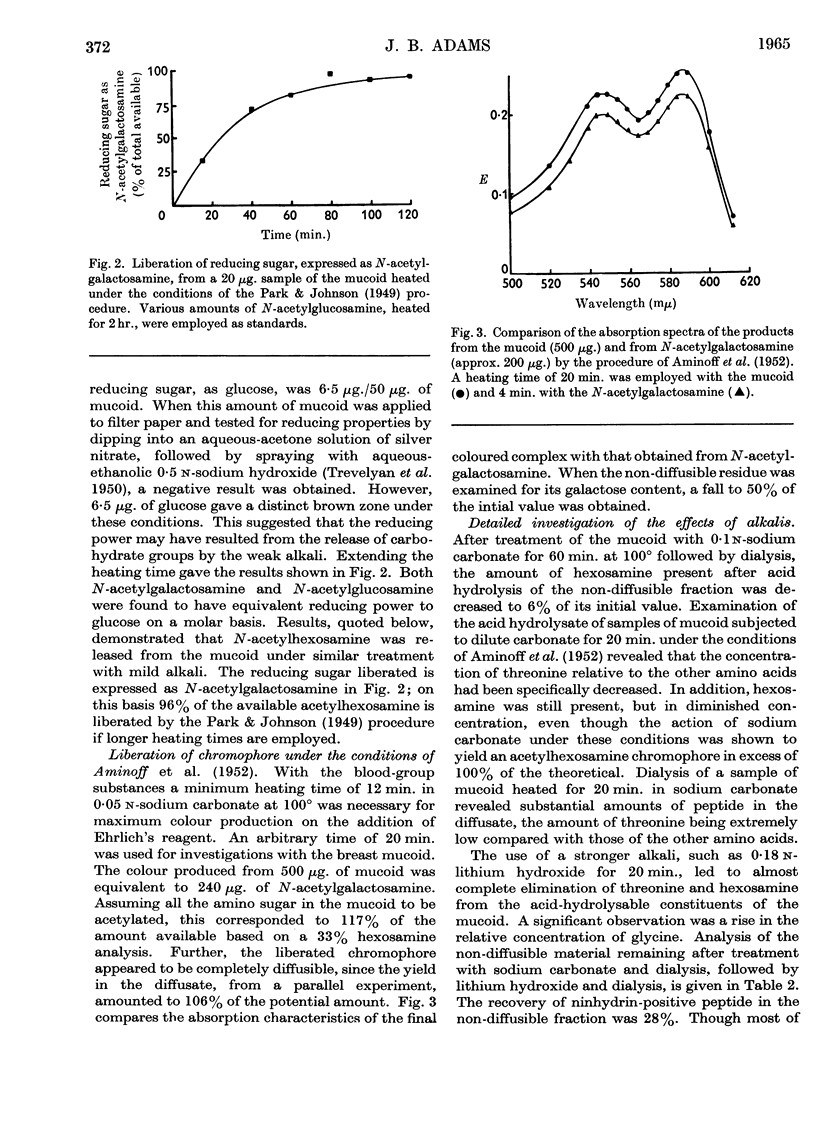
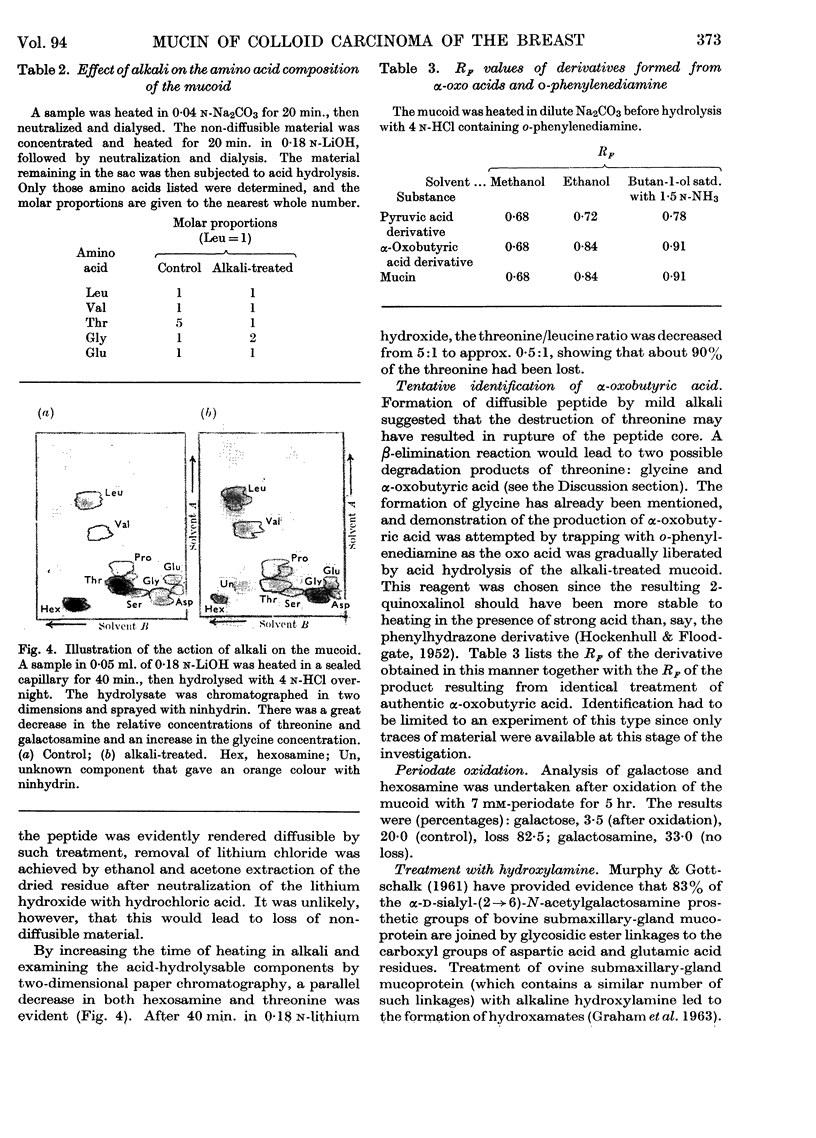
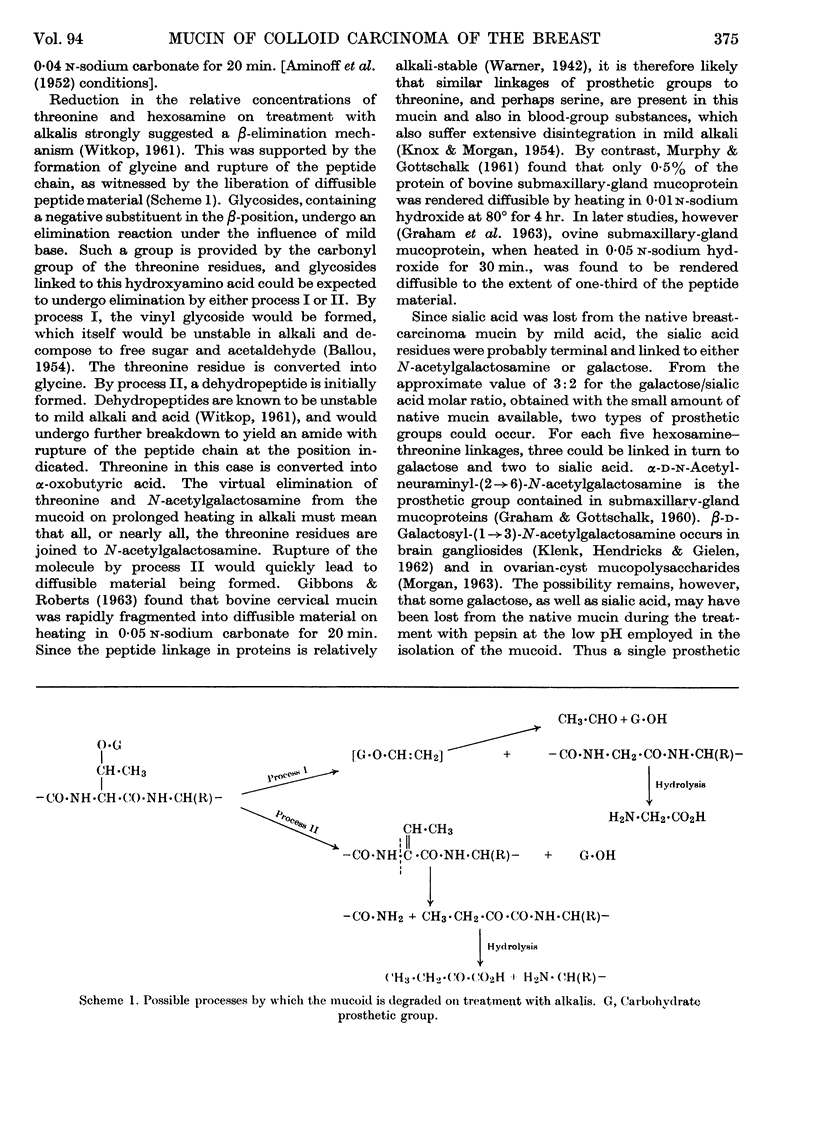
Abstract
1. A non-diffusible mucoid, showing a single peak in the ultracentrifuge, was isolated from human colloid breast carcinoma by treatment with trypsin and pepsin. The material contained threonine, leucine (isoleucine), valine, proline, glycine and glutamic acid in the approximate molar proportions 5:1:1:2:1:1. Smaller amounts of aspartic acid and serine were also found. For each 5 threonine residues, 6 N-acetylgalactosamine and 3–4 galactose residues were present. 2. The mucoid possessed reducing properties by the Park & Johnson (1949) procedure; these were attributable to the action of mild alkali, as employed in this procedure. Mild alkaline treatment by the Aminoff, Morgan & Watkins (1952) procedure gave rise to a diffusible N-acetylgalactosamine chromophore that gave an enhanced colour with Ehrlich's reagent. That galactosyl-(1→3)-N-acetylgalactosamine residues were liberated was supported by periodate studies. 3. Alkaline liberation of hexosamine residues was accompanied by a specific destruction of threonine. After 40 min. at 100° in 0·18 n-lithium hydroxide, both moieties had almost completely disappeared from the ninhydrin-positive components formed on subsequent acid hydrolysis. Glycine and α-oxobutyric acid were present in the acid hydrolysate, showing that both possible pathways of a β-elimination reaction were involved. Formation of diffusible peptide on very mild alkaline treatment was attributable to the rupture of the original peptide core, necessitated by the second of these two pathways. 4. Hydroxamate formation on treatment with hydroxylamine showed the presence of carbohydrate linkage to glutamic acid or aspartic acid residues or both. This could account for the single N-acetylgalactosamine residue not linked to threonine. 