Abstract
Objective
To review and synthesize existing evidence on the effect of mirror therapy (MT) on motor and functional recovery and the effect of unimanual and bimanual MT in individuals with subacute stroke.
Methodology
PubMed, Physiotherapy Evidence Database, Cochrane, and Airiti Library were searched for relevant studies. Randomized and pilot randomized controlled trials comparing MT with sham MT or conventional therapy were included. Three researchers independently reviewed eligible studies for study design, participants' characteristics, intervention, and outcome measures and assessed study quality. The Physiotherapy Evidence Database scale was used to evaluate the methodological quality of included studies, and the Cochrane Risk of Bias Tool was used to assess the risk of bias.
Synthesis
Fifteen studies with 546 participants were included. An overall effect of MT was found for motor impairment (effect size [95% confidence interval]: 0.473 [0.274–0.673], p < .001), motor function (0.266 [0.059−0.474], p = .012), and activities of daily living (ADL) (0.461 [0.25–0.671], p < .001), compared with controls. There was a significant difference in motor impairment (0.39 [0.134–0.647], p = .003), motor function (0.298 [0.003–0.593], p = .048), and ADL (0.461 [0.157–0.766], p = .003) in favor of bimanual MT compared with controls. No significant effect was found for unimanual MT.
Conclusion
MT, specifically bimanual MT, is an effective intervention for improving motor recovery, motor function, and ADL in individuals with subacute stroke, whereas unimanual MT does not show significant benefits in these areas.
INTRODUCTION
Stroke is the third‐leading cause of disability in adults worldwide. 1 Motor impairment is one of the causes of disability after stroke. More than 50% of people who have had a stroke report a loss or impairment in upper limb mobility, 2 , 3 which affects their ability to perform activities of daily living (ADL) and affects their quality of life. 4 , 5 Of the stroke survivors with mild upper extremity paresis and severe upper extremity paresis, 79% and 18% achieved full upper limb function, respectively. 6 Therefore, it is critical to have stroke rehabilitation for improvement in upper limb mobility.
To help stroke survivors regain their motor function, various rehabilitation methods have been developed. Some of these methods are using only the affected arm (constraint‐induced movement therapy), using a mirror to create a visual illusion of the affected arm (mirror therapy), imagining moving the affected arm (motor imagery training), applying electric currents to stimulate the muscles (functional electric stimulation), applying magnetic pulses to stimulate the brain (repetitive transcranial magnetic stimulation), using a robotic device to assist or resist movement (robot‐assisted therapy), and using a computer‐generated environment to simulate real‐life tasks (virtual reality training). These methods are all aimed at improving upper extremity function. Mirror therapy (MT) is simple, easy to use, and inexpensive among rehabilitation methods. Dr Ramachandran first used MT to create an illusion of movement in the phantom limb of a patient. 7 This was a method to trick the patient's brain and reduce the symptoms of phantom limb. 8 In MT, the affected limb is hidden behind a mirror. The unaffected limb is moved in front of the mirror, creating a visual illusion that the affected limb is moving, too. MT procedures can be applied unimanually to the unaffected limb or bimanually to both limbs. Unimanual MT consists of moving the unaffected limb and seeing its reflection in the mirror while pretending that the affected limb is also moving. The therapist guides the patient through this process. Alternatively, the patient can try bimanual MT, moving both limbs to the best of their ability.
Three possible mechanisms have been proposed to explain the neurophysiological basis of mirror therapy: (1) visually oriented motor imagery, (2) the mirror neuron system, and (3) the unlearning of learned paralysis. First, motor imagery training refers to mentally imitating the movement without actual execution. It improves motor ability by producing brain activity similar to actual movement actions, such as in the premotor area, parietal areas, basal ganglia, and cerebellum. Recent studies found significant improvements in motor performance after motor imagery training in individuals with stroke. 9 The visual perception of movement during MT activates the occipital area on the ipsilesional side. The brain areas deal with visuomotor information directly to the anterior intraparietal sulcus or from the superior occipital gyrus to the posterior parietal cortex. After receiving visuomotor information, the ipsilesional primary somatosensory cortex activates afterward, whereas the premotor area activates later following MT sessions. Meanwhile, increased callosal communication between the hemispheres also occurs to balance the interhemispheric inhibition. 10 Second, the mirror neuron system can identify movement complexity and imitate what we see, hear, or perceive unconsciously. 11 , 12 , 13 It is mainly located in the frontal lobe, parietal lobe, ventral premotor area, and inferior frontal gyrus. 14 , 15 , 16 Therefore, MT used the mirror's reflective surface to project the movements of the unaffected side and observe the activities in the mirror to activate mirror neurons to achieve the effect of motor learning. Third, the affected arm provides negative visual feedback and causes learned paralysis. Conversely, MT provides positive visual input from the unaffected arm, which reactivates cortical motor neurons. 17
MT has become a common rehabilitation technique for individuals with stroke. Several studies suggested that MT may have positive effects on individuals with stroke. A few systematic reviews and meta‐analyses have been conducted to summarize the findings of individual studies and reexamine the effect of MT on motor function in individuals with stroke. 18 , 19 , 20 , 21 , 22 , 23 These systematic review and meta‐analysis studies included participants who had experienced a stroke, regardless of whether they were in the subacute phase (within 6 months of the stroke) or the chronic phase (more than 6 months after the stroke). Two systematic reviews suggested that MT showed a better effect on recovery of upper limb function in individuals with subacute and chronic stroke than control intervention (sham MT or conventional rehabilitation). 18 , 22 The other four systematic reviews performed a meta‐analysis. Three of them showed that MT may significantly improve motor impairment, upper limb function, or ADL in individuals with acute, subacute, and chronic stroke, but the results showed moderate to high heterogeneity. 19 , 20 , 21 Although Zeng et al. reported that the onset time of stroke might not cause heterogeneity, 21 one of these meta‐analytic studies reported that MT did not show a significant advantage over control, but it seems to benefit individuals in the subacute phase of the stroke. 23 This statement is speculative and lacks direct evidence to support it. Further studies are needed to confirm the effects of MT in different poststroke phases. Moreover, there is a lack of consensus on unimanual and bimanual training in MT studies. 23
The recovery from a stroke is often described by the poststroke phase. According to the Stroke Roundtable Consortium, the different phases of stroke recovery are the hyperacute phase (the first 24 hours), the acute phase (the first week), the early subacute phase (the first 3 months), the late subacute phase (months 4–6), and the chronic phase (from 6 months onwards). 24 Spontaneous recovery is defined by Kwakkel et al. 25 to describe how function and activity improve over time for stroke survivors. Previous studies reported that most spontaneous motor function recovery happens within the first 3 months post stroke. 6 , 25 , 26 This time perspective is a crucial target for intervention to optimize the effectiveness of rehabilitation. Therefore, it is necessary to clarify the effects of training in different phases after a stroke. A recent systematic review reported that most studies showed that MT improved upper limb recovery among individuals with chronic stroke. 27 However, there are limited systematic reviews and/or meta‐analyses emphasizing the effects of MT in individuals with subacute stroke.
