em18 and determination of N-terminal amino acid sequence.
The native Em18 antigen was immunoaffinity purified with recombinant EmII/3-10-immunized rabbit serum (3). For N-terminal sequencing, the sample was subjected to SDS-PAGE and transferred onto a PVDF membrane. Bands were visualized in 0.2% Coomassie blue R-250, excised, and washed with distilled water. Sequencing was performed by Takara Shuzo Co., Ltd., Tokyo, Japan, by using the Edman degradation technique.
Construction of E. multilocularis metacestodes cDNA library.
A λSCREEN cDNA library was constructed from poly(A)+ RNA isolated from E. multilocularis metacestodes by FastTrack 2.0 kit (Invitrogen, Carlsbad, Calif.). The oligo(dT)-primed cDNA was synthesized from 5 μg of poly(A)+ RNA by using cDNA Synthesis Kit (Takara). The resulting cDNA fragments were ligated to directional EcoRI/HindIII linker DNA (Novagen, Madison, Wis.), digested with restriction enzymes (EcoRI and HindIII), and finally ligated with λSCREEN arms (Novagen). The recombinant DNA was packaged by using Phage Maker In Vitro Packaging System (Novagen).
Cloning of EM10 cDNA.
The EM10 cDNA used for construction of recombinant EmII/3-10 or Em18 expression vector was amplified by PCR. Primers EmF1 (5′-ATGTTGAAGAGGAGTAAGAAT-3′) and EmR3 (5′-CTACATCGACTCAAACTGTTCAAC-3′) used were designed from the published nucleotide sequence (5) (GenBank accession number M61186). The PCRs were performed in a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, a 0.1 μM concentration of each primer, 0.2 mM concentrations of each deoxynucleoside triphosphate, 2 μl of E. multilocularis metacestodes cDNA phage solution (ca. 4 × 105 PFU), and 0.5 U of Taq DNA polymerase (AmpliTaq Gold; Perkin Elmer, Foster City, Calif.), and the cycling conditions were 30 s at 94°C (first cycle of 10 min at 94°C), 30 s at 55°C, and 2 min at 72°C for 35 cycles. The resultant PCR product was ligated into a pGEM-T plasmid vector (Promega, Madison, Wis.) and sequenced. The nucleotide sequence obtained in this study was identical to the published sequence.
Expression and purification of recombinant EmII/3-10 and Em18.
The coding region of EmII/3-10 (4, 5) and the putative coding region of Em18 was amplified by PCR with a primer set contained a restriction enzyme BamHI recognition sequence (underlined) added to 5′ end to facilitate cloning of the PCR products. The primers used were 5′-CGGGATCCGTTTTTCTTCTTGGTGGAAAAATC-3′ (EmII/3-10F), 5′-CGGGATCCTACTTGTGCTTTGCATCGTGTTT-3′ (EmII/3-10R), 5′-CGGGATCCGAAGGAGTCTGACTTAGCGGAT-3′ (Em18F), and 5′-CGGGATCCTATTTGAGGTTGGCCAGCTTCGT-3′ (Em18R). The PCR products were digested with BamHI and cloned into bacterial expression vector pET-16b(+) (Novagen) for producing a fusion protein with His tag. The orientation of insert DNA was confirmed by sequencing. The cloned plasmid was transfected into E. coli BL21(DE3)pLysS strain. Expression of the recombinant protein was induced by addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to the culture. The expressed recombinant proteins were purified by using a His Trap kit (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) and then dialyzed against phosphate-buffered saline (PBS). Protein concentration was determined by BCA Protein Assay kit (Pierce, Rockford, Ill.).
ELISA.
ELISA plates (Nunc-Immuno Plate MaxiSorp Surface; Nalge Nunc International, Tokyo, Japan) were coated with 0.1 μg of recombinant proteins. The wells were blocked with 300 μl of casein buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% casein) at 37°C for 1 to 2 h. After the wells were rinsed twice with PBS containing 0.05% Tween 20 (PBST), 100-μl serum samples diluted 1:100 in PBST containing 1% bovine serum albumin were added, and the mixtures were incubated at 37°C for 1 h. The wells were rinsed five times with PBST, incubated with 100 μl of anti-human IgG antibodies conjugated with peroxidase (Cappel) at 37°C for 1 h, and rinsed five times with PBST. After incubation with 100 μl of substrate (0.4 mM 2,2′-azino-di-[3-ethyl-benzthiazoline sulfonate] in 0.2 M citric acid buffer; pH 4.7) for 15 min at room temperature, the optical density at 405 nm (OD405) of each well was determined by using the ELISA plate reader (Model 450; Bio-Rad Laboratories, Hercules, Calif.). Serum samples giving OD405 values greater than the three mean OD405 values for normal human controls were considered seropositive.