5. The native mucin contained sialic acid, which was cleaved by the acid environment used in the treatment with pepsin. A statistical model of the mucin would require each prosthetic group to be linked, via N-acetylgalactosamine, to threonine, which would occupy every alternate position among the amino acids in the peptide core.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D., MORGAN W. T. J. Studies in immunochemistry; the oxidation of the human blood-group A substance with the periodate ion. Biochem J. 1951 Jan;48(1):74–88. [PMC free article] [PubMed] [Google Scholar]
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. Studies in immunochemistry. 11. The action of dilute alkali on the N-acetylhexosamines and the specific blood-group mucoids. Biochem J. 1952 Jun;51(3):379–389. doi: 10.1042/bj0510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON A. J., MACLAGAN N. F. The isolation and estimation of urinary mucoproteins. Biochem J. 1955 Apr;59(4):638–644. doi: 10.1042/bj0590638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLOU C. E. Alkali-sensitive glycosides. Adv Carbohydr Chem. 1954;9:59–95. doi: 10.1016/s0096-5332(08)60372-0. [DOI] [PubMed] [Google Scholar]
- BLIX G. The linkage between hexosamine and amino acids in ovine submaxillary mucin. Ann N Y Acad Sci. 1963 Mar 30;106:164–167. doi: 10.1111/j.1749-6632.1963.tb16635.x. [DOI] [PubMed] [Google Scholar]
- DAVIDSON E., HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 1956 Sep;21(3):506–518. doi: 10.1016/0006-3002(56)90188-3. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- FLETCHER A. P., MARKS G. S., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 5. Procedures for the isolation of glycopeptides from hen's-egg albumin and their oxidation by periodate. Biochem J. 1963 May;87:265–273. doi: 10.1042/bj0870265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER A. P., MARSHALL R. D., NEUBERGER A. Further investigations on the carbohydrate moiety of egg albumin. Biochim Biophys Acta. 1963 May 14;71:505–508. doi: 10.1016/0006-3002(63)91122-3. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. A., ROBERTS G. P. Some aspects of the structure of macromolecular constituents of epithelial mucus. Ann N Y Acad Sci. 1963 Mar 30;106:218–232. doi: 10.1111/j.1749-6632.1963.tb16640.x. [DOI] [PubMed] [Google Scholar]
- GRAHAM E. R., GOTTSCHALK A. Studies on mucoproteins. I. The structure of the prosthetic group of ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1960 Mar 11;38:513–524. doi: 10.1016/0006-3002(60)91286-5. [DOI] [PubMed] [Google Scholar]
- GRAHAM E. R., MURPHY W. H., GOTTSCHALK A. Studies of mucoproteins. IX. On the susceptibility to alkali and to hydroxylamine of the predominant carbohvdrate-peptide linkage in ovine-submaxillary-gland glycoprotein. Biochim Biophys Acta. 1963 Jul 16;74:222–238. doi: 10.1016/0006-3002(63)91361-1. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., HASHIMOTO S., PIGMAN W. PURIFICATION AND PROPERTIES OF PORCINE SUBMAXILLARY MUCIN. Arch Biochem Biophys. 1964 Feb;104:282–291. doi: 10.1016/s0003-9861(64)80015-1. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., TSUIKI S., NISIZAWA K., PIGMAN W. Action of proteolytic enzymes on purified bovine submaxillary mucin. Ann N Y Acad Sci. 1963 Mar 30;106:233–246. doi: 10.1111/j.1749-6632.1963.tb16641.x. [DOI] [PubMed] [Google Scholar]
- HEYWORTH R., PERKINS H. R., WALKER P. G. Paper chromatography of hexosamines and N-acetylhexosamines. Nature. 1961 Apr 15;190:261–262. doi: 10.1038/190261a0. [DOI] [PubMed] [Google Scholar]
