Abstract
The M phenotype is by far the most common mechanism of erythromycin resistance among Streptococcus pyogenes isolates in Spain. A geographic analysis of the relationship between within-country differences in the prevalence of M-type resistance to erythromycin in S. pyogenes and the level of consumption of 14- and 15-membered macrolides within different provinces was carried out. From 1998 to 1999, a nationwide multicenter surveillance study yielded 2,039 consecutive pharyngeal isolates of S. pyogenes. Data on antibiotic consumption for the same period were gathered from IMS Health, and the corresponding daily defined doses per 1,000 inhabitants per day were calculated according to the Anatomic Therapeutic Classification index. Macrolide use was subdivided into dosages given three times a day (TID), twice a day (BID), or once a day (OD). Spearman nonparametric correlation coefficients (R) were calculated, and variables proving to be significantly associated (P < 0.1) were introduced into a linear regression model. The total consumption of macrolides presented a significant correlation with the prevalence of resistance (R = 0.527; P = 0.032). Neither TID nor BID macrolide consumption showed significant correlations. Only OD consumption had a significance below 0.1. These data are consistent with the hypothesis that only the total consumption of macrolides influences the local rates of M-type erythromycin resistance in S. pyogenes, and subgroups of macrolides seem to have an additive rather than a selective effect by contributing to increasing the final amount of macrolides used. Local variations in total consumption were associated only with BID consumption (R = 0.849; P = 0.004). The simple linear regression with total macrolide consumption showed a considerable determination coefficient (R2 = 0.678; P = 0.006). The model explains up to 68% of the measured variation and is clearly better as a predictor of the prevalence of resistance than the mere mean is. By solving the regression equation, the resultant value of 2.2 defined doses per 1,000 inhabitants per day fits with the existence of a critical threshold of selective pressure.
Streptococcus pyogenes, which still surprises us with its extraordinary and constant susceptibility to penicillin (making this drug the preferred treatment for streptococcal infections), has not been spared from development of resistance. For patients who are allergic to penicillin, macrolides have been recommended as an alternative treatment (14, 27), but in some instances their favorable pharmacokinetics, ensuring better compliance, has led to their displacing penicillin even in the absence of a justifiable indication.
Since its first description in 1955 in the United Kingdom (20), resistance to erythromycin developed abruptly in the 1980s and has escalated in many countries in the 1990s (5, 13, 16, 21, 24, 25, 28, 29, 30, 31). Several Asian countries have been identified at some time as hot spots for erythromycin resistance, with rates exceeding 60% (13, 21). In Spain, a temporal association with macrolide consumption has been described (11), and the most recent data in 1999 show an average prevalence of erythromycin resistance of 20% (25). This figure is among the highest in Europe, together with rates in Italy (5) and Greece (32). Interestingly, after France, the three Mediterranean countries also lead Europe in terms of macrolide consumption (2, 3).
This study aimed at determining whether the within-country differences in the prevalence of erythromycin resistance in S. pyogenes in Spain are due to a different level of antimicrobial agent consumption in the different provinces. Likewise, we wanted to assess whether differences are due to an additive or selective effect of the consumption of subgroups of macrolides.
MATERIALS AND METHODS
From November 1998 to October 1999, a nationwide multicenter surveillance study was carried out at 17 different hospitals in Spain (25). At each center, every consecutive pharyngeal isolate of S. pyogenes was kept at −70°C. Once a month they were thawed, seeded onto an enriched transport medium, incubated overnight at 35 to 37°C, and shipped to a central laboratory (Instituto Valenciano de Microbiología, Valencia, Spain). The central laboratory undertook confirmation of the initial identification and susceptibility testing, by a semiautomated microdilution method (Sensititre; Trek Diagnostics Inc. Westlake, Ohio), in cation-adjusted Mueller-Hinton broth with 3% lysed horse blood as recommended by the National Committee for Clinical Laboratory Standards (23). Overall, 2,039 S. pyogenes isolates were available for analysis.