RESULTS
Confirmation of an antigenic relationship between Em18 and EmII/3.
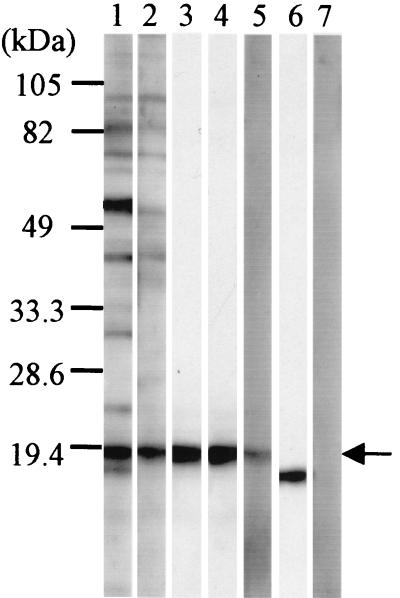
Em18 which had been partially purified by IEFE was analyzed in immunoblots with AE patients' monospecific antibodies that had been affinity purified on recombinant EmII/3-10 and a rabbit antiserum raised against recombinant EmII/3-10 (Fig. 1). Both sources of EmII/3-10-specific antibodies specifically bind to the Em18 antigen (Fig. 1, lanes 3, 4, and 5, respectively), indicating a clear antigenic relationship between Em18 and EmII/3.
FIG. 1.
Immunoblot analysis of Em18 partially purified by isoelectric-focusing electrophoresis with AE patients' and rabbit monospecific antibodies to recombinant EmII/3-10. Lane 1, serum from patient A; lane 2, serum from patient B; lane 3, monospecific antibodies purified from patient A; lane 4, monospecific antibodies purified from patient B; lane 5, recombinant EmII/3-10-immunized rabbit serum; lane 6, monoclonal antibody to 16-kDa antigen (Em16); lane 7, normal human serum. Molecular size markers are indicated on the left. Em18 band is indicated by arrow.
Determination of the N-terminal amino acid sequence of Em18.
The relationship between Em18 and the EmII/3 fraction of EM10 was investigated further with native Em18 which had been was affinity purified from E. multilocularis protoscolex extract by using rabbit anti-EmII/3-10 antibodies. The purified Em18 was subjected to N-terminal amino acid sequencing which identified the sequence KESDLAD. A homology search revealed that this amino acid sequence was identical to that of EM10 (349K to 355D, indicated by a number sign [#] in Fig. 2), indicating the likelihood that Em18 was a proteolytic breakdown product of EM10.
FIG. 2.
Alignment of amino acid sequences of EM10 and human moesin (GenBank accession number BC017293) and designation of the RecEm18 fragment. The amino acid sequences were aligned by CLUSTALV program (12). An asterisk indicates identical residues, while gaps introduced by CLUSTAL V program are symbolized by a dash. N-terminal amino acid residues of Em18 determined in this study are indicated by a number sign (#). Sequence for expression of recombinant Em18 is shaded in gray.
Proteinase inhibition analysis.
