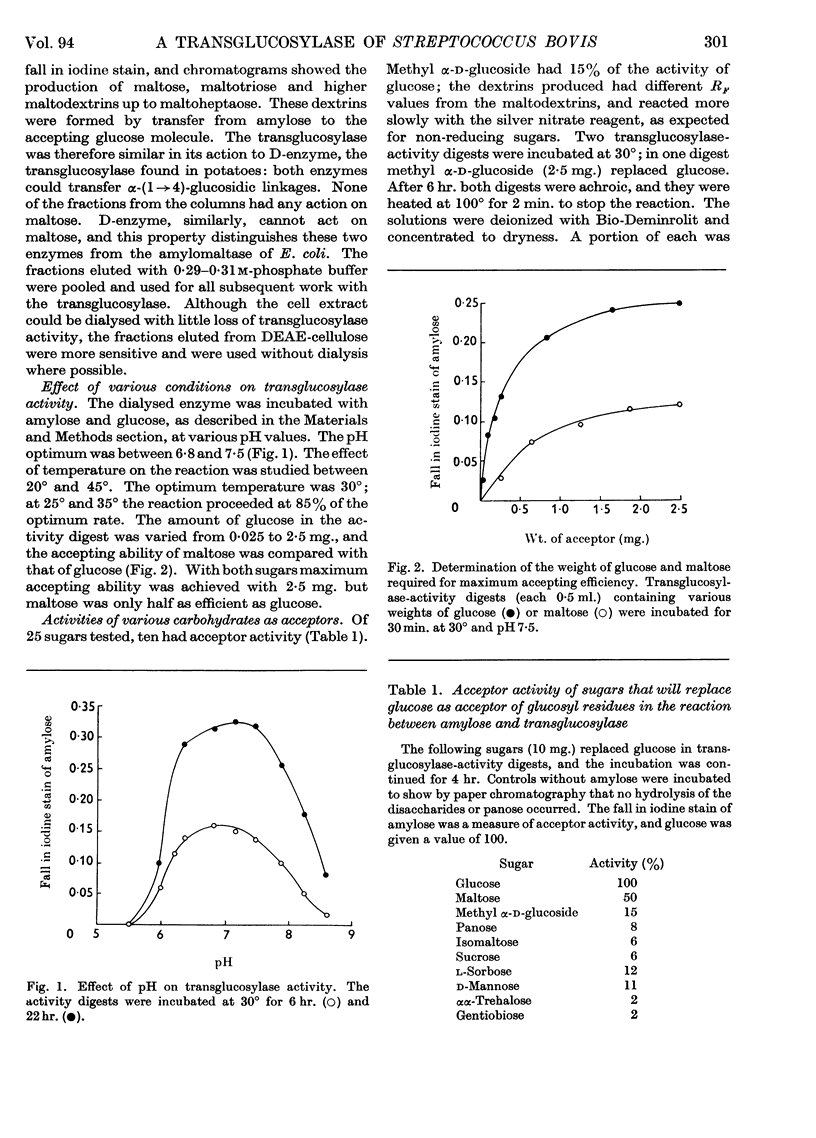
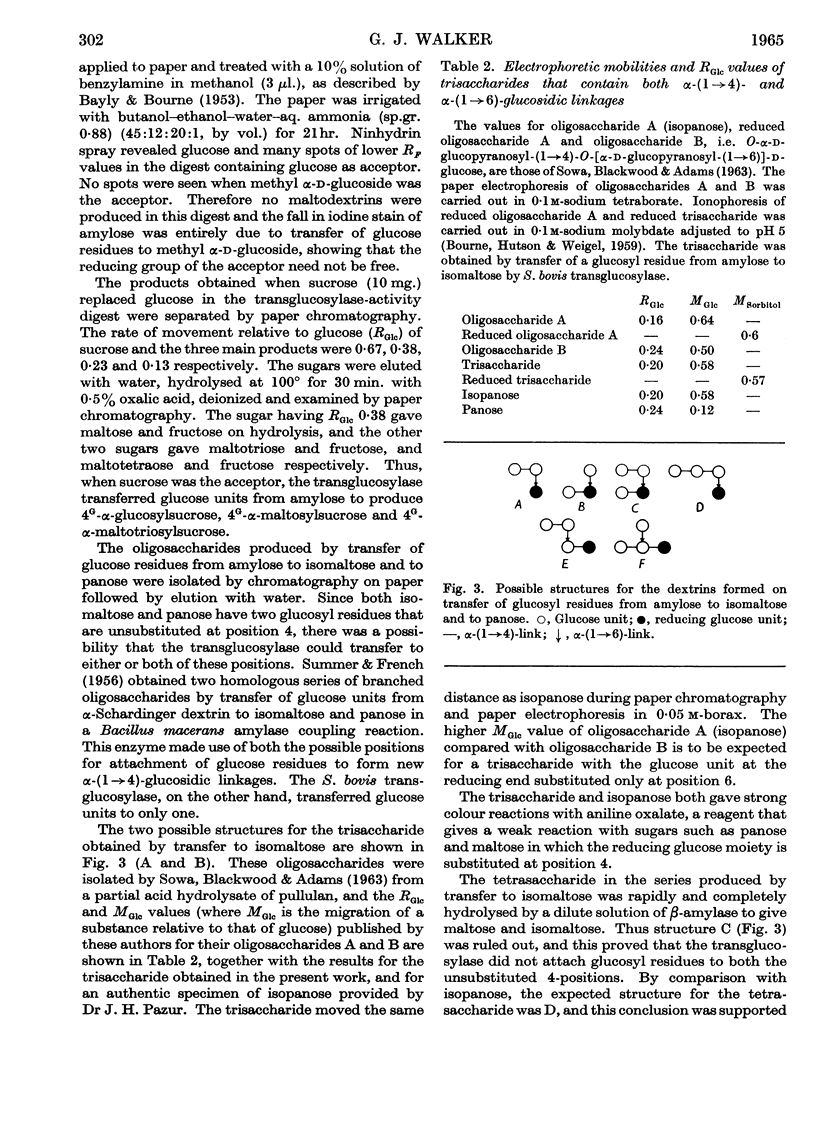
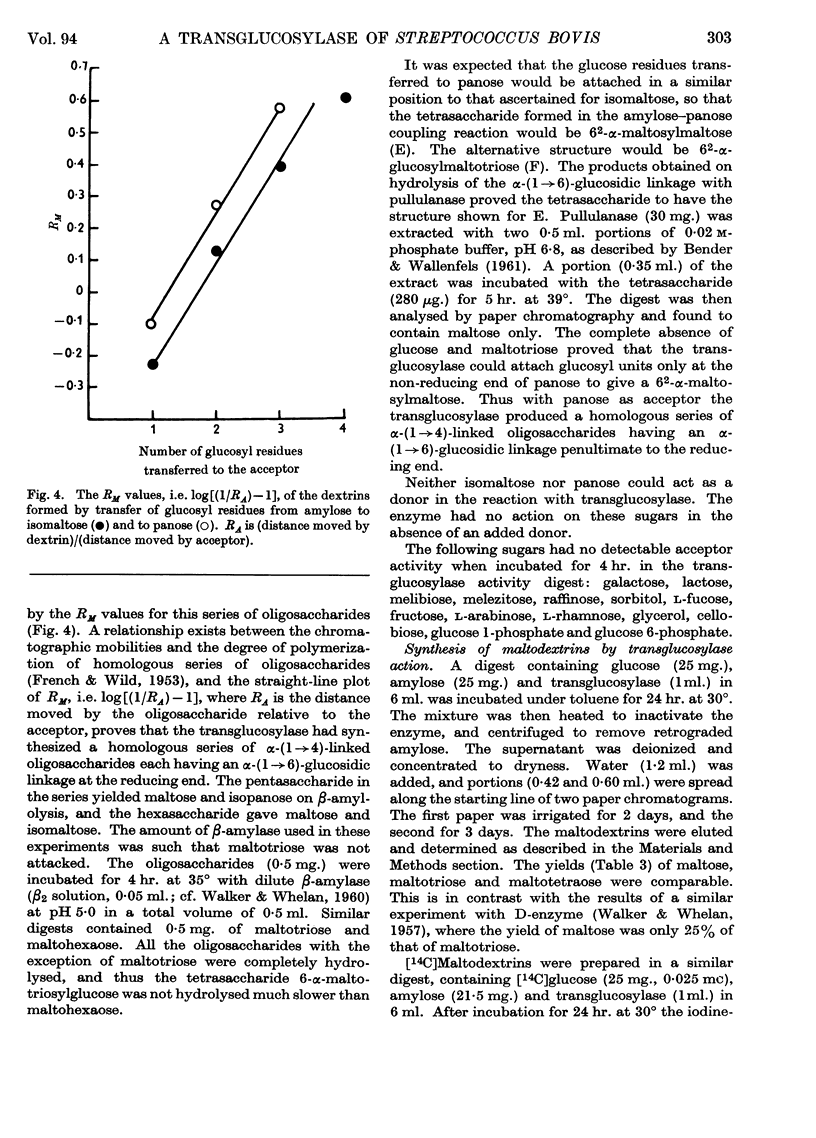
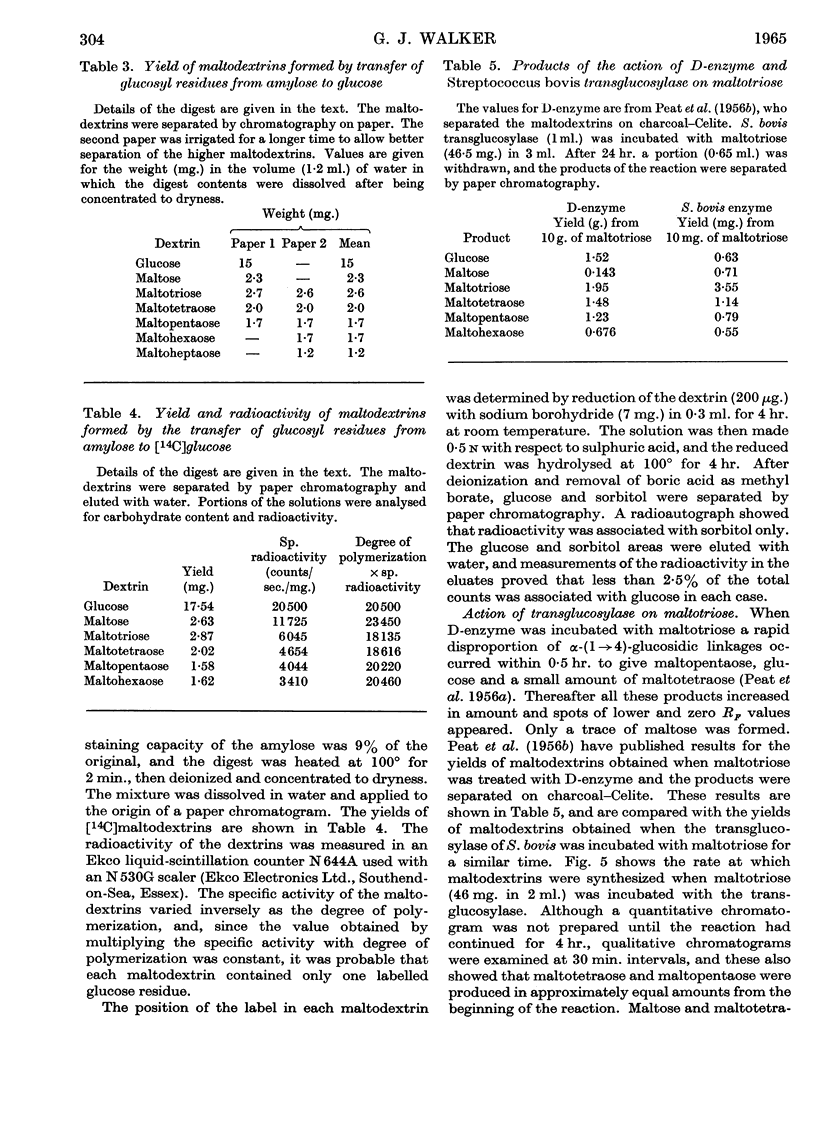
Abstract
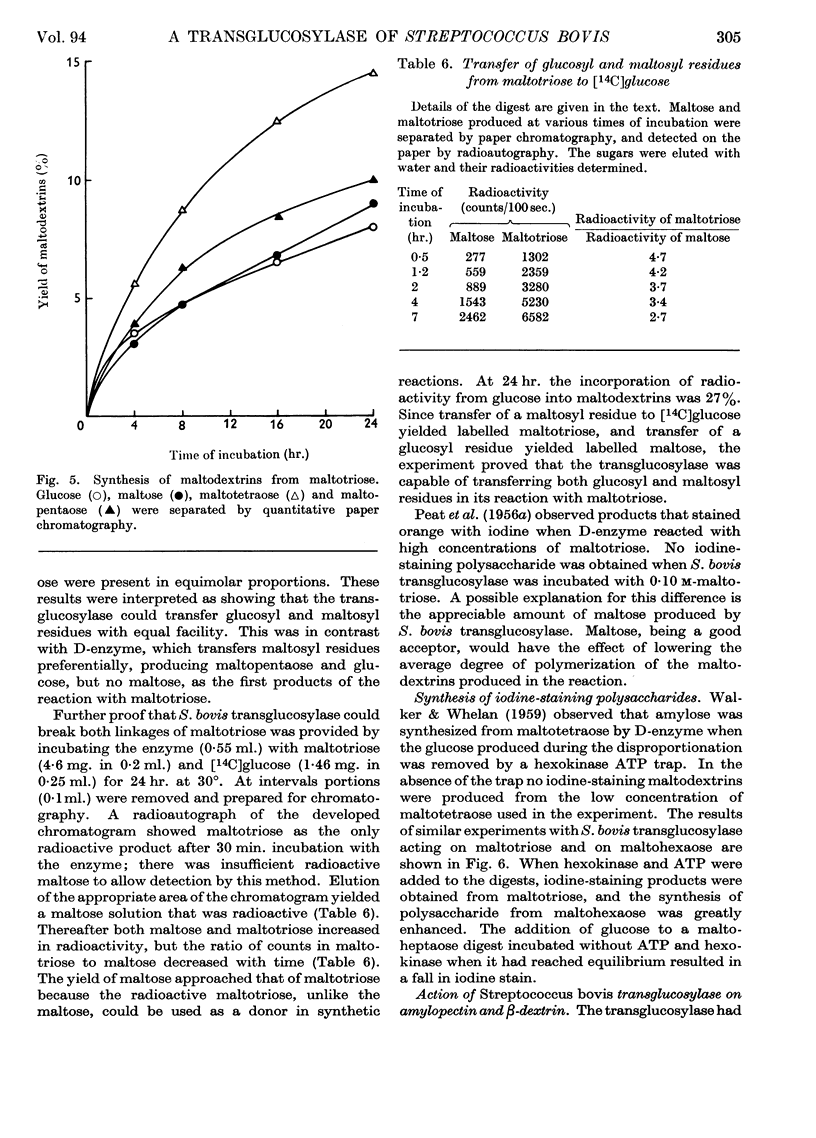
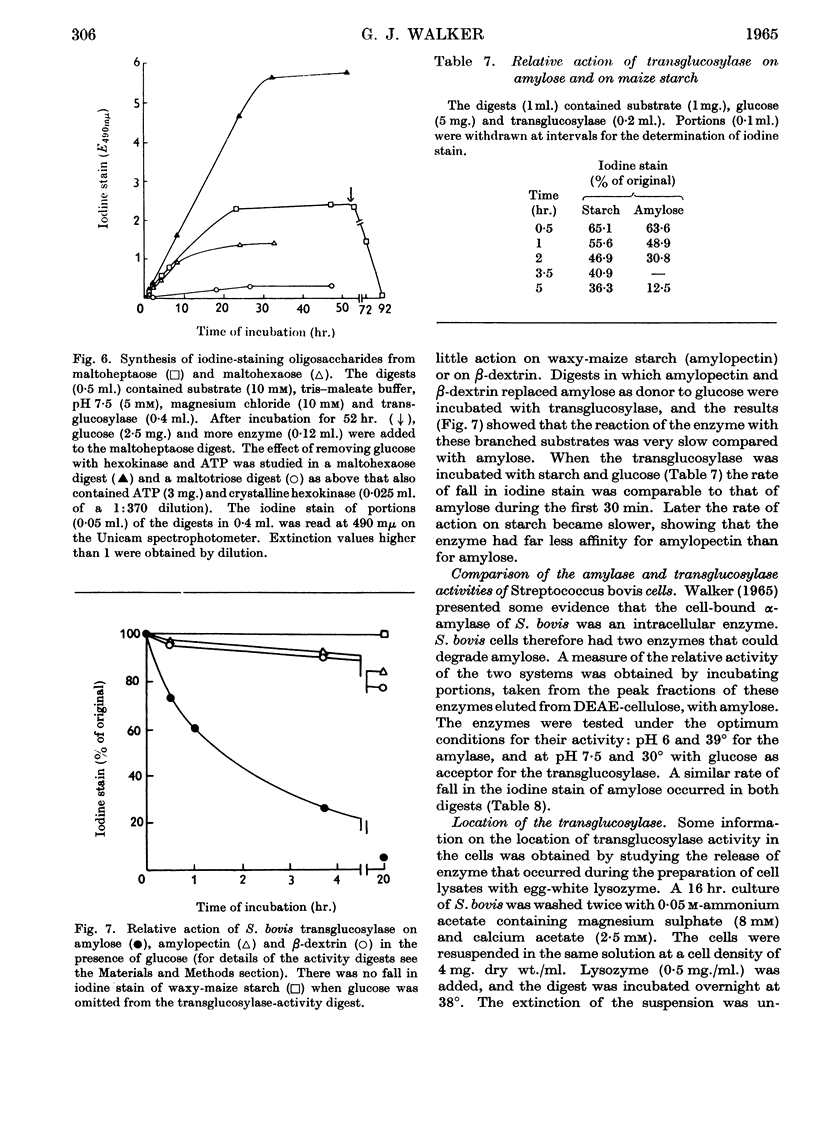
1. A transglucosylase has been separated from the α-amylase of Streptococcus bovis by chromatography of the cell extract on DEAE-cellulose. 2. The transglucosylase can synthesize higher maltodextrins from maltotriose, but maltose, isomaltose and panose do not function as donors. 3. Iodine-staining polysaccharide may be synthesized from maltotriose provided that glucose is removed. Synthesis from maltohexaose results in dextrins of sufficient chain length to stain with iodine, but again maltodextrins of longer chain length are formed when glucose is removed from the system. 