Data on antibiotic consumption for the same 365-day period in which the surveillance was conducted were gathered from IMS Health (Intercontinental Marketing Services, Madrid, Spain) as the total number of antibiotic wholesaler sales per presentation per year in the retail market. The consumption of each antibiotic was then calculated as daily defined doses per 1,000 inhabitants per day (DID), following the 2002 World Health Organization (WHO) Collaborating Center for Drug Statistics Methodology recommendation (35). Official demographic data on the Spanish population as of 1 January 1998 were obtained from the Spanish National Statistics Institute.
Of the possible mechanisms of erythromycin resistance in S. pyogenes, the M phenotype (efflux pump) is acknowledged to be far more predominant (>90%) in Spain (25) than the MLSB phenotype (ribosomal target modification). Therefore, since M phenotype resistance involves 14- and 15-membered lactone ring macrolides but does not affect the activity of 16-membered macrolides or lincosamides, erythromycin resistance of the M phenotype and provincial differences in the consumption of 14- and 15-membered macrolides were investigated.
The local prevalence of erythromycin resistance (MIC ≥ 0.5 μg/ml) was obtained by dividing the number of resistant isolates by the total number of strains collected at a given hospital, and 90% confidence intervals (90% CI) were calculated by the binomial method. To enter the analyses, centers had to provide a minimum number of isolates, which had to represent at least 2% of the whole collection under study; this meant a minimum of 40 isolates.
The consumption of 14- and 15-membered macrolides as a whole was considered separately from that of the groups into which they were further divided, namely, those administered three times a day (TID) (erythromycin), twice a day (BID) (clarithromycin and roxithromycin), and once a day (OD) (azithromycin and dirithromycin). The prevalence of erythromycin resistance (dependent variable) in each hospital was plotted against the corresponding provincial antibiotic consumption values (predictor variables).
Spearman nonparametric correlation coefficients (R), with two-tailed significance to minimize the impact of outliers, between provincial prevalence of resistance and their antibiotic consumption were calculated in bivariate correlations. Only in the event that all the variables reached a significant association (P < 0.1) in the previous correlations were they introduced into a linear regression model by the enter method. The SPSS for Windows Release 8.0 statistical package was used to carry out the analyses.
RESULTS
Nine hospitals in eight cities located in eight different provinces fulfilled the criteria for inclusion in this study. The number of isolates, rate and 90% CI of erythromycin resistance, consumption of total macrolides and subgroups, and province populations are provided in Table 1.
TABLE 1.
Total and resistant S. pyogenes isolates, prevalence of M phenotype erythromycin resistance, and macrolide consumption by hospital
| Hospital—code (province) | No. of isolates (no. resistant) | Measured prevalence of resistance (%) | Predicted prevalence of resistance (%) | 90% CI (%) | Consumption of macrolidesa (DID)
|
Population (103) | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | TID | BID | OD | ||||||
| Hospital de Donosti—HD (Guipúzcoa) | 403 (39) | 9.7 | 10.5 | 7.4-12.5 | 2.635 | 0.235 | 1.532 | 0.868 | 676 |
| Hospital de Valdecilla—HV (Cantabria) | 509 (53) | 10.4 | 26.3 | 8.2-12.9 | 3.241 | 0.329 | 2.159 | 0.753 | 527 |
| Hospital de Cruces—HC (Vizcaya) | 197 (31) | 15.7 | 23.9 | 11.7-20.6 | 3.151 | 0.283 | 1.854 | 1.014 | 1,137 |
| Hospital Virgen Macarena—HMS (Sevilla) | 133 (26) | 19.5 | 11.1 | 14.1-26.1 | 2.658 | 0.659 | 1.235 | 0.764 | 1,714 |
| Hospital La Paz—HPM (Madrid) | 80 (16) | 20.0 | 18.0 | 13.0-28.8 | 2.925 | 0.605 | 1.360 | 0.960 | 5,091 |
| Hospital Gregorio Marañón—HGM (Madrid) | 326 (74) | 22.7 | 18.0 | 18.9-26.8 | 2.925 | 0.605 | 1.360 | 0.960 | 5,091 |
| Hospital Virgen de las Nieves—HNG (Granada) | 54 (21) | 38.9 | 33.6 | 27.7-51.0 | 3.522 | 0.719 | 1.944 | 0.859 | 801 |
| Hospital Sant Joan de Deu—HJD (Barcelona) | 81 (35) | 43.2 | 39.7 | 33.8-53.0 | 3.758 | 0.434 | 2.258 | 1.066 | 4,666 |
| Hospital Clinico Salamanca—HCS (Salamanca) | 45 (20) | 44.4 | 39.2 | 31.7-57.7 | 3.739 | 0.576 | 2.054 | 1.109 | 349 |
TID, erythromycin; BID, clarithromycin and roxithromycin; OD, azithromycin and dirithromycin.