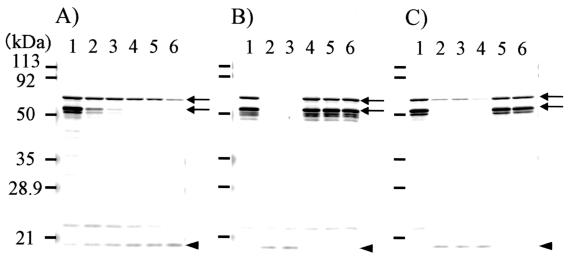
The hypothesis that Em18 was a product of the proteolysis of EM10 was investigated by performing proteinase inhibition studies with E. multilocularis antigen extracts. Immunoblot analyses were carried out with rabbit antisera raised against EmII/3-10 after incubation of crude E. multilocularis antigen extract at 37°C in the presence or absence of proteinase inhibitors (Fig. 3). The 65- and 52-kDa proteins reported by Frosch et al. (5) and Felleisen and Gottstein (3) were readily detected in freshly extracted protocoleces (Fig. 3, arrows). Incubation of crude E. multilocularis antigen extract in the absence of proteinase inhibitors caused a time-dependent decrease of 65- and 52-kDa proteins (Fig. 3A, arrows) and an increase in the relative amount of Em18 (Fig. 3A, arrowhead). At neutral pH in the presence of chelating reagent (Fig. 3B, lane 4) and cysteine proteinase inhibitors (Fig. 3B, lanes 5 and 6), the banding patterns were very similar to that of preincubation extract. However, the inclusion of a serine proteinase inhibitor (Fig. 3B, lane 3) and an aspartic proteinase inhibitor (data not shown) had no effect on the changes in the proportions of EM10 and Em18, which could be detected in amounts indistinguishable form those seen with the extract incubated without proteinase inhibitors (Fig. 3B, lane 2). At an acidic pH, only the cysteine proteinase inhibitors prevented the degradation of the 65- and 52-kDa antigens and the appearance of Em18 (Fig. 3C). These data indicate that Em18 is the proteolytic product of EM10 following the action of by cysteine proteinases.
FIG. 3.
Results of proteinase inhibition assays. (A) E. multilocularis crude extracts were incubated at neutral pH condition in the absence of proteinase inhibitors for different time intervals. Lane 1, 0 min; lane 2, 5 min; lane 3, 10 min; lane 4, 20 min; lane 5, 30 min; lane 6, 60 min. (B) Incubation at neutral pH (pH 7.4) for 60 min in the presence of proteinase inhibitor. (C) Incubation at acidic pH (pH 5.0) for 60 min in the presence of proteinase inhibitor. Lane 1, preincubation; lane 2, incubated in the absence of proteinase inhibitors; lane 3, incubated in the presence of AEBSF (10 mM); lane 4, incubated in the presence of EDTA (5 mM); lane 5, incubated in the presence of leupeptin (50 μM); lane 6, incubated in the presence of E64 (5 μM). After incubation, each extract was characterized by immunoblot analysis with recombinant EmII/3-10-immunized rabbit serum. Molecular size markers are indicated on the left. The 65- and 52-kDa bands are indicated by arrows, and Em18 is indicated by an arrowhead.
Expression of recombinant Em18 (RecEm18) and its evaluation for serodiagnostic value.
Recombinant Em18 (RecEm18) was prepared by subcloning the appropriate segment of a cDNA encoding this fragment of the EM10 antigen and expression in an E coli-based expression system. The predicted molecular mass from 349K to the C terminus of EM10 was 24.5 kDa, indicating that Em18 was produced by additional cleavage in the vicinity of the C terminus. Since we did not determine the C-terminal amino acid sequence of Em18 in this study, it remains unclear if RecEm18 exactly corresponds to native Em18. However, based on molecular size and isoelectric point information obtained from the primary structure of EM10 and native Em18, a polypeptide from 349K to 508K of EM10 was chosen (Fig. 2) for expression as RecEm18. The region selected for expression as RecEm18 excluded those parts of EM10 which show homology to the human ezrin, radixin, and moesin (ERM) family molecules (Fig. 2) and thus might be more likely to contain epitopes recognized by AE patients.
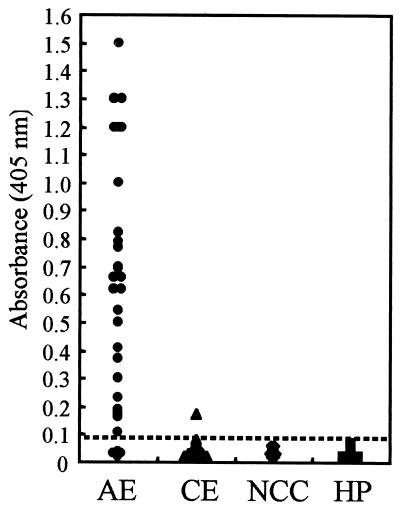
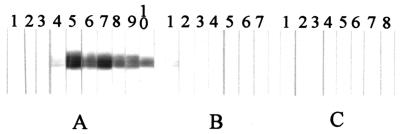
The diagnostic value of RecEm18 was tested by both ELISA (Fig. 4) and immunoblotting (Fig. 5) with individual sera from patients with AE, CE, and NCC. ELISA showed a positive reaction to RecEm18 that was observed in 87.1% (27 of 31 cases) of sera from clinically confirmed AE patients based on a cutoff value of 0.09. One serum sample was judged as negative by ELISA but was recorded as a relatively weak positive by immunoblot (Fig. 5A, lane 4). Of the 33 CE patient sera, 1 exclusively reacted to RecEm18 by both methods. This was an Australian CE case showing a strong positive response against E. granulosus cyst fluid antigens by ELISA and against Arc 5 by immunoelectrophoresis. Five other CE samples showing similar high titers were negative against RecEm18. No positive results were observed with sera from patients with NCC (n = 10) or healthy persons (n = 15).