4. The transglucosylase degrades amylose in the presence of a suitable acceptor, transferring one or more glucosyl residues from the non-reducing end of the donor to the non-reducing end of the acceptor. With [14C]glucose as acceptor the maltodextrins produced were labelled in the reducing glucose unit only. 5. The acceptor activities of 25 sugars have been compared with that of glucose. Maltose has 50%, methyl α-glucoside has 15%, isomaltose and panose each has 8% and sucrose has 6% of the accepting efficiency of glucose. Mannose and sorbose also had detectable activity. With the exception of maltose all these sugars produced a different series of dextrins from that obtained with glucose. 6. It was concluded that S. bovis transglucosylase transfers α-(1→4)-glucosidic linkages in the same manner as D-enzyme, but some differences in specificity distinguish the two enzymes. Unlike D-enzyme, S. bovis transglucosylase can transfer glucosyl units, producing appreciable amounts of maltose both during synthesis from maltotriose and during transfer from amylose to glucose. 7. No evidence was found that the transglucosylase was extracellular. The enzyme is cell-bound, and is released by treatment of the cells with lysozyme and by suspension of the spheroplasts in dilute buffer. 8. The transglucosylase may be responsible for the storage of intracellular iodophilic polysaccharide that occurs when the cells are grown in the presence of suitable carbohydrate sources.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. Transglucosidase activity of rumen strains of Streptococcus bovis. 2. Isolation and properties of dextransucrase. Biochem J. 1959 May;72(1):42–49. doi: 10.1042/bj0720042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLY R. J., BOURNE E. J. A new method for the paper chromatography of oligosaccharides. Nature. 1953 Feb 28;171(4348):385–387. doi: 10.1038/171385a0. [DOI] [PubMed] [Google Scholar]
- CROWLEY N., JEVONS M. P. The formation of a starch-like polysaccharide from maltose by strains of Streptococcus pyogenes. J Gen Microbiol. 1955 Oct;13(2):226–234. doi: 10.1099/00221287-13-2-226. [DOI] [PubMed] [Google Scholar]
