Abstract
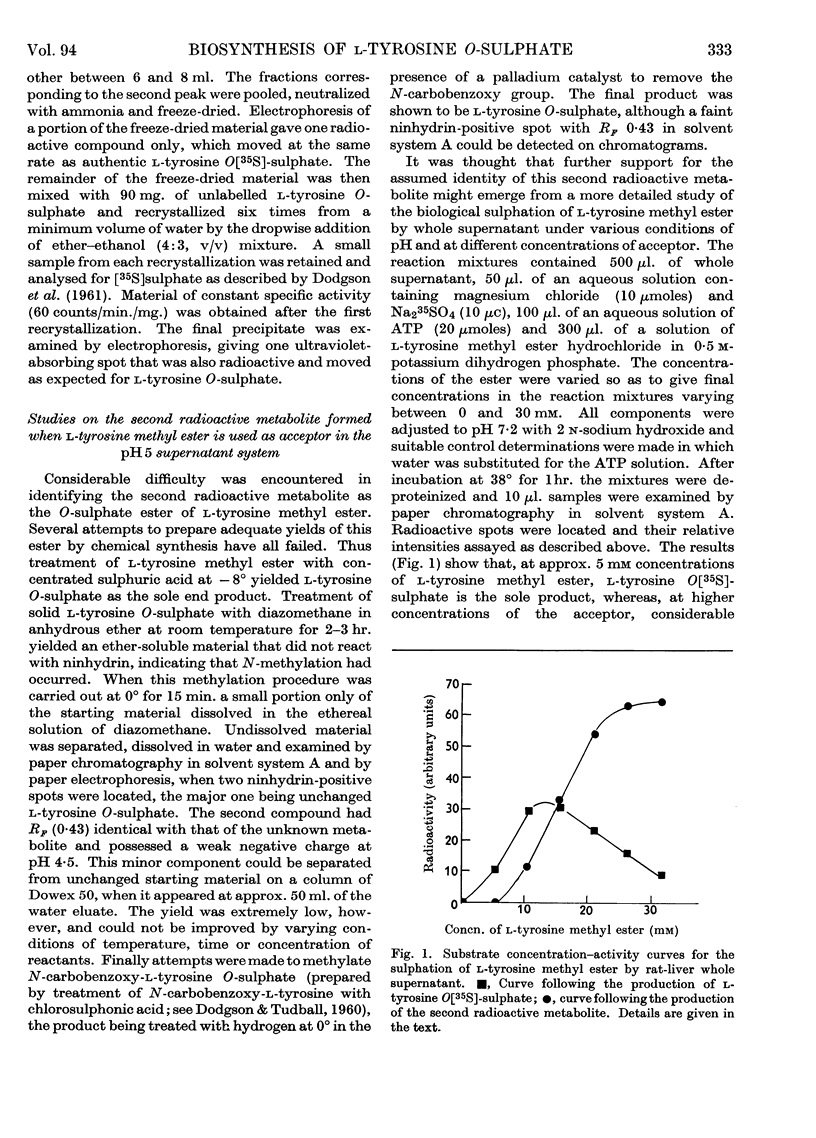
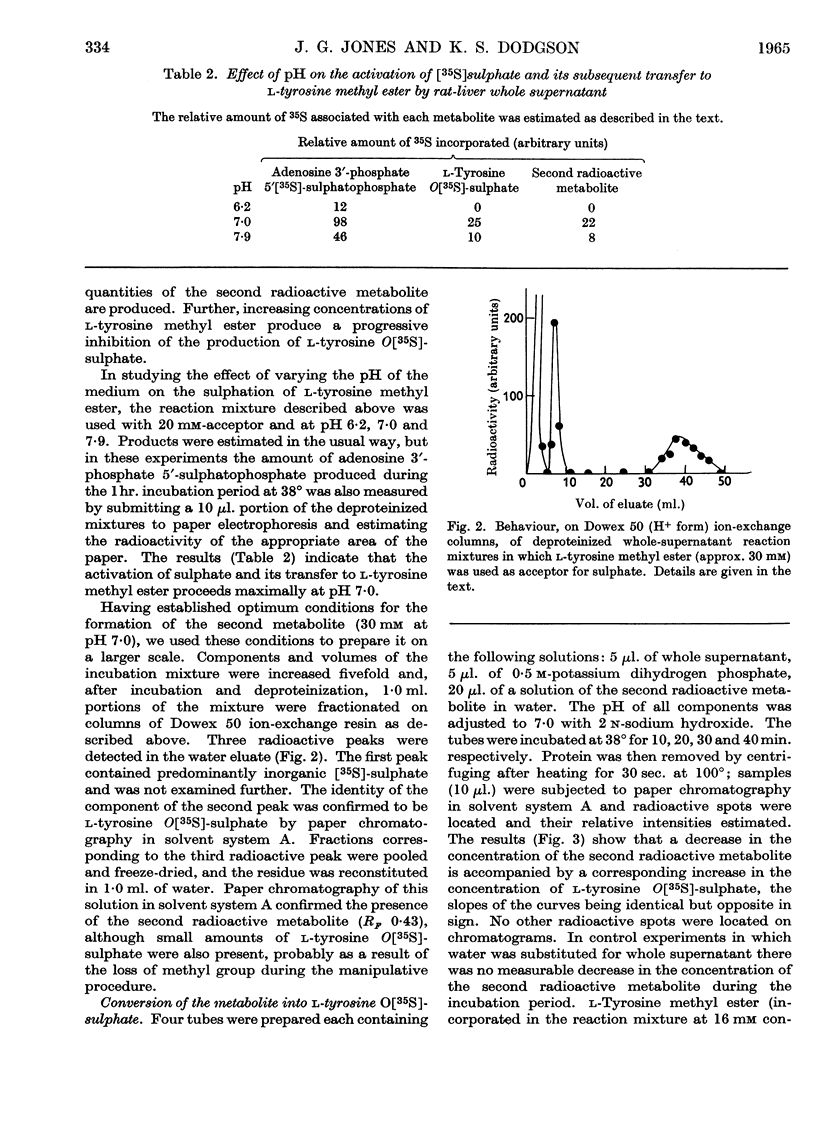
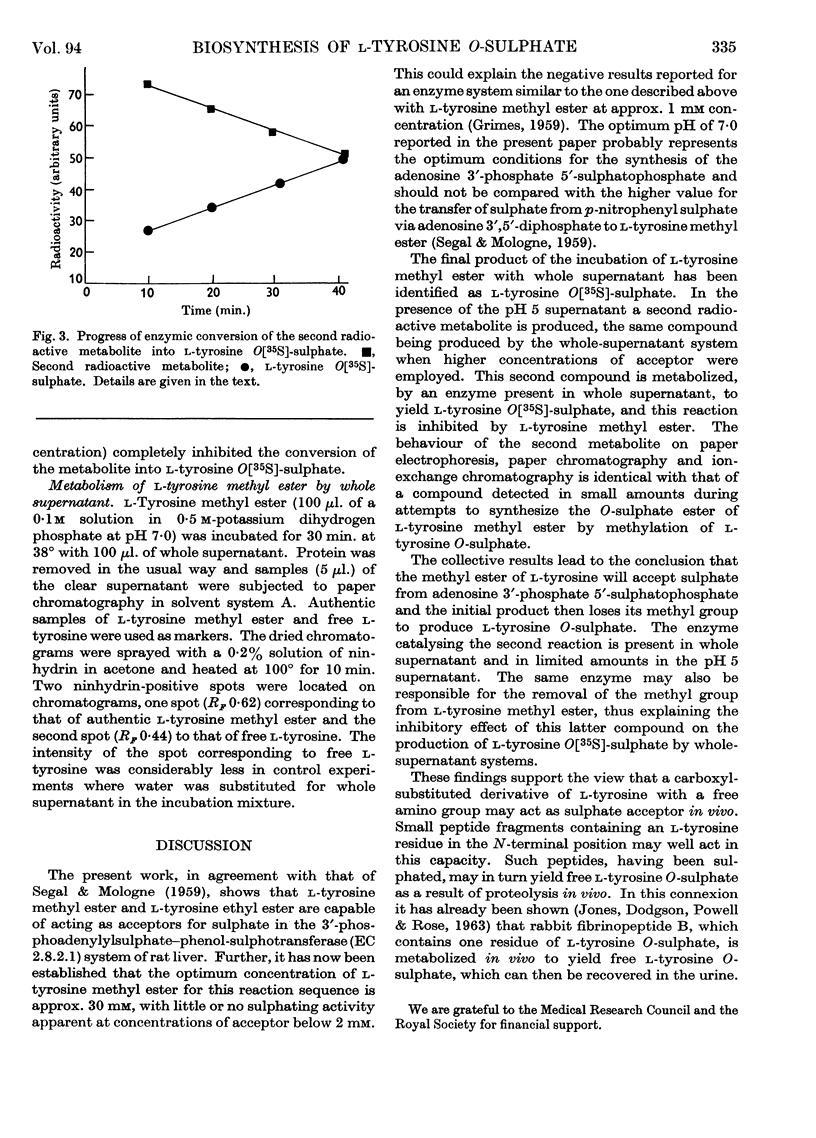
1. Rat-liver supernatant preparations are capable of achieving the biological sulphation of l-tyrosine methyl ester, the reaction proceeding maximally at a substrate concentration of 30 mm and at pH 7·0. 2. Two sulphated products are formed, one of which has been identified as l-tyrosine O-sulphate. On the basis of indirect evidence the other product can be assumed to be l-tyrosine O-sulphate methyl ester. 3. An enzyme present in rat-liver supernatant preparations is capable of converting l-tyrosine O-sulphate methyl ester into l-tyrosine O-sulphate. This enzyme is inhibited by l-tyrosine methyl ester. 4. l-Tyrosine ethyl ester also yields two sulphated products when used as an acceptor in the liver sulphating system. One of these has been identified chromatographically as l-tyrosine O-sulphate and the other may be presumed to be l-tyrosine O-sulphate ethyl ester.