FIG. 4.
Results of ELISA with recombinant Em18 with sera from 31 patients with AE, 33 patients with CE, 10 patients with NCC, and 15 healthy persons (HP). The dotted line shows the cutoff value (i.e., 0.09).
FIG. 5.
Immunoblot analysis of recombinant Em18 with sera from patients with AE, CE, or NCC. Each strip was probed with an individual serum sample. The figure shows a representative subset of the data (10 AE, 7 CE, and 8 NCC samples) that were obtained for each patient group.
DISCUSSION
Evidence presented in this study, indicates that Em18 is the product of proteinase-mediated proteolysis of EM10 by cysteine proteinase (Fig. 3). Rabbit antisera raised against the EmII/3-10 fragment of the EM10 antigen identified two molecules with molecular masses of 65 and 52 kDa (Fig. 3) in fresh extracts of E. multilocularis protoscoleces, as shown in previous studies (3, 5). The full-length EM10 cDNA encodes a predicted protein of 65 kDa and is believed to be encoded by a single-copy gene (11), which suggests that the 52-kDa molecule seen in immunoblots is itself a degradation product of the 65-kDa molecule. Frosch et al. (5) highlighted the homology between EM10 and the ERM family of proteins in eukaryotes, which are known as molecules linking between cell surface protein and actin filaments. In mammalian cells, biological activities of ERM molecules are reflected by their conformations (2, 7, 15, 28, 40) and EM10 has been shown to have functional similarity to the ERM family of molecules (14). Several studies have identified the sensitivity of the ERM family of proteins to Ca2+-activated neutral cysteine proteinase (calpain)-dependent proteolysis and the importance of the interaction between ERM molecules and calpain for the regulation of ERM family molecule functions (31, 36, 42, 43) and for the pathogenesis of some kinds of tumors (13, 23). Further research would be required on the EM10 protein in E. multilocularis in order to determine whether the protein undergoes proteolytic degradation in intact parasites and whether this process is involved in the normal function of the protein in the parasite's biology.
The C-terminal sequence of native Em18 was not determined in this study, and thus a recombinant protein exactly corresponding to native Em18 could not be expressed. However, based on the molecular size and isoelectric point (pI 5.2 to 5.5) of native Em18 (20), a recombinant protein containing from 349K to 508K of EM10 was expressed (Fig. 2) and evaluated as a serodiagnostic antigen. Of 31 serum samples from clinically confirmed AE patients, 27 (87.1%) were positive by ELISA (Fig. 4) and 1 of the serum samples judged to be negative by ELISA, but was positive in the immunoblot (28 of 31, 90.3%) (Fig. 5A, lane 4). Of 33 CE patient sera, 1 was recorded as showing a positive reaction to RecEm18 (Fig. 5B, lane 1). This patient had undergone recent surgery in an attempt to evacuate a large hydatid cyst of the liver which was unable to be removed completely. In another study evaluating RecEm18 for serodiagnosis of AE, we have determined that a single patient with CE became weakly positive by both ELISA and immunoblot. This CE patient had multiple and complicated cysts with rupture (A. Ito, X. Ning, M. Liance, M. O. Sato, Y. Sako, M. Wulamu, M. Nakao, H. Yamasaki, K. Nakaya, and D. A. Vuitton, unpublished data).