The prevalence of resistance to erythromycin averaged around 20%, ranging from a minimum of 10% (90% CI, ≈8% to 12%) to a maximum of 40% (90% CI, ≈30% to 50%). The lowest rates were found in the three northern provinces (Guipúzcoa, Cantabria, and Vizcaya), whereas the three highest rates were geographically scattered (Granada, Barcelona, and Salamanca).
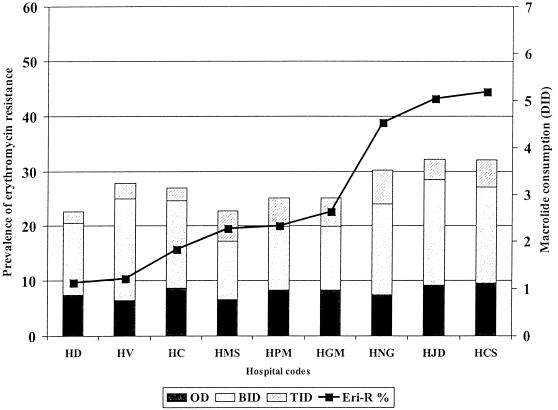
The consumption of macrolides ranged from 2.6 DID in Guipúzcoa to over 3.5 DID in the three provinces with mean rates of erythromycin resistance of over 40%. However, in provinces with lower rates of resistance, the relationship with macrolide consumption was not so evident (Fig. 1).
FIG. 1.
Prevalence of M phenotype erythromycin resistance (Eri-R) in S. pyogenes and consumption of 14- and 15-membered macrolides by hospital and province.
Table 2 shows the bivariate correlation coefficients and their associated P values for local rates of erythromycin resistance and the corresponding values for macrolide consumption. The total consumption of macrolides presented a significant correlation with the prevalence of resistance (R = 0.527; P = 0.032). Neither TID (P = 0.145) nor BID (P = 0.417) macrolide consumption showed significant correlations, and only OD consumption (P = 0.081) had a significance below 0.1. On the other hand, local variations in total consumption were associated with BID consumption (R = 0.849; P = 0.004), but not with TID or OD macrolide consumption.
TABLE 2.
Relationships between local rates of M phenotype erythromycin resistance among S. pyogenes isolates and consumption of macrolides in Spain
| Test | Variable | R | Pa | R2 | Constant | B (slope) | Equation |
|---|---|---|---|---|---|---|---|
| Bivariate correlations | Total macrolides | 0.527 | 0.032** | ||||
| TID | 0.310 | 0.145 (NS) | |||||
| BID | 0.611 | 0.417 (NS) | |||||
| OD | 0.711 | 0.081* | |||||
| Simple linear regression | Total macrolides | 0.006 | 0.678 | −58 | 26 | y = 26x − 58 |
∗∗, statistically significant; ∗, trend towards statistical significance; NS, not significant.