Therefore, the current study conducted a systematic review and meta‐analysis to investigate the effect of MT on motor and functional recovery of the upper limb and to examine the effect of unimanual or bimanual MT in individuals with subacute stroke. This focused approach to subacute stroke allowed for a more detailed analysis, clearer conclusions, and more precise clinical application. Additionally, the present study examined the effects of unimanual and bimanual MT, which may differ from previous reviews and could provide new insights.
METHODS
The review protocol was prospectively registered in International Prospective Register of Systematic Reviews (PROSPERO) in August 2022 (CRD42022354060). The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 28
Eligibility criteria
Study design
Randomized controlled trials (RCTs), quasi‐RCTs, pilot RCTs, and controlled clinical trials that compared MT with sham MT or conventional therapy were included. Review articles, case reports, qualitative studies, and studies that did not describe their procedures were excluded.
Participants
Studies conducted in individuals with a clinical diagnosis of stroke in the subacute stage (<6 months) were included regardless of age or stroke subtypes.
Intervention
Conventional MT or task‐specific MT with or without conventional therapy was the primary intervention in the experimental group. Conventional therapy with or without sham MT was the control intervention in the control group. Studies with different forms of MT or interventions not routinely used in clinical settings performed in the control group were excluded.
Outcomes
Primary outcomes were motor recovery evaluated by the upper extremity part of the Fugl‐Meyer Assessment (FMA‐UE) and upper limb function assessed by the action research arm test (ARAT), Bhakta scale, box and block test (BBT), or Wolf motor function test (WMFT). Secondary outcomes were ADL evaluated by the Barthel index (BI), modified Barthel index (MBI), functional independence measure (FIM), or instrumental activities of daily living (IADL), and quality of life evaluated by stroke impact scale (SIS). According to the International Classification of Functioning, Disability, and Health framework, motor recovery falls under the Body Functions domain; upper limb function is categorized within the Activities and Participation domain; ADL also fits into the Activities and Participation domain; and quality of life aligns with both the Activities and Participation domain and the Environmental Factors domain.
Search strategy
A literature search was performed in the following databases for studies published in English or Chinese and the publication year from January 1, 2005, to September 8, 2024: PubMed, Physiotherapy Evidence Database (PEDro), Cochrane, Airiti Library, and National Digital Library of Theses and Dissertations in Taiwan. To build the search strategy, the following keywords were used to search for possible studies on the effects of MT on motor and functional recovery in individuals with stroke: “(mirror therapy)” AND “(stroke) OR (CVA) OR (cerebrovascular accident) OR (hemiplegia)” AND “(upper limb).” The PubMed search strategies are outlined in Appendix A. Reference lists of included studies were examined for potentially eligible studies.
Study selection
After the literature search, all studies were recorded on a Microsoft Excel spreadsheet; 6 duplicates were found and removed. Then, titles and abstracts of the remaining studies were assessed independently by three researchers (T.Y.Y., Y.L.H., and Z.Y.P.) to exclude irrelevant studies based on the eligibility criteria. Finally, full texts were independently reviewed by the same researchers to determine the eligibility for inclusion. Disagreements were resolved through discussions among the three researchers or by consulting another researcher (Y.R.Y.) until a consensus was reached.
Data extraction
The same three researchers independently reviewed eligible studies. The following data were extracted from each study: publication information (authors and year), demographic characteristics (number, age, paretic side, time since stroke onset, and severity by FMA‐UE), methods (study design, intervention details, and outcome measures), and results. For grading of severity, FMA‐UE > 45 was defined as mild, ≥30 and ≤45 was described as moderate, and <30 was defined as severe. 29
Quality appraisal
The methodological quality of the included studies was independently evaluated by two researchers using the PEDro scale and the Cochrane risk‐of‐bias tool for randomized trials (RoB). The PEDro scale contains 11 items, and items 2–11 are scored for a total score between 0 and 10. 30 , 31 It has been suggested that studies scoring 9–10 are considered excellent, 6–8 are considered good, 4–5 are considered fair, and <4 are considered poor quality. 30 , 31 A PEDro score of less than four indicates poor methodological quality, which can lead to biased results and unreliable conclusions. 31 , 32 Therefore, any study with a PEDro score of <4 was excluded from further analysis. RoB contains seven bias items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. 33 The risk‐of‐bias judgment of each domain and overall bias were determined as low risk of bias, unclear risk of bias, or high risk of bias. 33 Any evaluation discrepancies between the two researchers were resolved through discussion to reach a consensus.
Data synthesis and analysis
A meta‐analysis was performed using Comprehensive Meta‐Analysis version 2.0. Selected outcomes were compared between the experimental and control groups, with the effect size described as the standardized mean difference (SMD) with a 95% confidence interval (CI). SMD of 0.2, 0.5, and 0.8 were considered small, medium, and large effects, respectively. 34 Heterogeneity among included studies was assessed using the I 2 statistics, with I 2 of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. 35 A p <.1 was considered to be the existence of significant heterogeneity. Egger's regression test was performed to examine the possibility of publication bias. Due to the variation of participants' characteristics, intervention, and outcome measures, a random‐effects model was used for meta‐analysis. 36 P values <.05 were considered statistically significant.
RESULTS
Search results
The database searches identified 3169 potentially relevant articles. Six articles were removed due to repeated appearance in each database. By filtering publication year and article type, 2197 articles remained. After reviewing the titles and abstracts, we excluded 2182 articles that did not meet the inclusion criteria, leaving 15 for full‐text review. A total of 15 articles with 546 participants were included in the systematic review and meta‐analysis. Figure 1 shows the flow chart of the study selection process.
FIGURE 1.

Flow chart of the study selection process. PEDro, Physiotherapy Evidence Database.
Characteristics of included studies
Characteristics of participants in the included studies are summarized in Table 1. The 15 included studies recruited 546 participants, 288 in the experimental group and 258 in the control group. The range of the mean age of the participants in the included studies was 46.4–68.2 years in the experimental group and 53.9–71.1 years in the control group. The range of the mean time since stroke in the studies was 13.9 days to 4.9 months in the experimental group and 12.7 days to 3.6 months in the control group. The disease severity, as indicated by the average FMA‐UE score in the studies, ranged from 3.2 to 43.3 in the experimental group and from 4.1 to 39.6 in the control group, with three studies not providing FMA‐UE scores. The severity of the conditions in the included studies falls into the category of moderate to severe.
TABLE 1.
