Abstract
Background
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease with increasing mortality worldwide. Traditional obesity indicators inadequately predict the mortality risk in this population. Thus, the research aimed to evaluate new obesity indicators to explore their close association with RA mortality.
Methods
This study analyzed 101,316 National Health and Nutrition Examination Survey participants (1999–2018) to evaluate alternative adiposity indices for RA mortality prediction. Missing data were imputed using the random forest method. Key covariates were selected using the Boruta algorithm and weighted univariate Cox regression. Multivariable-adjusted models generated hazard ratios (95% confidence interval), validated by time-dependent receiver operating characteristic curves and Harrell’s C-index. Survival patterns were assessed with restricted cubic splines (RCS) and Kaplan–Meier curves. Threshold effects and robustness were analyzed via segmented Cox models and sensitivity analyses. Extreme gradient boosting (XGBoost) identified A Body Shape Index (ABSI) as the strongest predictor.
Results
Among the 1,266 individuals, 299 deaths occurred during follow-up (190 all-cause, 59 cardiovascular, 50 cancer). ABSI predicted the 5-, 10-, and 20-year mortality (area under the curve: 0.823, 0.801, 0.752, respectively) and outperformed other indices in the Harrell’s C-index. Weighted multivariable Cox regression linked higher ABSI × 100 values with increased mortality; Kaplan–Meier curves confirmed reduced survival in the highest quartile (P < 0.001). RCS revealed a U-curve association linking ABSI × 100 to mortality. Moreover, the mediating effects analysis indicated the Monocyte-to-High-Density Lipoprotein Cholesterol Ratio, Neutrophil-to-Lymphocyte Ratio, Advanced Lung Cancer Inflammation Index, and Systemic Immune-Inflammation Index played significant roles as mediators, with mediation ratios of 4.9%, 5.1%, 8.5%, and 4.5%, respectively. Additional sensitivity analyses validated these results. Quartile stratification revealed a pronounced risk amplification in the highest quartile (Q4), particularly in the fully adjusted specification (Hazard ratio = 3.43, 1.45–8.14; P = 0.005). Furthermore, XGBoost results indicate that ABSI is the best obesity metric for predicting the prognosis of patients with RA.
Conclusions
This study revealed a potential clinical value of a new obesity index, specifically the ABSI, in predicting the survival rates among individuals with RA. Inflammatory markers appear to play a partial mediating role in this relationship.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02584-9.
Keywords: Rheumatoid arthritis, Mortality, A Body Shape Index, Alternative adiposity indices, Inflammatory
Introduction
The prototype of inflammatory polyarthritis, rheumatoid arthritis (RA) affects nearly 1 in 100 adults worldwide, featuring progressive joint destruction through autoimmune pathogenesis, significantly driving morbidity and mortality—especially that attributed to cardiovascular disease (CVD) and cancer [1, 2]. The impact of RA goes beyond joint inflammation, with patients being predisposed to CVD—a leading mortality factor in this population [3, 4]. This elevated risk is partly attributable to well-established cardiovascular risk contributors, such as obesity, elevated blood pressure, and high lipid levels. However, the distinct pathophysiological aspects of RA make risk assessment more challenging [5]. For instance, traditional adiposity metrics particularly the body mass index (BMI) demonstrate limited capacity to characterize visceral adiposity distribution or cardiometabolic risk profiles in RA populations. This highlights the need for alternative adiposity indices that accurately reflect obesity distribution and associated health risks [6, 7].