Given the above results, only a valid simple linear regression with total macrolide consumption could be carried out (Table 2). The determination coefficient was 0.678 (F estimate, 14.767; P = 0.006). The model explains up to 68% of the measured variation and is clearly better as a predictor of the prevalence of resistance than is the mere mean. The slope (the coefficient of the regression equation) for the consumption of macrolides was ≈26, and the constant of the equation was −58. The expected value for macrolide consumption that would have avoided the upsurge of erythromycin resistance was 2.2 DID.
The predicted prevalence of resistance by site resulting from the application of the simple linear equation is given in Table 1, where some results are overestimated in provinces with a low prevalence of resistance (Cantabria and Vizcaya), whereas others are underestimated (Sevilla).
DISCUSSION
Two different mechanisms for the spread of resistance are usually given: (i) clonal expansion of resistant isolates and (ii) horizontal exchange of resistance traits between different bacteria; in short, bacterium- and gene-related resistance. Clearly, neither mechanism is completely exclusive, and a combination of both is more likely, but each one may have a different weight depending on the population structure of a given species and the resistance trait under study.
With S. pyogenes, several facts must be taken into account. (i) Its population structure level has not yet been unequivocally elucidated at an ecological level because some data (1, 7, 15) do not reconcile well with a network-like structure due to high rates of recombination (8). (ii) The relative prevalence of the two main mechanisms of erythromycin resistance can vary among different communities. (iii) The mef genes encoding the efflux pump responsible for the M phenotype of erythromycin resistance may be horizontally transmitted, since they have been found in multiple gram-positive bacteria. (iv) The level of protection achieved by a given mechanism of resistance must be balanced against its biological cost. In this way, efflux pumps (protecting only against low drug concentrations) could impose a different fitness cost on the bacterium than alterations of ribosomal targets (which render bacteria virtually immune to macrolides at any concentration). However, the diverse prevalence of the MLSB phenotype in other countries regardless of their level of macrolide use indicates that both mechanisms are likely to have a similar fitness cost. (v) The lack of coresistance to β-lactams may weaken the strength of macrolides in the spread of erythromycin resistance in S. pyogenes.
The role of antibiotics in the selection of resistance has been studied mostly in the hospital context (17, 19). However, in the larger, open, dynamic, and more complex community setting, we need larger and more geographically dispersed samples of clinical isolates to be representative of what is being measured, along with reliable and standardized data on antibiotic consumption. This may explain why until recently have there been few works that shed light on the issue. They have offered a starting point and a formal framework for the qualitative and quantitative study of the temporal and geographical aspects of the problem of resistance in several bacterial species (2, 6, 9, 10, 11, 12, 22, 30).
In the case of S. pyogenes, published results appear conflicting, since sometimes they attribute a role to antibiotic use in the level of resistance (4, 11, 25, 29, 30), but sometimes they do not (26, 34). The fact that only a few clones seem to drive resistance to erythromycin makes clonal spread a variable worth considering (M12 in Japan and M4 and M75 in Spain and Finland). Besides, the prevalence of erythromycin resistance reported in France does not match its high level of macrolide use (3, 33). The truth possibly lies somewhere in between. In any case, the selective pressure imposed by antibiotic use may favor clonal spread.
In our study, the simple linear regression had a considerable determination coefficient (R2 = 0.678), but the model failed for provinces with low resistance rates. This inconsistency could have various explanations. (i) It may reflect the independent contribution of clonal spread not linked to antibiotic use. (ii) As S. pyogenes does not exhibit coresistance to β-lactams, much higher values of macrolide consumption than expected may be needed to overcome the resistance due to clonal spread. (iii) In certain regions, some prevailing clones carrying mef gene determinants may be outcompeted by erythromycin-susceptible clones in the event of a level of macrolide use above the protection conferred by the resistance trait (the competitive advantage would disappear but the fitness cost would remain). This possibility would be more difficult a priori with erm gene determinants. (iv) Considering the inertia with which resistance seems to follow changes in antibiotic consumption (18), the inconsistent values may in fact be a predictor of the prevalence to come if the consumption is not modified.