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DODGSON K. S., POWELL G. M., ROSE F. A., TUDBALL N. Observations on the metabolism of tyrosine O[35S]-sulphate in the rat. Biochem J. 1961 May;79:209–213. doi: 10.1042/bj0790209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., ROSE F. A., TUDBALL N. Studies on sulphatases. 23. The enzymic desulphation of tyrosine O-sulphate. Biochem J. 1959 Jan;71(1):10–15. doi: 10.1042/bj0710010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., TUDBALL N. The metabolic fate of the ester sulphate group of potassium p-nitrophenyl [35S]sulphate. Biochem J. 1960 Jan;74:154–159. doi: 10.1042/bj0740154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIMES A. J. Synthesis of 35S-labelled arylsulphates byintact animals and by tissue preparations, with particular reference to L-tyrosine O-sulphate. Biochem J. 1959 Dec;73:723–729. doi: 10.1042/bj0730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOAGLAND M. B., KELLER E. B., ZAMECNIK P. C. Enzymatic carboxyl activation of amino acids. J Biol Chem. 1956 Jan;218(1):345–358. [PubMed] [Google Scholar]
- JONES J. G., DODGSON K. S., POWELL G. M., ROSE F. A. Studies on L-tyrosine O-sulphate. 3. The metabolic fate of the L-tyrosine O[35S]-sulphate residue of 35S-labelled rabbit fibrinopeptide B. Biochem J. 1963 Jun;87:548–553. doi: 10.1042/bj0870548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSE Y., LIPMANN F. Separation of steroid sulfokinases. J Biol Chem. 1958 Dec;233(6):1348–1351. [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- SEGAL H. L., MOLOGNE L. A. Enzymatic sulfurylation of tyrosine derivatives. J Biol Chem. 1959 Apr;234(4):909–911. [PubMed] [Google Scholar]
- SUZUKI S., STROMINGER J. L. Enzymatic sulfation of mucopolysaccharides in hen oviduct. I. Transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to mucopolysaccharides. J Biol Chem. 1960 Feb;235:257–266. [PubMed] [Google Scholar]
- Spencer B. Endogenous sulphate acceptors in rat liver. Biochem J. 1960 Nov;77(2):294–304. doi: 10.1042/bj0770294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLAN H. H., BELLA S. T., STEIN W. H., MOORE S. Tyrosine-O-sulfate as a constituent of normal human urine. J Biol Chem. 1955 Dec;217(2):703–708. [PubMed] [Google Scholar]