Adiposity indices are essential for assessing nutritional status and quickly identifying disease risks. Conventional anthropometric measures include BMI, waist circumference (WC), and hip size. Nevertheless, alternative phenotyping approaches demonstrate that conventional measures poorly capture the cardiometabolic risk–associated adiposity patterning in autoimmune arthritis populations [8]. Ongoing evidence has highlighted the limitations of traditional adiposity metrics in capturing visceral adiposity dynamics linked to adverse cardiometabolic outcomes. To advance risk stratification precision, alternative adiposity indices—A Body Shape Index (ABSI), and Conicity Index (C-index)—have emerged as promising tools for quantifying inflammation-mediated fat redistribution patterns in chronic autoimmune conditions [9, 10]. For instance, ABSI’s unique ability to quantify central adiposity-driven inflammatory load enables precise prediction of metabolically unhealthy phenotypes, particularly in identifying early-stage cardiovascular remodeling before clinical manifestation [11]. Similarly, the body roundness index (BRI) has shown potential as a forecasting measure for visceral fat and body fat percentages, highlighting the need for improved metrics within this patient group [12]. Moreover, the relationship between alternative adiposity indices and fat mass varies notably based on age, sex, and conditions, such as rheumatoid cachexia, thus complicating the interpretation of these metrics in patients with RA [8].
Pro-inflammatory cytokines are central to RA pathogenesis, notably tumor necrosis factor-alpha (TNF-α)-mediated signaling, which drives metabolically adverse body composition remodeling characterized by ectopic lipid deposition and sarcopenic alterations–phenotypic shifts inadequately captured by conventional anthropometrics [13]. This indicates the need to use various alternative adiposity indices for analyzing body composition and its impact on cardiovascular health in patients with RA [14, 15]. In summary, while traditional cardiovascular risk contributors play a major role in elevating CVD risk among patients with RA, the unique pathophysiological traits of the disease call for alternative anthropometric methods for evaluating the physique profile and associated health risks better.
This study hypothesizes that alternative adiposity indices, such as ABSI and the C-index, are superior to conventional metrics such as BMI or WC in predicting adverse health outcomes—including all-cause mortality, CVD events, and cancer incidence—within the unique inflammatory and metabolic context of patients with RA. Additionally, this investigation explores potential mediating pathways involving inflammatory biomarkers that may link emerging adiposity measures with mortality outcomes in RA populations, an area that remains underexplored in current research. To test this hypothesis, the present study utilized nationally representative National Health and Nutrition Examination Survey (NHANES) datasets (1999–2018) to systematically assess the correlations between emerging adiposity metrics and overall mortality, CVD outcomes, and cancer incidence within the RA population.
Methods
Study population and design
This investigation utilized NHANES datasets—a nationally representative survey that evaluates U.S. adults’ health and nutritional status—by combining questionnaire responses with extensive physical examinations for a multidimensional perspective, which was directed by the National Center for Health Statistics (NCHS) [16]. During the original data collection, written informed consent was obtained from all the participants, and the study was approved by the NCHS Ethics Review Board.
Data from 10 NHANES cycles (1990–2018) were pooled, excluding participants with unrecorded arthritis from a total of 101,316 individuals. The research excluded 46,235 respondents under 20 years of age. A total of 1,623 patients with RA were selected from participants who identified"Rheumatoid arthritis"in response to the MCQ190, MCQ191, and MCQ195 questions regarding their type of arthritis. RA diagnosis was self-reported through three questions: MCQ190 (‘Which type of arthritis?’), MCQ191 (‘Which type of arthritis was it?’), and MCQ195 (‘Which type of arthritis was it?’). This approach has been widely employed in various NHANES studies related to RA [17, 18].
Furthermore, the study excluded 199 patients lacking specific sample weight information, 156 patients without ABSI data, and 2 patients lacking mortality information. Ultimately, 1,266 individuals with RA were considered in the research. Figure 1 presents a flowchart detailing the participant election process.
Fig. 1.

Patient selection flow diagram
Alternative adiposity indices
Alternative adiposity indices, including the ABSI, weight-adjusted waist index (WWI), conicity index (C-index), waist height ratio (WHtR), BRI, visceral adiposity index (VAI), lipid accumulation product (LAP), and relative fat mass (RFM), are better indicators of the body’s nutritional status [6]. These indices are derived from WC, which is adjusted for height and weight. All the anthropometric assessments were performed using standardized techniques (http://cdc.gov/nchs/nhanes). The following formulas were used to calculate these values [9, 12, 19–22]:
To facilitate analysis, the ABSI, WWI, and C-index were categorized into quartiles:
Mortality
Data regarding the mortality status and duration of follow-up (until December 31, 2019) were collected from the NHANES through the National Death Index Mortality Database. Mortality outcomes were evaluated based on the International Classification of Diseases, 10 th Revision (ICD–10). The primary emphasis of the research was all-cause deaths, while CVD and cancer-related deaths were regarded as secondary endpoints. CVD–affiliated deaths were classified under ICD–10 codes 054–068, while cancer–affiliated deaths were segmented under ICD–10 codes 019–043 [23].