Our data show that in any case, only the total consumption of macrolides influences the local rates of M-type erythromycin resistance in S. pyogenes, and subgroups of macrolides seem to have an additive rather than a selective effect by contributing to increasing the final amount of macrolides used in the community.
Interestingly, a hypothetical level of macrolide use of 2.2 DID would solve the equation and make the expected prevalence of erythromycin resistance zero in theory. This finding is in line with the hypothesis of a critical threshold of selective pressure already suggested by other authors and coincides with a value around 2 DID (9, 14, 18). The present mean macrolide consumption of the eight provinces studied was 3.2 DID, which implies a predicted average prevalence of 25.3% for M-type resistance to erythromycin in Spain. On the other hand, once resistance has appeared, a subsequent decrease is probably unlikely as long as the intensity of consumption remains above the threshold limit. In any case, for a decrease in resistance to occur, macrolide use should probably be below the trigger limit, and even if this were to happen, perhaps we would be able only to retard the rate of increase of resistance. In this context, the fitness costs associated with the maintenance of the resistant trait relative to fully susceptible isolates would be of the utmost importance in any decrease in the rate of resistance.
Obviously, the present work is no more than an ambitious oversimplified distortion of the complex reality under study. Even so, it would be highly desirable that other countries with a different prevalence of erythromycin-resistant S. pyogenes or with a different ratio of MLSB to M phenotype erythromycin resistance undertake similar studies. Variables of potential importance have not been taken into account for the sake of easier interpretation. Other factors such as (i) a temporal lag between changes in antibiotic pressure and the expected resistance rate (accounting for stable or fading oscillations with a different width), (ii) random ambient and demographic fluctuations (amenable to modeling with Montecarlo simulations), and (iii) the spatial population heterogeneity (paths between subpopulations and recolonization) could be tried and modeled in the future.
Well-organized resistance surveillance networks and reliable data on antibiotic consumption are needed in each country to obtain meaningful information with the sufficient geographic precision to draw conclusions that could lead to measures worth implementing. Regarding resistance to erythromycin in S. pyogenes, the present analysis points to an additive effect of the consumption of different macrolides and a critical threshold of consumption for the explosive spread of resistance. Factors such as the bacterial population structure (clonal versus nonclonal) and the basal prevalence of resistance (and that of its mechanisms) at a given time point and location are also likely to have a significant impact on the equation consumption and resistance for this pathogen.
Acknowledgments
We thank members of the Spanish Surveillance Group for Respiratory Pathogens for priceless dedication to updating resistance data. We are also indebted to Rafael Dal-Ré for critical comments and Veronica A. Maguire for assistance with the English version of the manuscript.