Characteristics of participants in included studies (n = 15).
| Study | N all | N group | Age (years), mean (SD) | Paretic side (n: right/left) | Stroke onset duration, mean (SD) | Disease severity (FMA‐UE), mean (SD) |
|---|---|---|---|---|---|---|
| Antoniotti et al., 2019 37 | 40 |
EG: 20 CG: 20 |
EG: 68.2 (14.4) CG: 69.5 (14.1) |
EG: 7/13 CG: 7/13 |
EG: 23.3 (6.57) days CG: 22 (9.28) days |
EG: 28.5 (21.8) CG: 30.9 (23.9) |
| Bai et al., 2019 38 | 34 |
EG 1: 12 EG 2: 11 CG: 11 |
EG 1: 56.08 (13.61) EG 2: 54.36 (11.56) CG: 58.27 (15.44) |
EG 1: 6/6 EG 2: 8/3 CG: 5/6 |
EG 1: 61.92 (35.35) days EG 2: 60.00 (44.41) days CG: 93.45 (59.75) days |
EG 1: 34.25 (12.21) EG 2: 37.55 (14.19) CG: 35.36 (10.62) |
| Chan and Au‐Yeung, 2018 39 | 35 |
EG: 15 CG: 20 |
EG: 65.3 (11.8) CG: 64.1 (13.3) |
EG: 5/10 CG: 8/12 |
EG: 13.9 (6.7) days CG: 12.7 (6.6) days |
EG: 19.2 (16.0) CG: 21.7 (15.1) |
| Dohle et al., 2009 40 | 36 |
EG: 18 CG: 18 |
EG: 54.9 (13.8) CG: 58.0 (14.0) |
NA |
EG: 26.2 (8.3) days CG: 27.8 (12.1) days |
NA |
| Gurbuz et al., 2016 41 | 31 |
EG: 16 CG: 15 |
EG: 60.9 (10.9) CG: 60.8 (20.0) |
EG: 8/8 CG: 9/6 |
EG: 46.1 (43.3) days CG: 42.4 (37.8) days |
EG: 12.8 (7.8) CG: 12.4 (8.9) |
| Hsieh et al., 2020 42 | 21 |
EG 1: 7 EG 2: 7 CG: 7 |
EG 1: 46.41 (13.45) EG 2: 52.77 (11.25) CG: 54.30 (13.61) |
EG 1: 4/3 EG 2: 2/5 CG: 3/4 |
EG 1: 4.86 (1.95) months EG 2: 2.86 (1.77) months CG: 2.57 (1.72) months |
EG 1: 43.29 (13.72) EG 2: 42.29 (11.03) CG: 39.57 (6.55) |
| Invernizzi et al., 2013 43 | 26 |
EG: 13 CG: 13 |
EG: 62 (25.87) CG: 71.1 (8.81) |
EG: 6/7 CG: 7/6 |
EG: 22 (3) days CG: 24 (2) days |
NA |
| Lee et al., 2012 44 | 26 |
EG: 13 CG: 13 |
EG: 58.8 (12.1) CG: 55.4 (12.2) |
EG: 8/5 CG: 7/6 |
EG: 3.5 (1.5) months CG: 3.6 (1.3) months |
NA |
| Lim et al., 2016 45 | 60 |
EG: 30 CG: 30 |
EG: 65.3 CG: 64.5 |
EG: 15/15 CG: 14/16 |
EG: 49.4 days CG: 53.7 days |
EG: 26.93 (6.32) CG: 26.90 (6.32) |
| Madhoun et al., 2020 46 | 30 |
EG: 15 CG: 15 |
EG: 49.33 (10.43) CG: 53.93 (8.76) |
EG: 6/9 CG: 5/10 |
EG: 4.13 (1.84) months CG: 3.60 (1.76) months |
EG: 19.33 (7.62) CG: 20.60 (12.07) |
| Mirela Cristina et al., 2015 47 | 15 |
EG: 7 CG: 8 |
EG: 58.2 (7.2) CG: 56.8 (8.3) |
EG: 5/2 CG: 5/3 |
EG: 54.3 days (7.9) CG: 52.2 days (12.7) |
EG: 34.1 (8.4) CG: 38.6 (6.2) |
| Samuelkamaleshkumar et al., 2014 48 | 20 |
EG: 10 CG: 10 |
EG: 48.4 (15.58) CG: 53.9 (11.57) |
EG: 6/4 CG: 4/6 |
EG: 3.7 (1.1) weeks CG: 4.4 (1.4) weeks |
EG: 9.7 (10) CG: 4.3 (9.9) |
| Thieme et al., 2013 49 | 60 |
EG 1: 18 EG 2: 21 CG: 21 |
EG 1: 63.8 (12.1) EG 2: 69.1 (10.2) CG: 68.3 (8.9) |
EG 1: 14/4 EG 2: 11/8 CG: 11/10 |
EG 1: 47.6 (25.8) days EG 2: 36.2 (21.1) days CG: 51.4 (22.5) days |
EG 1: 5.3 (8.6) EG 2: 3.2 (4.1) CG: 4.1 (4.6) |
| Wen et al., 2022 50 | 52 |
EG: 25 CG: 27 |
EG: 53.76 (11.76) CG: 57.89 (10.74) |
EG: 11/14 CG: 12/15 |
EG: 31 days CG: 30 days |
EG: 19.48 (16.62) CG: 20.59 (18.65) |
| Zhang et al., 2021 51 | 60 |
EG: 30 CG: 30 |
EG: 57.0 (10.4) CG: 59.9 (11.8) |
NA |
EG: 29.5 (7.8) days CG: 31.5 (8.9) days |
EG: 16.8 (5.0) CG: 15.6 (3.9) |
Abbreviations: CG, control group; EG, experimental group; FMA‐UE, Fugl‐Meyer assessment upper limb subscale; NA, not available.
A summary of the study design, intervention, assessment, and results of the included studies is presented in Table 2. Twelve included studies were RCTs, and three were pilot RCTs. Ten studies applied MT with both upper limbs moving simultaneously, 38 , 39 , 40 , 42 , 44 , 45 , 47 , 48 , 49 , 50 whereas two studies applied MT with the unaffected upper limb moving only, 37 , 46 and the remaining three studies did not mention unimanual or bimanual movement in the methods. 41 , 43 , 51 Among the included studies, eight compared MT plus conventional therapy versus sham MT plus conventional therapy 37 , 39 , 40 , 41 , 42 , 43 , 46 , 49 ; six compared MT plus conventional therapy versus conventional therapy alone 38 , 44 , 47 , 48 , 50 , 51 ; and one compared MT versus sham MT. 45 The intervention content for MT included conventional techniques, such as elbow flexion/extension and wrist flexion/extension, as well as task‐specific MT involving functional tasks like grasping objects or flipping cards. Each session lasted between 20 and 60 minutes, with an intervention frequency of 4–10 sessions per week and a total intervention duration of 3–6 weeks. The total duration of the intervention of MT in the included studies ranged from 400 to 1200 minutes.
TABLE 2.