Despite there being a protein expressed by E. granulosus metacestodes which has a high level of homology to EM10 (98.6% identity at amino acid level) (4, 6), this protein does not induce significant levels of cross-reacting antibodies to RecEm18 in CE patients except in a small minority of patients. Similar results were obtained by Felleisen and Gottstein (4), who found that sera from either AE or CE patients reacted similarly to both E. multilocularis EM10 (referred to by them as II/3) or its E. granulosus homologue and that AE patients more frequently raised antibodies to this antigen. It is interesting to speculate about the reasons why these similar proteins should seem to have distinctly different immunogenicities in AE and CE patients. The two parasites have quite different pathologies, with E. multilocularis being invasive and having intimate contact with host tissues (38) in comparison to E. granulosus which has relatively less direct contact with the host except after cyst rupture (26). For this reason, B-cell responses in CE patients to the EM10 homologue of E. granulosus might be low. Further investigations will be needed to clarify the relationship between different pathogenesis of parasites and the B-cell response to Em18.
Hereafter, we will characterize the proteinase(s) involved in degradation of EM10, leading to production of Em18 and determine the nature of the B-cell epitopes on Em18 toward the development of synthetic peptide-based ELISA system that may be suitable for supplying stable and high-quality diagnoses.
Acknowledgments
This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan and the Japan Society of Promotion of Science to A.I. (10557029, 11694259, and 12557024) and to Y.S. (12770122), by the National Institutes of Health (1 R01 TW01565-01; principal investigator, P. S. Craig), and by the Australian National Health and Medical Research Council.
REFERENCES
- 1.Ammann, R. W., R. Hirsbrunner, J. Cotting, U. Steiger, P. Jacquier, and J. Eckert. 1990. Recurrence rate after discontinuation of long-term mebendazole therapy in alveolar echinococcosis (preliminary results). Am. J. Trop. Med. Hyg. 43:506-515. [DOI] [PubMed] [Google Scholar]
- 2.Andreoli, C., M. Martin, R. Le Borgne, H. Reggio, and P. Mangeat. 1994. Ezrin has properties to self-associate at the plasma membrane. J. Cell. Sci. 107:2509-2521. [DOI] [PubMed] [Google Scholar]
- 3.Felleisen, R., and B. Gottstein. 1993. Echinococcus multilocularis: molecular and immunochemical characterization of diagnostic antigen II/3-10. Parasitology 107:335-342. [DOI] [PubMed] [Google Scholar]
- 4.Felleisen, R., and B. Gottstein. 1994. Comparative analysis of full-length antigen II/3 from Echinococcus multilocularis and E. granulosus. Parasitology 109:223-232. [DOI] [PubMed] [Google Scholar]
- 5.Frosch, P. M., M. Frosch, T. Pfister, V. Schaad, and D. Bitter-Suermann. 1991. Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol. Biochem. Parasitol. 48:121-130. [DOI] [PubMed] [Google Scholar]
- 6.Frosch, P. M., F. Muhlschlegel, L. Sygulla, M. Hartmann, and M. Frosch. 1994. Identification of a cDNA clone from the larval stage of Echinococcus granulosus with homologies to the E. multilocularis antigen EM10-expressing cDNA clone. Parasitol. Res. 80:703-705. [DOI] [PubMed] [Google Scholar]
- 7.Gary, R., and A. Bretscher. 1995. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 6:1061-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottstein, B. 1985. Purification and characterization of a specific antigen from Echinococcus multilocularis. Parasite Immunol. 7:201-212. [DOI] [PubMed] [Google Scholar]
- 9.Gottstein, B., C. Lengeler, P. Bachmann, P. Hagemann, P. Kocher, M. Brossard, F. Witassek, and J. Eckert. 1987. Sero-epidemiological survey for alveolar echinococcosis (by Em2-ELISA) of blood donors in an endemic area of Switzerland. Trans. R. Soc. Trop. Med. Hyg. 81:960-964. [DOI] [PubMed] [Google Scholar]