REFERENCES
- 1.Bessen, D. E., M. W. Izzo, T. R. Fiorentino, R. M. Caringal, S. K. Hollingshead, and B. Beall. 1999. Genetic linkage of exotoxin alleles and emm gene markers for tissue tropism in group A streptococci. J. Infect. Dis. 179:627-636. [DOI] [PubMed] [Google Scholar]
- 2.Bronzwaer, S. L., O. Cars, U. Buchholz, S. Mölstad, W. Goettsch, I. K. Veldhuijzen, J. L. Kool, M. JW. Sprenger, J. E. Degener, and the European Antimicrobial Resistance Surveillance System. 2002. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 8:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cars, O., S. Molstad, and A. Melander. 2001. Variation in antibiotic use in the European Union. Lancet 357:1851-1853. [DOI] [PubMed] [Google Scholar]
- 4.Cizman, M., M. Pokorn, K. Seme, A. Orazem, and M. Paragi. 2001. The relationship between trends in macrolide use and resistance to macrolides of common respiratory pathogens. J. Antimicrob. Chemother. 47:475-477. [DOI] [PubMed] [Google Scholar]
- 5.Cornaglia, G., M. Ligozzi, A. Mazzariol, L. Masala, G. Lo Cascio, G. Orefici, R. Fontana, et al. 1998. Resistance of Streptococcus pyogenes to erythromycin and related antibiotics in Italy. Clin. Infect. Dis. 1:S87-S92. [DOI] [PubMed] [Google Scholar]
- 6.de Neeling, A. J., B. P. Overbeek, A. M. Horrevorts, E. E. Ligtvoet, and W. G. Goettsch. 2001. Antibiotic use and resistance of Streptococcus pneumoniae in the Netherlands during the period 1994-1999. J. Antimicrob. Chemother. 48:441-444. [DOI] [PubMed] [Google Scholar]
- 7.Enright, M. C., B. G. Spratt, A. Kalia, J. C. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feil, E. J., E. C. Holmes, D. E. Bessen, M.-S. Chan, N. P. J. Day, M. C. Enright, R. Goldstein, D. W. Hood, A. Kalia, C. E. Moore, J. Zhou, and B. G. Spratt. 2001. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc. Natl. Acad. Sci. USA 98:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Rey, C., L. Aguilar, F. Baquero, J. Casal, and R. Dal-Ré. 2002. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 40:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Lus, R., J. J. Granizo, L. Aguilar, E. Bouza, A. Gutierrez, J. García-de-Lomas, and the Spanish Surveillance Group for Respiratory Pathogens. 1999. Is there an ecological relationship between rates of antibiotic resistance of species of the genus Streptococcus? J. Clin. Microbiol. 37:3384-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granizo, J. J., L. Aguilar, J. Casal, R. Dal-Ré, and F. Baquero. 2000. Streptococcus pyogenes resistance to erythromycin in relation to macrolide consumption in Spain (1986-1997). J. Antimicrob. Chemother. 46:959-964. [DOI] [PubMed] [Google Scholar]
- 12.Granizo, J. J., L. Aguilar, J. Casal, C. García-Rey, R. Dal-Ré, and F. Baquero. 2000. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and beta-lactam consumption in Spain (1979-1997). J. Antimicrob. Chemother. 46:767-773. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh, P. R., H. M. Chen, A. H. Huang, and J. J. Wu. 1995. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob. Agents Chemother. 39:2239-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huovinen, P., H. Seppälä, J. Kataja, T. Klaukka, and the Finnish Study Group of Antimicrobial Resistance. 1997. The relationship between erythromycin consumption and resistance in Finland. Ciba Found. Symp. 207:36-46. [PubMed] [Google Scholar]
- 15.Kalia, A., B. G. Spratt, M. C. Enright, and D. E. Bessen. 2002. Influence of recombination and niche separation on the population genetic structure of the pathogen Streptococcus pyogenes. Infect. Immun. 70:1971-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan, E. L. 1991. The resurgence of group A streptococcal infections and their sequelae. Eur. J. Clin. Microbiol. Infect. Dis. 10:55-57. [DOI] [PubMed] [Google Scholar]
- 17.Leibovici, L., R. Berger, T. Gruenewald, J. Yahav, Y. Yehezkelli, G. Milo, M. Paul, Z. Samra, and S. D. Pitlik. 2001. Departmental consumption of antibiotic drugs and subsequent resistance: a quantitative link. J. Antimicrob. Chemother. 48:535-540. [DOI] [PubMed] [Google Scholar]