Summary of study design, intervention, assessment, and results in included studies (n = 15).
| Study design | Interventions | Frequency and duration of MT | Assessment | Relevant findings |
|---|---|---|---|---|
|
Antoniotti et al., 2019 37 RCT |
EG: unimanual MT + CT CG: sham MT + CT |
30 min/session 5 sessions/week 6 weeks TTD: 900 min |
FMA‐UE ARAT FIM |
Both groups showed significant improvement in all relevant outcomes. No significant differences in improvement were observed between groups for all relevant outcomes. |
|
Bai et al., 2019 38 Pilot RCT |
EG 1: bimanual MMT + CT EG 2: bimanual TMT + CT CG: CT |
30 min/session 5 sessions/week 4 weeks TTD: 600 min |
FMA‐UE WMFT MBI |
Significant time effects were shown in all relevant outcomes. A significant time‐by‐group interaction effect was noted in the FMA. Improved FMA in the EG 1 was significantly more than in the other two groups. |
|
Chan and Au‐Yeung, 2018 39 RCT |
EG: bimanual MT + CT CG: sham MT + CT |
30 min/session 2 sessions/day 5 days/week 4 weeks TTD: 1200 min |
FMA‐UE WMFT |
Both groups showed significant improvement in all relevant outcomes. No significant differences in improvement were observed between groups for all relevant outcomes. |
|
Dohle et al., 2009 40 RCT |
EG: bimanual MT + CT CG: sham MT + CT |
30 min/session 5 sessions/week 6 weeks TTD: 900 min |
FMA‐UE ARAT FIM |
Both groups showed significant improvement in the ARAT. |
|
Gurbuz et al., 2016 41 RCT |
EG: MT + CT (didn't inform unimanual or bimanual) CG: sham MT + CT |
20 min/session 5 sessions/week 4 weeks TTD: 400 min |
FMA‐UE FIM |
Both groups showed significant improvement in all relevant outcomes. Improved FMA in the EG was significantly more than that in the CG. |
|
Hsieh et al., 2020 42 Pilot RCT |
EG 1: bimanual MT + CT EG 2: AOT + CT CG: sham MT + CT |
60 min/session 5 sessions/week 3 weeks TTD: 900 min |
FMA‐UE BBT FIM SIS |
Both the EG 2 and CG showed similar improvements in the FMA, BBT, and SIS. The EG 2 improved the FIM more than the other 2 groups. The EG 1 gained the most minor improvements in the outcomes. |
|
Invernizzi et al., 2013 43 RCT |
EG: MT + CT (didn't inform unimanual or bimanual) CG: sham MT + CT |
30 min/session for week 1 & 2 60 min/session for week 3 & 4 5 sessions/week 4 weeks TTD: 900 min |
ARAT FIM |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in all relevant outcomes than the CG. |
|
Lee et al., 2012 44 RCT |
EG: bimanual MT + CT CG: CT |
25 min/session 2 sessions/day 5 days/week 4 weeks TTD: 1000 min |
FMA‐UE MFT |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in all relevant outcomes than the CG. |
|
Lim et al., 2016 45 RCT |
EG: bimanual MT CG: Sham MT |
20 min/session 5 sessions/week 4 weeks TTD: 400 min |
FMA‐UE MBI |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in all relevant outcomes than the CG. |
|
Madhoun et al., 2020 46 RCT |
EG: unimanual MT + CT CG: sham MT + CT |
25 min/session 7 sessions/week 25 sessions TTD: 625 min |
FMA‐UE MBI |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in the FMA than the CG. |
|
Mirela Cristina et al., 2015 47 RCT |
EG: bimanual MT + CT CG: CT |
30 min/session 5 sessions/week 6 weeks TTD: 900 min |
FMA‐UE Bhakta scale |
The EG showed significant improvement in all relevant outcomes. The CG showed substantial improvement in the FMA. The EG had greater improvement in the Bhakta scale than the CG. |
|
Samuelkamaleshkumar et al., 2014 48 Pilot RCT |
EG: bimanual MT + CT CG: CT |
30 min/session 2 sessions/day 5 days/week 3 weeks TTD: 900 min |
FMA‐UE BBT |
The EG showed significant improvement in all relevant outcomes. The CG showed substantial improvement in the FMA. The EG had greater improvement in all relevant outcomes than the CG. |
|
Thieme et al., 2013 49 RCT |
EG 1: individual bimanual MT + CT EG 2: group bimanual MT+ CT CG: sham MT + CT |
30 min/session 20 sessions 3–5 sessions/week 5 weeks TTD: 600 min |
FMA‐UE ARAT BI SIS |
Three groups showed significant improvement in all relevant outcomes. No significant differences in improvement were observed between groups for all relevant outcomes. |
|
Wen et al., 2022 50 RCT |
EG: bimanual MT + CT CG: CT |
30 min/session 6 sessions/week 3 weeks TTD: 540 min |
FMA‐UE ARAT IADL |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in the FMA and IADL than the CG. |
|
Zhang et al., 2021 51 RCT |
EG: MT + CT (didn't inform unimanual or bimanual) CG: CT |
60 min/session 5 sessions/week 4 weeks TTD: 1200 min |
FMA‐UE MBI |
Both groups showed significant improvement in all relevant outcomes. The EG had more remarkable improvement in all relevant outcomes than the CG. |
Abbreviations: AOT, action observation therapy; ARAT, action research arm test; BBT, box and block test; BI, Barthel index; CG, control group; CT, conventional therapy; EG, experimental group; FIM, functional independence measure; FMA, Fugl‐Meyer assessment upper limb subscale; IADL, instrumental activities of daily living; MBI, modified Barthel index; MFT, manual function test; MMT, movement‐based mirror therapy; MT, mirror therapy; RCT, randomized controlled trial; SIS, stroke impact scale; TMT, task‐based mirror therapy; TTD, total training duration; WMFT, Wolf motor function test.
Quality of included studies
The methodologic quality of the included studies is summarized in Table 3. The quality score of the included studies ranged from 5 to 8; 10 studies were good (PEDro score = 6–8), and five were fair (PEDro score = 5). One study was excluded overall due to low methodological quality (PEDro score = 3). 52 All studies met these criteria: (1) item 2: participants were randomly allocated to groups; (2) item 10: the results of between‐group statistical comparisons are reported for at least one key outcome; and (3) item 11: the study provides both point measures and measures of variability for at least one key outcome. On the other hand, all studies did not meet the following criteria: (1) item 5: there was blinding of all participants, and (2) item 6: there was blinding of all therapists who administered the therapy.
TABLE 3.
Quality assessment using the PEDro scale.

|
Figure 2 presents the risk of bias for the included studies. The method for generating the allocation sequence was reported in detail in 14 of 15 studies. Only three studies described the method used to conceal the allocation sequence sufficiently. None of the studies blinded the participants and the therapists who delivered the therapy. Blinding of outcome assessment was reported in 11 studies. Nine studies described the completeness of outcome data for each primary outcome. Fourteen studies were assessed as having a low risk of reporting bias, and 13 were assessed as having a low risk of other bias.
FIGURE 2.