- 10.Helbig, M., P. Frosch, P. Kern, and M. Frosch. 1993. Serological differentiation between cystic and alveolar echinococcosis by use of recombinant larval antigens. J. Clin. Microbiol. 31:3211-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmings, L., and D. P. McManus. 1991. The diagnostic value and molecular characterization of an Echinococcus multilocularis antigen gene clone. Mol. Biochem. Parasitol. 44:56-62. [DOI] [PubMed] [Google Scholar]
- 12.Higgins, D. G., A. J. Bleasby, and R. Fuchs. 1992. CLUSTAL V: improved software for multiple sequence alignment. Comput. Appl. Biosci. 8:189-191. [DOI] [PubMed] [Google Scholar]
- 13.Hovens, C. M., and A. H. Kaye. 2001. The tumour suppressor protein NF2/merlin: the puzzle continues. J. Clin. Neurosci. 8:4-7. [DOI] [PubMed] [Google Scholar]
- 14.Hubert, K., E. Cordero, M. Frosch, and F. Solomon. 1999. Activities of the EM10 protein from Echinococcus multilocularis in cultured mammalian cells demonstrate functional relationships to ERM family members. Cell Motil. Cytoskeleton 42:178-188. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa, H., A. Tamura, T. Matsui, H. Sasaki, T. Hakoshima, S. Tsukita, and S. Tsukita. 2001. Structural conversion between open and closed forms of radixin: low-angle shadowing electron microscopy. J. Mol. Biol. 310:973-978. [DOI] [PubMed] [Google Scholar]
- 16.Ito, A., M. Nakao, H. Kutsumi, M. W. Lightowlers, M. Itoh, and S. Sato. 1993. Serodiagnosis of alveolar hydatid disease by Western blotting. Trans. R. Soc. Trop. Med. Hyg. 87:170-172. [DOI] [PubMed] [Google Scholar]
- 17.Ito, A., P. M. Schantz, and J. F. Wilson. 1995. Em18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am. J. Trop. Med. Hyg. 52:41-44. [DOI] [PubMed] [Google Scholar]
- 18.Ito, A., L. Ma, M. Itoh, S. Y. Cho, Y. Kong, S. Y. Kang, T. Horii, X. L. Pang, M. Okamoto, T. Yamashita, M. W. Lightowlers, X. G. Wang, and Y. H. Liu. 1997. Immunodiagnosis of alveolar echinococcosis by enzyme-linked immunosorbent assay using a partially purified Em18/16 enriched fraction. Clin. Diagn. Lab. Immunol. 4:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, A., A. Plancarte, L. Ma, Y. Kong, A. Flisser, S. Y. Cho, Y. H. Liu, S. Kamhawi, M. W. Lightowlers, and P. M. Schantz. 1998. Novel antigens for neurocysticercosis: simple method for preparation and evaluation for serodiagnosis. Am. J. Trop. Med. Hyg. 59:291-294. [DOI] [PubMed] [Google Scholar]
- 20.Ito, A., L. Ma, P. M. Schantz, B. Gottstein, Y. H. Liu, J. J. Chai, S. K. Abdel-Hafez, N. Altintas, D. D. Joshi, M. W. Lightowlers, and Z. S. Pawlowski. 1999. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18). Am. J. Trop. Med. Hyg. 60:188-192. [DOI] [PubMed] [Google Scholar]
- 21.Ito, A., L. Ma, M. Paul, J. Stefaniak, and Z. S. Pawlowski. 1998. Evaluation of Em18-, Em16-, antigen B-Western blots, Em2plus-ELISA and four other tests for differential serodiagnosis of alveolar and cystic echinococcosis patients in Poland. Parasitol. Int. 47:95-99. [Google Scholar]
- 22.Jiang, L., H. Wen, and A. Ito. 2001. Immunodiagnostic differentiation of alveolar and cystic echinococcosis using ELISA test with 18-kDa antigen extracted from Echinococcus protoscoleces. Trans. R. Soc. Trop. Med. Hyg. 95:285-288. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, Y., H. Koga, N. Araki, N. Mugita, N. Fujita, H. Takeshima, T. Nishi, T. Yamashima, T. C. Saido, T. Yamasaki, K. Moritake, H. Saya, and M. Nakao. 1998. The involvement of calpain-dependent proteolysis of the tumor suppressor NF2 (merlin) in schwannomas and meningiomas. Nat. Med. 4:915-922. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Liance, M., V. Janin, S. Bresson-Hadni, D. A. Vuitton, R. Houin, and R. Piarroux. 2000. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial Western Blot. J. Clin. Microbiol. 38:3718-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lightowlers, M. W., M. D. Rickard, and R. D. Honey. 1986. Serum antibody response following parenteral immunization with hydatid cyst fluid in sheep infected with Echinococcus granulosus. Am. J. Trop. Med. Hyg. 35:818-823. [DOI] [PubMed] [Google Scholar]