- 18.Levin, B. R. 2001. Minimizing potential resistance: a population dynamics view. Clin. Infect. Dis. 33(Suppl. 3):S161-S169. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Lozano, J. M., D. L. Monnet, A. Yague, A. Burgos, A. Gonzalo, P. Campillos, and J. M. Saez. 2000. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: a time series analysis. Int. J. Antimicrob. Agents 14:21-31. [DOI] [PubMed] [Google Scholar]
- 20.Lowbury, E. J. L., and L. Hurst. 1959. The sensitivity of staphylococci and other wound bacteria to erythromycin, oleandomycin and spiramycin. J. Clin. Pathol. 12:163-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama, S., H. Yoshioka, K. Fujita, M. Takimoto, and Y. Satake. 1979. Sensitivity of group A streptococci to antibiotics. Prevalence of resistance to erythromycin in Japan. Am. J. Dis. Child. 133:151-154. [DOI] [PubMed] [Google Scholar]
- 22.Melander, E., K. Ekdahl, G. Jonsson, and S. Molstad. 2000. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr. Infect. Dis. J. 19:1172-1177. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Orden, B., E. Pérez-Trallero, M. Montes, and R. Martínez. 1998. Erythromycin resistance of Streptococcus pyogenes in Madrid. Pediatr. Infect. Dis. J. 17:470-473. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Trallero, E., C. Fernández-Mazarrasa, C. García-Rey, E. Bouza, L. Aguilar, J. García-de-Lomas, F. Baquero, and the Spanish Surveillance Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 45:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Trallero, E., J. M. Marimón, M. Montes, B. Orden, and M. de Pablos. 1999. Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg. Infect. Dis. 5:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peter, G. 1992. Streptococcal pharyngitis: current therapy and criteria for evaluation of new agents. Clin. Infect. Dis. 2:S218-223, S231-232. [DOI] [PubMed] [Google Scholar]
- 28.Reinert, R. R., A. Al-Lahham, M. Lemperle, C. Tenholte, C. Briefs, S. Haupts, H. H. Gerards, and R. Lutticken. 2001. Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany. J. Antimicrob. Chemother. 49:61-68. [DOI] [PubMed] [Google Scholar]
- 29.Seppala, H., A. Nissinen, H. Jarvinen, S. Huovinen, T. Henriksson, E. Herva, S. E. Holm, M. Jahkola, M. L. Katila, T. Klaukka, et al. 1992. Resistance to erythromycin in group A streptococci. N. Engl. J. Med. 326:292-297. [DOI] [PubMed] [Google Scholar]
- 30.Seppala, H., T. Klaukka, K. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, et al. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]
- 31.Stingemore, N., G. R. Francis, M. Toohey, and D. B. McGechie. 1989. The emergence of erythromycin resistance in Streptococcus pyogenes in Fremantle, Western Australia. Med. J. Aust. 150:626-627, 630-631. [DOI] [PubMed] [Google Scholar]
- 32.Syrogiannopoulos, G. A., I. N. Grivea, F. Fitoussi, C. Doit, G. D. Katopodis, E. Bingen, and N. G. Beratis. 2001. High prevalence of erythromycin resistance of Streptococcus pyogenes in Greek children. Pediatr. Infect. Dis. J. 20:863-868. [DOI] [PubMed] [Google Scholar]
- 33.Weber, P., J. Filipecki, E. Bingen, F. Fitoussi, G. Goldfarb, J. P. Chauvin, C. Reitz, and H. Portier. 2001. Genetic and phenotypic characterization of macrolide resistance in group A streptococci isolated from adults with pharyngo-tonsillitis in France. J. Antimicrob. Chemother. 48:291-294. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, K., J. de Azavedo, C. Restieri, L. A. Galarneau, M. Gourdeau, P. Harvey, J. F. Paradis, K. Salim, and D. E. Low. 2001. Phenotypic and genotypic characterization of macrolide-resistant group A Streptococcus strains in the province of Quebec, Canada. J. Antimicrob. Chemother. 47:345-348. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization Collaborating Center for Drug Statistic Methodology. 2002. ATC index with DDDs. World Health Organization Collaborating Center for Drug Statistic Methodology, Oslo, Norway.