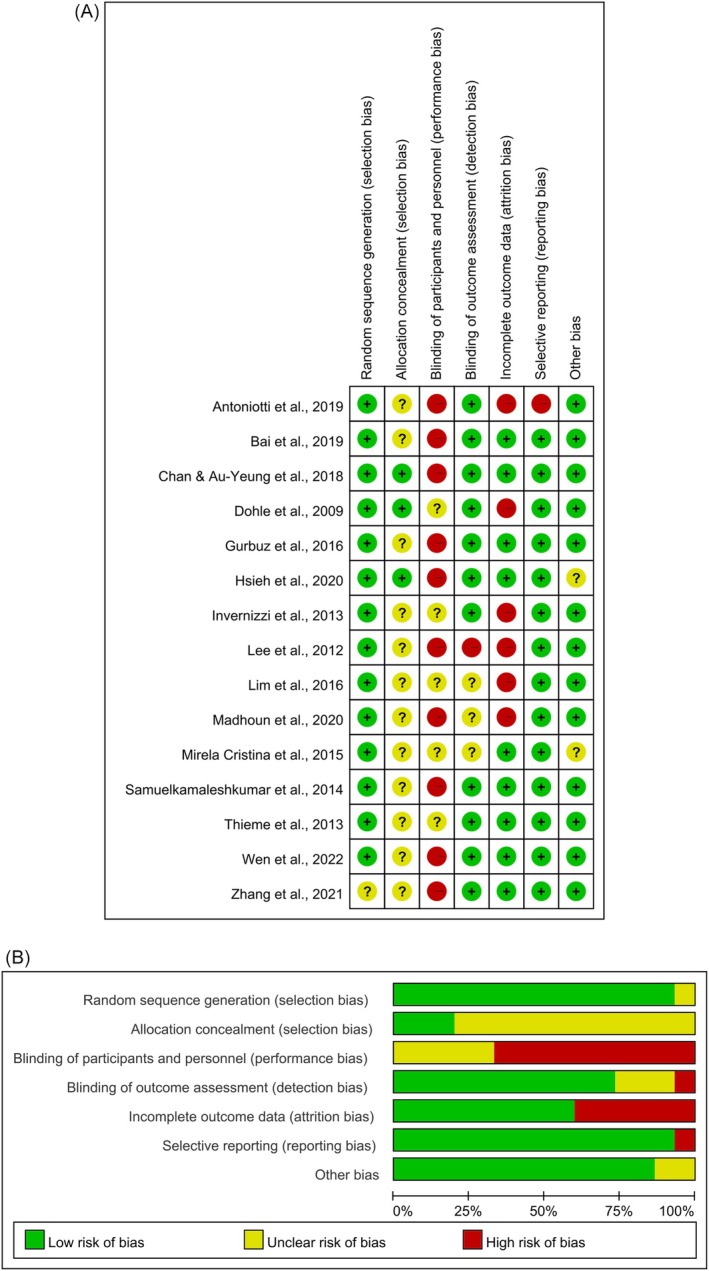
Risk of bias summary (A) and risk of bias graph (B).
Effect of mirror therapy on selected outcomes
Motor recovery
Fourteen studies evaluated motor recovery of the upper limb by measuring the FMA‐UE. Due to the absence of data 40 or the unavailability of the FMA‐UE total score, 44 , 49 three studies were not included in the meta‐analysis. Data from 11 studies with 12 comparisons, 37 , 38 , 39 , 41 , 42 , 45 , 46 , 47 , 48 , 50 , 51 regardless of whether they involved bimanual or unimanual MT, were included in the meta‐analysis. Figure 3A presents a more significant effect of MT on motor recovery than the control intervention (SMD = 0.473, 95% CI = [0.274–0.673]) with low heterogeneity (I 2 = 0%, p = .529), and the value of overall effect was 4.646 (p < .001). The Egger's regression test showed no significant publication bias among the 11 studies (t = 0.098, 95% CI = [−2.916 to 2.670], p = .924).
FIGURE 3.

Forest plots illustrating the effects of mirror therapy, regardless of whether bimanual or unimanual, on motor recovery (A), the effects of bimanual mirror therapy on motor recovery (B), and the effects of unimanual mirror therapy on motor recovery (C). CI, confidence interval; CT, conventional therapy; MMT, movement‐based mirror therapy; MT, mirror therapy; TMT, task‐based mirror therapy.
Ten studies applied bimanual MT. Seven studies with eight comparisons 38 , 39 , 42 , 45 , 47 , 48 , 50 reported total scores of the FMA‐UE and were included for meta‐analysis. Figure 3B shows that MT has a significantly better effect on motor recovery (SMD = 0.390, 95% CI = [0.134–0.647]) with low heterogeneity (I 2 = 0%, p = .859), and the value of overall effect was 2.986 (p = .003). The Egger regression test detected no significant publication bias among the seven studies (t = 0.458, 95% CI = [−1.966 to 2.871], p = .663).
Two studies applied unimanual MT and reported the effect on motor recovery. These two studies with two comparisons 37 , 46 were included for meta‐analysis. Figure 3C shows no significant difference between MT and control intervention (SMD = 0.238, 95% CI = [−0.284 to 0.760]) with low heterogeneity (I 2 = 17.508%, p = .271) and the value of overall effect was 0.892 (p = .372).
Upper limb function
Ten studies evaluated upper limb function using the ARAT, Bhakta scale, BBT, or WMFT. Data from the 10 studies with 12 comparisons 37 , 38 , 39 , 40 , 42 , 43 , 47 , 48 , 49 , 50 regardless of whether they involved bimanual or unimanual MT, were included in the meta‐analysis. Figure 4A presents that MT achieved a more significant effect on improving upper limb function than the control intervention (SMD = 0.266, 95% CI = [0.059–0.474]) with low heterogeneity (I 2 = 0%, p = .838) and the value of overall effect was 2.512 (p = .012). The Egger regression test did not reveal any significant evidence of publication bias among the 10 studies (t = 1.741, 95% CI = [−0.496 to 4.046], p = .112).
FIGURE 4.

Forest plots illustrating the effects of mirror therapy, regardless of whether bimanual or unimanual, on upper limb function (A), the effects of bimanual mirror therapy on upper limb function (B), and the effects of unimanual mirror therapy on upper limb function (C). CI, confidence interval; CT, conventional therapy; MMT, movement‐based mirror therapy; MT, mirror therapy; TMT, task‐based mirror therapy.
Six studies applying bimanual MT with seven comparisons 38 , 39 , 42 , 47 , 48 , 50 reported the related measures of upper limb function and were included in the meta‐analysis. Figure 4B shows that MT has a significantly better effect on upper limb function (SMD = 0.298, 95% CI = [0.003–0.593]) with low heterogeneity (I 2 = 0%, p = .861), and the value of overall effect was 1.981 (p = .048). The Egger regression test detected no significant publication bias among the six studies (t = 0.983, 95% CI = [−1.735 to 3.885], p = .371).
Only one study reported the effect of the unimanual MT on upper limb function, and the data were included in the meta‐analysis. 37 Figure 4C shows no significant difference between MT and control intervention (SMD = 0.012, 95% CI = [−0.607 to 0.632]) and the value of overall effect was 0.039 (p = .969).
Activities of daily living
Eleven studies evaluated ADL by the BI, MBI, FIM, or IADL. Data from the 11 studies with 13 comparisons 37 , 38 , 40 , 41 , 42 , 43 , 45 , 46 , 49 , 50 , 51 regardless of whether they involved bimanual or unimanual MT, were included for meta‐analysis. Figure 5A shows a significant overall beneficial effect of MT on ADL (SMD = 0.461, 95% CI = [0.250–0.671]) with low heterogeneity (I 2 = 21.43%, p = .227), and the value of overall effect was 4.294 (p < .001). The Egger regression test detected no significant publication bias among the 11 studies (t = 0.445, 95% CI = [−4.571 to 3.033], p = .665).