- HOCKENHULL D. J. D., FLOODGATE G. D. o-Phenylenediamine and 1:2-diamino-4-nitrobenzene as reagents for a-keto acids. Biochem J. 1952 Sep;52(1):38–40. doi: 10.1042/bj0520038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMMERS J., VASSEUR E. Influence of sugars and amines on the colorimetric hexosamine method of elson and morgan and its possible elimination. Nature. 1950 Jun 3;165(4205):898–898. doi: 10.1038/165898a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON W. C., HELWIG E. B. Histochemistry of primary. Ann N Y Acad Sci. 1963 Mar 30;106:794–803. [PubMed] [Google Scholar]
- KLENK E., HENDRICKS U. W., GIELEN W. [beta-D-Galactosido-(1-3)-N-acetyl-D-galactosamine, a crystallized disaccharide from human brain gangliosides]. Hoppe Seylers Z Physiol Chem. 1962 Dec 15;330:140–144. doi: 10.1515/bchm2.1962.330.1.140. [DOI] [PubMed] [Google Scholar]
- KNOX K. W., MORGAN W. T. The alkaline degradation of the human blood-group substances. Biochem J. 1954 Jun 19;58(330TH):v–v. [PubMed] [Google Scholar]
- MURPHY W. H., GOTTSCHALK A. Studies on mucoproteins. VII. The linkage of the prosthetic group to aspartic and glutamic acid residues in bovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1961 Sep 16;52:349–360. doi: 10.1016/0006-3002(61)90684-9. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- POPENOE E. A. The linkage neuraminic acid in orosomucoid. Biochim Biophys Acta. 1959 Apr;32:584–585. doi: 10.1016/0006-3002(59)90651-1. [DOI] [PubMed] [Google Scholar]
- RAACKE I. D. On the reaction of hydroxylamine with esters of amino acids. Biochim Biophys Acta. 1958 Feb;27(2):416–416. doi: 10.1016/0006-3002(58)90354-8. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]