Explanations of the covariates
In this study, an extensive array of covariates was employed to address potential confounding factors, thereby enhancing the reliability and precision of the analysis. The examined covariates encompassed demographic details, including age, sex, marital status, ethnicity, education, and the level of family income in relation to the poverty guideline (PIR). Moreover, health-related factors, including smoking and alcohol consumption, were also assessed. The medical condition data included congestive heart failure (CHF), diabetes mellitus (DM), coronary heart disease (CHD), hyperlipidemia, hypertension, chronic kidney disease (CKD), angina, stroke, and chronic obstructive pulmonary disease (COPD). Medication data included antidiabetic medications, antihypertensive agents, anti-infective drugs, and antihyperlipidemic therapies. Laboratory parameters assessed included fasting plasma glucose (FPG), urinary albumin (u-ALB), HDL-C, glycosylated hemoglobin (HbA1c), lymphocyte percentage, TG, albumin (ALB), uric acid, serum creatinine (SCR), urine creatinine (Ucr), total cholesterol (TC), neutrophil percentage, monocyte percentage, and low-density lipoprotein cholesterol (LDL-C).
Ethnicity was classified as Mexican Hispanic, non-Hispanic Black, non-Mexican Hispanic, non-Hispanic White, and other. The married population included individuals who were married, widowed, divorced, or in a committed relationship. Family income was categorized as low, middle, or high, corresponding to a family PIR (< 1.0, 1.0–3.0, > 3.0), respectively. Participants’ smoking was classified into three categories: nonsmokers, former smokers, and current smokers. Education levels were divided into three groups. Alcohol consumption was designated as five categories: never, former, heavy, moderate, and mild drinkers [24].
The height, weight, WC, and BMI of the participants were assessed by the NHANES staff at the Mobile Examination Center. BMI was divided into four types [23]. In addition, serum samples were collected during laboratory assessments in the initial phase to evaluate clinical indicators, including FPG, HDL-C, and TC. Information regarding the use of prescription medications was collected through the interview sections of the NHANES. Detailed definitions of comorbidities are available in Supplementary file 1.
Inflammatory markers
We derived multiple inflammatory indices to assess systemic inflammation based on the peripheral blood cell counts. These indices include the Systemic Immune-Inflammation Index (SII), Neutrophil Percentage-to-Albumin Ratio (NPAR), Monocyte-to-High-Density Lipoprotein Cholesterol Ratio (MHR), Platelet-to-Lymphocyte Ratio (PLR), C-Reactive Protein-to-Lymphocyte Ratio (CLR), Lymphocyte-to-High-Density Lipoprotein Cholesterol Ratio (LHR), Neutrophil-to-Lymphocyte Ratio (NLR), Advanced Lung Cancer Inflammation Index (ALI), and Systemic Inflammatory Response Index (SIRI) [25].
Data analysis
Given the intricate sampling framework of the NHANES dataset, we applied sampling weights and made adjustments for stratification and clustering in all the analyses to ensure robust estimates and enhance the applicability of the results. We used three indicators of alternative adiposity indices, namely ABSI, WWI, and C-index, as independent variables and all-cause, CVD, and cancer mortality as outcome variables. To control for statistical differences, we multiplied the ABSI and C-index by 100 for data analysis. An introductory descriptive assessment was undertaken to capture the main traits of the study population.
The Boruta algorithm, a supervised machine-learning technique designed to identify all relevant features for constructing a model, was used for feature selection. Following feature selection, we conducted a weighted univariate Cox regression analysis, with the results integrated alongside the final selections from the Boruta algorithm to ensure the inclusion of all relevant covariates in the analysis. To enhance statistical efficacy and minimize potential bias caused by the exclusion of individuals due to lacking covariate values, we utilized the random forest imputation technique for multiple imputations to handle missing covariate data [26].