FIGURE 5.

Forest plots illustrating the effects of mirror therapy, regardless of whether bimanual or unimanual, on activities of daily living (A), the effects of bimanual mirror therapy on activities of daily living (B), and the effects of unimanual mirror therapy on activities of daily living (C). CI, confidence interval; CT, conventional therapy; MMT, movement‐based mirror therapy; MT, mirror therapy; TMT, task‐based mirror therapy.
Four studies applying bimanual MT with five comparisons 38 , 42 , 45 , 50 reported the related measures of ADL and were included in the meta‐analysis. Figure 5B shows that MT has a significantly better effect on ADL (SMD = 0.461, 95% CI = [0.157–0.766]) with low heterogeneity (I 2 = 0%, p = .928), and the value of overall effect was 2.972 (p = .003). The Egger regression test detected no significant publication bias among the four studies (t = 1.411, 95% CI = [−3.274 to 1.262], p = .253).
Two studies reported the effect of the unimanual MT on ADL, and the data were included in the meta‐analysis. 37 , 46 Figure 5C shows no significant difference between MT and control intervention (SMD = 0.181, 95% CI = [−0.288 to 0.651]) with low heterogeneity (I 2 = 0%, p = .817) and the value of overall effect was 0.757 (p = .449).
Quality of life
Two studies evaluated quality of life by the SIS. Data from two studies with three comparisons 42 , 49 were included for meta‐analysis. Figure 6 shows that there is no significant effect of MT on quality of life (SMD = 0.110, 95% CI = [−0.300 to 0.520]) with low heterogeneity (I 2 = 0%, p = .932) and the value of overall effect was 0.526 (p = .599). Due to the lack of adequate studies, the meta‐analysis of bimanual and unimanual MT could not be conducted.
FIGURE 6.
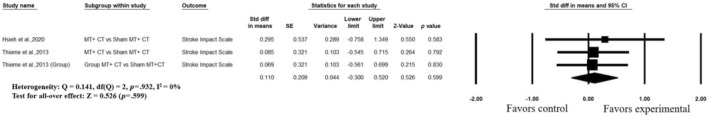
Forest plots illustrating the effects of mirror therapy on quality of life. CI, confidence interval.
DISCUSSION
This systematic review and meta‐analysis investigated the effect of MT on motor and functional recovery and the effect of unimanual and bimanual MT in individuals with subacute stroke. This study reviewed 12 RCTs and 3 pilot RCTs involving a total of 546 participants with subacute stroke. Of 15 studies, 10 used bimanual MT, and 2 used unilateral MT. The included studies had low heterogeneity and presented a moderate risk of selection, detection, and attrition bias and a high risk of performance bias. The results of the present meta‐analysis suggested that MT is more effective than control treatment on motor impairment, upper limb function, and ADL in individuals with subacute stroke. In addition, compared to the control, the bimanual procedure showed a more significant effect on motor impairment, upper limb function, and ADL, but the unimanual procedure did not.
FMA was the outcome measure used most often to measure the effectiveness of MT for upper‐limb motor recovery. The current finding was in line with the results of previous meta‐analyses that suggested that MT significantly improved FMA compared to control therapy. 19 , 20 , 21 Contrasting to the earlier meta‐analyses with high heterogeneity (I 2 = 61%–84.8%), 19 , 20 , 21 the present meta‐analysis showed low heterogeneity (I 2 = 0%). The enrolled studies in the present meta‐analysis included only studies conducted in individuals with stroke in the subacute stage, consequently leading to low heterogeneity. Furthermore, the results of the current meta‐analysis supported the inference of Nogueira et al. that MT has a beneficial effect on motor recovery of the upper extremities in the subacute phase after stroke. 23
Another critical outcome, besides motor recovery, is functional recovery. Various measures, including ARAT, Bhakta scale, BBT, or WMFT, were used by previous studies to evaluate upper limb function. These functional‐related outcomes were frequently pooled together for meta‐analysis. Our finding that upper limb function improved with MT was in line with the meta‐analysis by Thieme et al. 19 Thieme et al. reported a statistically significant effect of MT on motor function compared to other interventions for subacute and chronic subgroups with high heterogeneity (I 2 = 59%–68%). 19 In contrast, upper limb function did not improve significantly with MT according to the meta‐analysis by Nogueira et al. 23 The inconsistency of the recent meta‐analysis with our results may be because Nogueira et al. mixed data of FMA, Brunnstrom stages, and ARAT as representations of motor and functional recovery and involved participants in different recovery stages (from acute to chronic). Nogueira et al. suggested that although the meta‐analyses were insignificant, MT was better than sham therapy, mainly in the subacute stage. 23 According to these meta‐analysis results, the main advantage of MT over control therapy may be in the subacute phases.
People with stroke may consider independence in ADL to be the most important thing. 53 Previous meta‐analyses had conflicting findings on the effect of MT on ADL. Although two studies found a positive impact of MT on ADL, 19 , 20 another study did not find any significant results. 23 To avoid heterogeneity from the recovery phase, a recent systematic review only focused on studies with people in the chronic phase. It reported no consistency in the improvement in ADL due to MT. 27 The current meta‐analysis differed from the previous systematic review by including only studies with people in the subacute phase. We found that MT improved ADL more than control therapy. The present study showed consistent results in the positive effects of MT on motor impairment, upper limb function, and ADL. It is reasonable to infer that MT improves motor impairment and upper limb function and, consequently, improves ADL.
Another outcome worthy of attention is quality of life. No previous meta‐analyses have explored the effect of MT on quality of life. Only a review by Gandhi et al. reported that quality of life did not improve with MT in one study measuring the EuroQol‐5 dimension. 22 This outcome was also included in the current research, and, consistently, our results did not find any positive indication for it. This might be because only two studies with 74 participants evaluated the outcomes relating to quality of life. More extensive analysis of MT's impact on quality of life after stroke is required in larger populations.
In clinical settings, stroke rehabilitation can involve either unimanual or bimanual MT. A secondary meta‐analysis of the 2018 Cochrane Review update found that unimanual and bimanual MT improved motor impairment and function in individuals with stroke throughout the acute to chronic stages. 54 Unimanual MT showed a more significant effect on motor impairment and function than bimanual MT. 54 The present study focused on studies with subacute phases after stroke and found positive results for the effects of bimanual MT on motor impairment, motor function, and ADL. However, unimanual MT did not produce the same results. Only two studies that applied unimanual MT were included in the present meta‐analysis. Both studies examined the effects of combining unimanual MT with conventional therapy in contrast to combining sham MT with conventional treatment but yielded inconsistent results. 37 , 46 The MT intervention in Antoniotti et al. included simple, complex, and functional movements with the mirror, 37 whereas the MT intervention in Madhoun et al. focused on functional tasks with the mirror. 46 Overall, the effects of unimanual MT did not show significant improvements in motor impairment, motor function, and ADL. Several factors may influence the effects of MT, including the level of impairment of the upper extremity, 55 spontaneous recovery after stroke, 37 , 49 mirror size, 54 MT exercises with manipulation, 54 and total training duration. 18 Both studies used small mirrors and exercises to manipulate objects, which may have resulted in a small or insignificant effect. 54 Because research on the effects of unimanual MT in the subacute stage after stroke is limited and inconclusive, further extensive randomized controlled trials are necessary.