- 27.Ma, L., A. Ito, Y. H. Liu, X. G. Wang, Y. Q. Yao, D. G. Yu, and Y. T. Chen. 1997. Alveolar echinococcosis: Em2plus-ELISA and Em18-Western blots for follow-up after treatment with albendazole. Trans. R. Soc. Trop. Med. Hyg. 91:476-478. [DOI] [PubMed] [Google Scholar]
- 28.Magendantz, M., M. D. Henry, A. Lander, and F. Solomon. 1995. Interdomain interactions of radixin in vitro. J. Biol. Chem. 270:25324-25327. [DOI] [PubMed] [Google Scholar]
- 29.Mamuti, W., H. Yamasaki, Y. Sako, K. Nakaya, M. Nakao, M. W. Lightowlers, and A. Ito. 2002. Usefulness of hydatid cyst fluid of Echinococcus granulosus developed in secondary infected mice for serodiagnosis of cystic echinococcosis in human. Clin. Diagn. Lab. Immunol. 9:573-579. [DOI] [PMC free article] [PubMed]
- 30.Muller, N., B. Gottstein, M. Vogel, K. Flury, and T. Seebeck. 1989. Application of a recombinant Echinococcus multilocularis antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of human alveolar echinococcosis. Mol. Biochem. Parasitol. 36:151-159. [DOI] [PubMed] [Google Scholar]
- 31.Potter, D. A., J. S. Tirnauer, R. Janssen, D. E. Croall, C. N. Hughes, K. A. Fiacco, J. W. Mier, M. Maki, and I. M. Herman. 1998. Calpain regulates actin remodeling during cell spreading. J. Cell Biol. 141:647-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickard, M. D., R. D. Honey, J. L. Brumley, and G. F. Mitchell. 1984. Serological diagnosis and postoperative surveillance of human hydatid disease. II. The enzyme-linked immunosorbent assay (ELISA) using various antigens. Pathology 16:211-215. [DOI] [PubMed] [Google Scholar]
- 33.Sarciron, E. M., S. Bresson-Hadni, M. Mercier, P. Lawton, C. Duranton, D. Lenys, A. F. Petavy, and D. A. Vuitton. 1997. Antibodies against Echinococcus multilocularis alkaline phosphatase as markers for the specific diagnosis and the serological monitoring of alveolar echinococcosis. Parasite Immunol. 19:61-68. [DOI] [PubMed] [Google Scholar]
- 34.Sato, C., and K. Furuya. 1994. Isolation and characterization of a diagnostic polysaccharide antigen from larval Echinococcus multilocularis. Jpn. J. Med. Sci. Biol. 47:65-71. [DOI] [PubMed] [Google Scholar]
- 35.Sato, C., S. Kawase, and S. Yano. 1999. Monoclonal antibodies specific to carbohydrates of Echinococcus multilocularis. Jpn. J. Infect. Dis. 52:156-159. [PubMed] [Google Scholar]
- 36.Shcherbina, A., A. Bretscher, D. M. Kenney, and E. Remold-O'Donnell. 1999. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 443:31-36. [DOI] [PubMed] [Google Scholar]
- 37.Siles-Lucas, M., and B. Gottstein. 2001. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop. Med. Int. Health 6:463-475. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, R. C. A. 1995. Biology and systematics of Echinococcus, p. 1-50. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 39.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsukita, S., and S. Yonemura. 1999. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 274:34507-34510. [DOI] [PubMed] [Google Scholar]
- 41.Vogel, M., B. Gottstein, N. Muller, and T. Seebeck. 1988. Production of a recombinant antigen of Echinococcus multilocularis with high immunodiagnostic sensitivity and specificity. Mol. Biochem. Parasitol. 31:117-125. [DOI] [PubMed] [Google Scholar]
- 42.Yan, B., D. A. Calderwood, B. Yaspan, and M. H. Ginsberg. 2000. Calpain cleavage promotes talin binding to the β3 integrin cytoplasmic domain. J. Biol. Chem. 276:28164-28170. [DOI] [PubMed] [Google Scholar]
- 43.Yao, X., A. Thibodeau, and J. G. Forte. 1993. Ezrin-calpain I interactions in gastric parietal cells. Am. J. Physiol. 265:C36-C46. [DOI] [PubMed] [Google Scholar]