- DUNICAN L. K., SEELEY H. W. Starch hydrolysis by Strepto-coccus equinus. J Bacteriol. 1962 Feb;83:264–269. doi: 10.1128/jb.83.2.264-269.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D., SUMMER R. Action of beta-amylase on branched oligosaccharides. J Biol Chem. 1956 Sep;222(1):469–477. [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N., MANN S. O. Some factors affecting the formation of iodophilic polysaccharide in group D streptococci from the rumen. J Gen Microbiol. 1955 Dec;13(3):420–435. doi: 10.1099/00221287-13-3-420. [DOI] [PubMed] [Google Scholar]
- MONOD J., TORRIANI A. M. De l'amylomaltase d'Escherichia coli. Ann Inst Pasteur (Paris) 1950 Jan;78(1):65–77. [PubMed] [Google Scholar]
- PEAT S., WHELAN W. J., REES W. R. D-enzyme: a disproportionating enzyme in potato juice. Nature. 1953 Jul 25;172(4369):158–158. doi: 10.1038/172158a0. [DOI] [PubMed] [Google Scholar]
- REES W. R., REYNOLDS T. A solvent for the paper chromatographic separation of glucose and sorbitol. Nature. 1958 Mar 15;181(4611):767–768. doi: 10.1038/181767a0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. Synthesis of amylose by potato D-enzyme. Nature. 1959 Jan 3;183(4653):46–46. doi: 10.1038/183046a0. [DOI] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 4. The mechanism of D-enzyme action. Biochem J. 1957 Dec;67(4):548–551. doi: 10.1042/bj0670548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 8. Structures of the muscle-phosphorylase limit dextrins of glycogen and amylopectin. Biochem J. 1960 Aug;76:264–268. doi: 10.1042/bj0760264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. II. General properties and mechanism of action of amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:427–439. doi: 10.1016/0006-3002(60)90195-5. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]