Ten studies that applied bimanual MT were included in the present study. Seven studies with eight comparisons reported the FMA‐UE, 38 , 39 , 42 , 45 , 47 , 48 , 50 six studies with seven comparisons reported the upper limb function, 38 , 39 , 42 , 47 , 48 , 50 and four studies with five comparisons reported the ADL 38 , 42 , 45 , 50 and were included for meta‐analysis. In line with a recent meta‐analysis, 54 our results showed that bimanual MT or combining bimanual MT with conventional therapy had a greater effect on motor impairment, function, and ADL than sham MT, conventional treatment, or the combination of sham MT with conventional therapy. There were three studies not included in the meta‐analysis. Lee et al. reported that MT, in combination with standard rehabilitation, had a more significant effect on motor impairment and motor function than standard rehabilitation. 44 Dohle et al. and Thieme et al. demonstrated no different effect on motor impairment, motor function, ADL, and quality of life when comparing a combination of MT with conventional therapy and a combination of sham MT with conventional treatment. 40 , 49 It is noteworthy that out of the six trials suggesting a more favorable impact of bimanual motor training compared to the control condition, five studies involved additional MT exposure for the experimental group beyond standard rehabilitation. In contrast, the control groups did not receive equivalent doses of alternative therapies. 38 , 44 , 47 , 48 , 50 Although our findings suggest that bimanual MT might yield superior outcomes compared to the control condition, it is crucial to interpret these results cautiously, as the higher training intensity in the experimental group may be a confounding factor.
Although the studies included in the present study did not examine the neurophysiological basis of MT, some evidence has been reported. Previous functional magnetic resonance imaging and magnetoencephalography studies have shown that MT can induce greater symmetry in the activity of the primary motor cortex between the two hemispheres. 56 , 57 Additionally, some electroencephalogram studies have demonstrated that MT modulates alpha, beta, and mu rhythms in individuals with stroke. 27 However, neurophysiological findings in MT studies remain limited and require further investigation to explain the underlying mechanisms and to advance the understanding of brain activity during MT.
The robustness of the present study lies in its homogeneity among included studies and its focus on analyzing only RCTs, ensuring high reliability and offering solid scientific evidence. However, this study has some limitations. First, the number of studies is small. Second, only studies published in English were included. Third, as only one study completed follow‐up data collection, we excluded follow‐up analysis from the present study.
The present systematic review and meta‐analysis provided evidence that MT can improve motor recovery of the upper extremity, upper limb function, and activities of daily living in individuals with subacute stroke. Bimanual MT showed a more significant effect on motor recovery, motor function, and activities of daily living than control intervention in individuals with subacute stroke. More well‐designed, randomized controlled studies are needed to determine the effects of unimanual MT on motor function of the upper extremity in subacute stroke.
DISCLOSURES
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
The review protocol was prospectively registered in PROSPERO in August 2022 (CRD42022354060).
ACKNOWLEDGMENTS
The authors express gratitude toward the original researchers for sharing the data required for the present study. This study was carried out with research funding from the National Science and Technology Council, Taiwan (MOST 110‐2314‐B‐A49A‐508‐MY3).
APPENDIX A. Search Strategy for PubMed
“(mirror therapy)” AND “(stroke) OR (CVA) OR (hemiplegia)” AND “(upper limb).”
Hsieh Y‐L, Yang T‐Y, Peng Z‐Y, Wang R‐Y, Shih H‐T, Yang Y‐R. Effects of mirror therapy on motor and functional recovery of the upper extremity in subacute stroke: Systematic review and meta‐analysis. PM&R. 2025;17(5):567‐581. doi: 10.1002/pmrj.13316
Tzu‐Ying Yang and Zi‐You Peng contributed equally to this paper.
REFERENCES
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease study 2019. Lancet Neurol. 2021;20:795‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker VM, Wade DT, Langton HR. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med. 1986;8:69‐73. [DOI] [PubMed] [Google Scholar]
- 3. Wade DT. Measuring arm impairment and disability after stroke. Int Disabil Stud. 1989;11:89‐92. [DOI] [PubMed] [Google Scholar]
- 4. Omar NH, Mohd Nordin NA, Chai SC, Abdul Aziz AF. Functionality among stroke survivors with upper limb impairment attending community‐based rehabilitation. Med J Malaysia. 2020;75:146‐151. [PubMed] [Google Scholar]
- 5. Franceschini M, La Porta F, Agosti M, et al. Is health‐related‐quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010;46:389‐399. [PubMed] [Google Scholar]
- 6. Nakayama H, Jørgensen HS, Raaschou HO, et al. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394‐398. [DOI] [PubMed] [Google Scholar]
- 7. Ramachandran VS. Phantom limbs, neglect syndromes, repressed memories, and Freudian psychology. Int Rev Neurobiol. 1994;37:291‐333. [DOI] [PubMed] [Google Scholar]
- 8. Ramachandran VS, Rogers‐Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377‐386. [DOI] [PubMed] [Google Scholar]
- 9. Bovonsunthonchai S, Aung N, Hiengkaew V, Tretriluxana J. A randomized controlled trial of motor imagery combined with structured progressive circuit class therapy on gait in stroke survivors. Sci Rep. 2020;10:6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arya KN. Underlying neural mechanisms of mirror therapy: implications for motor rehabilitation in stroke. Neurol India. 2016;64:38‐44. [DOI] [PubMed] [Google Scholar]
- 11. Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942‐951. [DOI] [PubMed] [Google Scholar]
- 12. Gazzola V, Aziz‐Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824‐1829. [DOI] [PubMed] [Google Scholar]
- 13. Le Bel RM, Pineda JA, Sharma A. Motor‐auditory‐visual integration: the role of the human mirror neuron system in communication and communication disorders. J Commun Disord. 2009;42:299‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Umiltà MA, Escola L, Intskirveli I, et al. When pliers become fingers in the monkey motor system. Proc Natl Acad Sci U S A. 2008;105:2209‐2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Der Werf YD, Van Der Helm E, Schoonheim MM, et al. Learning by observation requires an early sleep window. Proc Natl Acad Sci U S A. 2009;106:18926‐18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamzei F, Läppchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair. 2012;26:484‐496. [DOI] [PubMed] [Google Scholar]