The predictive accuracy of the newly developed adiposity indices for survival outcomes over various time intervals was assessed through time-dependent receiver operating characteristic (ROC) curve analysis using the time ROC package. Additionally, time-dependent Harrell’s C-index curves were created to estimate the prognostic performance of these alternative adiposity indices in mortality prediction [27].
A weighted multivariate Cox proportional hazards model was employed to develop three distinct models for estimating hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs), ensuring comprehensive adjustment for potential confounders. Model 1 was based on a univariate Cox proportional regression analysis and did not include any adjustments for variables. Model 2 was adapted for age, sex, ethnicity, educational, and marital status. Model 3 further incorporated additional covariates beyond those in Model 2, including PIR, smoking, alcohol, DM, hyperlipidemia, hypertension, CKD, COPD, stroke, use of antihypertensive and antihyperlipidemic medications, lymphocyte percentage, neutrophil percentage, HbA1c levels, and estimated glomerular filtration rate (eGFR).
Kaplan–Meier survival curves were created to demonstrate further the discrepancies in the survival rates among the alternative adiposity indices. In addition, restricted cubic spline (RCS) were utilized to explore potential nonlinear relationships between ABSI × 100, WWI, C-index × 100, and mortality outcomes. If the RCS suggested a nonlinear relationship, the turning point was determined using the “segmented” package, which employs a likelihood ratio test and bootstrap resampling method. Thereafter, a segmented Cox proportional hazards model was used on either side of the turning point to examine the association between the three indices and mortality. A follow-up subgroup analysis was conducted to investigate the association between ABSI × 100 and mortality outcomes within different stratified groups, using the likelihood ratio test to assess subgroup interactions.
To further investigate the potential mechanisms underlying this association, inflammatory markers (NPAR, NLR, PLR, SII, MHR, ALI, SIR, and CLR) were selected for the mediation analysis to identify potential mediators in the relationship between ABSI and mortality. A sensitivity analysis was conducted to enhance result validity by excluding participants who died within the first two years of monitoring. Finally, to further assess the significance of selected factors related to RA, the XGBoost algorithm, a machine-learning technique, was implemented. This approach enabled robust feature selection and enhanced predictive modeling. All the statistical analyses were conducted using R 4.4.2 (R Foundation for Statistical Computing, Vienna, Austria) along with EmpowerStats 4.2 (X&Y solutions, Inc. Boston, MA, US).
Results
Baseline characteristics
Significant variations in the initial features were observed across quartiles of ABSI × 100 among individuals with RA (Table 1). First, the average age of individuals demonstrated a significant upward trend (P < 0.001), with the average age in the Q1 group at 36.7 years and rising to 61.4 years in the Q4 group. In addition, racial distribution varied significantly with an increase in the ABSI quartile (P < 0.001), with a decrease in the percentage of African Americans and an increase in the proportion of non-Hispanic white patients. Marital status also demonstrated a notable growth in the percentage of married or cohabiting patients as the ABSI × 100 quartile increased (P < 0.001), while the percentage of single patients decreased. Smoking and alcohol consumption also demonstrated notable variations among the quartiles (P < 0.001). The rates of DM, chronic kidney disease (CKD), hyperlipidemia, and hypertension demonstrated a significant rise across the ABSI quartiles (P < 0.001). For example, the rate of DM was 3.4% in the Q1 group and 25.5% in Q4, respectively, indicating a significant difference. Similarly, the prevalence increased from 2.5% in Q1 to 11.9% in Q4. Additionally, BMI and WC were notably greater in the Q4 than in the Q1 (P < 0.001), with WC exhibiting the most pronounced difference. Regarding medication usage, individuals in the Q4 group exhibited a significantly higher likelihood of using antidiabetic drugs in comparison to those in the Q1 (P < 0.001). The baseline features of the survival and mortality groups regarding overall CVD and cancer deaths are summarized in Tables S1–S3.
Table 1.