- 18. Pérez‐Cruzado D, Merchán‐Baeza JA, González‐Sánchez M, Cuesta‐Vargas AI. Systematic review of mirror therapy compared with conventional rehabilitation in upper extremity function in stroke survivors. Aust Occup Ther J. 2017;64:91‐112. [DOI] [PubMed] [Google Scholar]
- 19. Thieme H, Morkisch N, Mehrholz J, et al. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev. 2018;7:Cd008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Y, Zhao Q, Zhang Y, Wu Q, Jiang X, Cheng G. Effect of mirror therapy on recovery of stroke survivors: a systematic review and network meta‐analysis. Neuroscience. 2018;390:318‐336. [DOI] [PubMed] [Google Scholar]
- 21. Zeng W, Guo Y, Wu G, Liu X, Fang Q. Mirror therapy for motor function of the upper extremity in patients with stroke: a meta‐analysis. J Rehabil Med. 2018;50:8‐15. [DOI] [PubMed] [Google Scholar]
- 22. Gandhi DB, Sterba A, Khatter H, et al. Mirror therapy in stroke rehabilitation: current perspectives. Ther Clin Risk Manag. 2020;16:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nogueira N, Parma JO, Leão S, et al. Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: a systematic review and meta‐analysis. Brain Res Bull. 2021;177:217‐238. [DOI] [PubMed] [Google Scholar]
- 24. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. 2017;12:444‐450. [DOI] [PubMed] [Google Scholar]
- 25. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181‐2186. [DOI] [PubMed] [Google Scholar]
- 26. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27‐32. [DOI] [PubMed] [Google Scholar]
- 27. Jaafar N, Che Daud AZ, Ahmad Roslan NF, Mansor W. Mirror therapy rehabilitation in stroke: a scoping review of upper limb recovery and brain activities. Rehabil Res Pract. 2021;2021:9487319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coscia M, Wessel MJ, Chaudary U, et al. Neurotechnology‐aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. 2019;142:2182‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foley NC, Teasell RW, Bhogal SK, Doherty T, Speechley MR. Stroke rehabilitation evidence‐based review: methodology. Top Stroke Rehabil. 2003;10:1‐7. [PubMed] [Google Scholar]
- 31. Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) scale. J Physiother. 2020;66:59. [DOI] [PubMed] [Google Scholar]
- 32. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713‐721. [PubMed] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta‐analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dettori JR, Norvell DC, Chapman JR. Fixed‐effect vs random‐effects models for meta‐analysis: 3 points to consider. Global Spine J. 2022;12:1624‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antoniotti P, Veronelli L, Caronni A, et al. No evidence of effectiveness of mirror therapy early after stroke: an assessor‐blinded randomized controlled trial. Clin Rehabil. 2019;33:885‐893. [DOI] [PubMed] [Google Scholar]
- 38. Bai Z, Zhang J, Zhang Z, Shu T, Niu W. Comparison between movement‐based and task‐based mirror therapies on improving upper limb functions in patients with stroke: a pilot randomized controlled trial. Front Neurol. 2019;10:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan WC, Au‐Yeung SSY. Recovery in the severely impaired arm post‐stroke after mirror therapy: a randomized controlled study. Am J Phys Med Rehabil. 2018;97:572‐577. [DOI] [PubMed] [Google Scholar]
- 40. Dohle C, Püllen J, Nakaten A, Küst J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. 2009;23:209‐217. [DOI] [PubMed] [Google Scholar]
- 41. Gurbuz N, Afsar SI, Ayaş S, Cosar SNS. Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. J Phys Ther Sci. 2016;28:2501‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsieh YW, Lin YH, Zhu JD, Wu CY, Lin YP, Chen CC. Treatment effects of upper limb action observation therapy and mirror therapy on rehabilitation outcomes after subacute stroke: a pilot study. Behav Neurol. 2020;2020:6250524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Invernizzi M, Negrini S, Carda S, Lanzotti L, Cisari C, Baricich A. The value of adding mirror therapy for upper limb motor recovery of subacute stroke patients: a randomized controlled trial. Eur J Phys Rehabil Med. 2013;49:311‐317. [PubMed] [Google Scholar]
- 44. Lee MM, Cho HY, Song CH. The mirror therapy program enhances upper‐limb motor recovery and motor function in acute stroke patients. Am J Phys Med Rehabil. 2012;91:689‐696. [DOI] [PubMed] [Google Scholar]
- 45. Lim KB, Lee HJ, Yoo J, Yun HJ, Hwang HJ. Efficacy of mirror therapy containing functional tasks in poststroke patients. Ann Rehabil Med. 2016;40:629‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Madhoun HY, Tan B, Feng Y, Zhou Y, Zhou C, Yu L. Task‐based mirror therapy enhances the upper limb motor function in subacute stroke patients: a randomized control trial. Eur J Phys Rehabil Med. 2020;56:265‐271. [DOI] [PubMed] [Google Scholar]
- 47. Mirela Cristina L, Matei D, Ignat B, Popescu CD. Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurol Belg. 2015;115:597‐603. [DOI] [PubMed] [Google Scholar]
- 48. Samuelkamaleshkumar S, Reethajanetsureka S, Pauljebaraj P, Benshamir B, Padankatti SM, David JA. Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2014;95:2000‐2005. [DOI] [PubMed] [Google Scholar]
- 49. Thieme H, Bayn M, Wurg M, Zange C, Pohl M, Behrens J. Mirror therapy for patients with severe arm paresis after stroke – a randomized controlled trial. Clin Rehabil. 2013;27:314‐324. [DOI] [PubMed] [Google Scholar]
- 50. Wen X, Li L, Li X, et al. Therapeutic role of additional mirror therapy on the recovery of upper extremity motor function after stroke: a single‐blind, randomized controlled trial. Neural Plast. 2022;2022:8966920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X, Zhang Y, Liu Y, Yao Q. Effectiveness of mirror therapy on upper limb function, activities of daily living, and depression in post‐stroke depression patients. Turk J Phys Med Rehabil. 2021;67:365‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bae SH, Jeong WS, Kim KY. Effects of mirror therapy on subacute stroke patients' brain waves and upper extremity functions. J Phys Ther Sci. 2012;24:1119‐1122. [Google Scholar]
- 53. Triantis E, Liu KPY. Activities of daily living interventions on activity performance of inpatients post‐stroke: a systematic review and meta‐analysis. Br J Occup Ther. 2024;87:598‐613. [DOI] [PubMed] [Google Scholar]
- 54. Morkisch N, Thieme H, Dohle C. How to perform mirror therapy after stroke? Evidence from a meta‐analysis. Restor Neurol Neurosci. 2019;37:421‐435. [DOI] [PubMed] [Google Scholar]
- 55. Kwakkel G, Kollen B. Predicting improvement in the upper paretic limb after stroke: a longitudinal prospective study. Restor Neurol Neurosci. 2007;25:453‐460. [PubMed] [Google Scholar]
- 56. Michielsen ME, Selles RW, van der Geest JN, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25:223‐233. [DOI] [PubMed] [Google Scholar]
- 57. Rossiter HE, Borrelli MR, Borchert RJ, Bradbury D, Ward NS. Cortical mechanisms of mirror therapy after stroke. Neurorehabil Neural Repair. 2015;29:444‐452. [DOI] [PubMed] [Google Scholar]