Baseline characteristics of participants with RA across ABSI (per 0.01 units) quartiles
| aVariables | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years |
36.705 (35.054,38.357) |
43.552 (41.606,45.498) | 49.068 (46.883,51.253) | 61.373 (59.598,63.148) | < 0.001 |
| Age group, years | < 0.001 | ||||
| ≤ 40 | 65.716 (59.196,71.693) | 44.797 (37.933,51.867) | 31.687 (25.260,38.898) | 9.946 (6.344,15.258) | |
| > 40, ≤ 60 | 27.692 (21.916,34.322) | 42.029 (35.581,48.761) | 44.102 (36.634,51.846) | 34.772 (28.189,41.995) | |
| > 60 | 6.592 (3.798,11.201) | 13.174 (9.443,18.084) | 24.212 (18.784,30.616) | 55.282 (48.284,62.077) | |
| Sex, n(%) | 0.159 | ||||
| Male | 42.072 (35.293,49.164) | 46.541 (39.993,53.211) | 49.642 (41.692,57.611) | 53.353 (46.427,60.152) | |
| Female | 57.928 (50.836,64.707) | 53.459 (46.789,60.007) | 50.358 (42.389,58.308) | 46.647 (39.848,53.573) | |
| Ethnicity, n(%) | < 0.001 | ||||
| Mexican American | 7.679 (5.317,10.970) | 10.074 (7.493,13.416) | 10.912 (7.716,15.213) | 6.729 (4.772,9.410) | |
| Other Hispanic | 7.421 (4.504,11.989) | 8.371 (5.327,12.916) | 5.403 (3.215,8.941) | 3.365 (1.586,6.997) | |
| Non-Hispanic White | 60.202 (52.880,67.094) | 65.104 (58.620,71.073) | 73.402 (68.526,77.767) | 75.605 (69.821,80.589) | |
| Non-Hispanic Black | 19.789 (15.723,24.599) | 11.378 (8.672,14.792) | 6.641 (4.802,9.116) | 4.352 (2.905,6.473) | |
| Other Race (Including Multi-Racial) | 4.909 (2.991,7.958) | 5.073 (2.964,8.550) | 3.642 (2.146,6.118) | 9.949 (6.473,14.991) | |
| Education level | 0.480 | ||||
| Under High School | 14.611 (11.138,18.937) | 21.838 (16.961,27.649) | 18.220 (13.728,23.776) | 21.144 (16.187,27.128) | |
| High School | 25.299 (19.136,32.647) | 21.575 (16.176,28.171) | 21.188 (15.789,27.823) | 23.829 (18.574,30.022) | |
| More Than High School | 60.090 (52.933,66.840) | 56.587 (49.715,63.215) | 60.592 (53.691,67.094) | 55.027 (47.591,62.246) | |
| PIR | 0.992 | ||||
| ≤ 1.5 | 23.605 (18.788,29.213) | 22.957 (18.852,27.652) | 21.781 (17.951,26.166) | 23.780 (18.263,30.346) | |
| > 1.5, ≤ 3.5 | 39.955 (33.558,46.713) | 40.191 (33.331,47.458) | 40.794 (33.773,48.211) | 42.273 (34.990,49.909) | |
| > 3.5 | 36.441 (29.999,43.408) | 36.852 (30.250,43.987) | 37.426 (30.019,45.473) | 33.947 (27.058,41.589) | |
| Marital status, n(%) | < 0.001 | ||||
| Married/Living with a partner | 59.927 (54.179,65.413) | 67.363 (60.736,73.363) | 70.763 (63.428,77.157) | 66.389 (59.305,72.805) | |
| Never Married | 14.521 (10.493,19.754) | 16.849 (12.777,21.893) | 16.863 (11.893,23.359) | 28.610 (22.146,36.085) | |
| Divorced/Separated/Widowed | 25.552 (20.357,31.549) | 15.788 (11.365,21.514) | 12.374 (8.407,17.848) | 5.002 (2.704,9.070) | |
| Smoking status | < 0.001 | ||||
| Never | 60.596 (53.833,66.976) | 62.552 (55.381,69.210) | 47.893 (39.670,56.231) | 48.209 (40.929,55.566) | |
| Former | 16.922 (12.725,22.151) | 16.692 (11.948,22.831) | 32.364 (25.191,40.475) | 32.637 (26.100,39.927) | |
| Now | 22.482 (17.590,28.268) | 20.756 (15.509,27.207) | 19.743 (14.342,26.547) | 19.154 (13.715,26.098) | |
| Alcohol consumption | < 0.001 | ||||
| Never | 9.478 (6.779,13.101) | 9.887 (6.794,14.174) | 8.307 (5.577,12.199) | 16.171 (11.632,22.041) | |
| Former | 8.522 (5.538,12.894) | 13.430 (9.468,18.708) | 15.797 (11.222,21.780) | 24.586 (18.972,31.220) | |
| Mild | 36.341 (29.467,43.823) | 43.029 (36.505,49.803) | 34.469 (27.695,41.940) | 35.906 (29.031,43.413) | |
| Moderate | 21.976 (17.046,27.853) | 11.760 (8.226,16.539) | 22.520 (16.346,30.184) | 10.972 (6.989,16.816) | |
| Heavy | 23.683 (18.919,29.215) | 21.894 (16.877,27.902) | 18.907 (13.554,25.745) | 12.365 (8.259,18.108) | |
| Physical examination | |||||
| BMI, kg/m2 | 27.900 (27.079,28.722) | 28.494 (27.666,29.322) | 28.668 (27.769,29.567) | 28.609 (27.650,29.568) | 0.551 |
| BMI group, kg/m2 | 0.063 | ||||
| Underweight (< 18.5) | 1.072 (0.393,2.889) | 2.185 (0.958,4.902) | 0.445 (0.149,1.319) | 2.862 (0.920,8.549) | |
| Normal (18.5 to < 25) | 40.722 (33.962,47.854) | 31.001 (24.732,38.055) | 28.128 (21.125,36.382) | 26.172 (19.135,34.687) | |
| Overweight (25 to < 30) | 29.483 (23.033,36.874) | 31.666 (25.552,38.487) | 34.993 (28.188,42.469) | 35.335 (28.943,42.299) | |
| Obese (30 or greater) | 28.722 (22.863,35.393) | 35.149 (29.155,41.650) | 36.433 (29.148,44.399) | 35.630 (28.643,43.289) | |
| WC, cm | 89.254 (87.594,90.913) | 96.349 (94.383,98.316) | 100.843 (98.662,103.025) | 106.645 (104.248,109.042) | < 0.001 |
| PA | 0.009 | ||||
| No | 66.870 (59.445,73.541) | 74.278 (67.267,80.229) | 74.306 (66.029,81.142) | 83.715 (77.013,88.748) | |
| Yes | 33.130 (26.459,40.555) | 25.722 (19.771,32.733) | 25.694 (18.858,33.971) | 16.285 (11.252,22.987) | |
| Chronic diseases | |||||
| DM, n (%) | < 0.001 | ||||
| No | 93.172 (89.342,95.693) | 85.588 (79.856,89.896) | 82.250 (75.640,87.365) | 65.033 (58.508,71.040) | |
| Prediabetes | 3.452 (1.808,6.491) | 4.116 (2.250,7.414) | 4.888 (2.580,9.069) | 9.436 (6.114,14.290) | |
| Yes | 3.376 (1.667,6.717) | 10.295 (6.562,15.794) | 12.863 (8.325,19.351) | 25.530 (19.891,32.127) | |
| CKD, n (%) | < 0.001 | ||||
| No | 98.177 (95.038,99.344) | 96.394 (93.819,97.921) | 94.443 (91.372,96.463) | 86.460 (81.438,90.285) | |
| Yes | 1.823 (0.656,4.962) | 3.606 (2.079,6.181) | 5.557 (3.537,8.628) | 13.540 (9.715,18.562) | |
| Hyperlipidemia, n (%) | < 0.001 | ||||
| No | 49.135 (41.772,56.536) | 35.766 (29.074,43.063) | 25.488 (19.057,33.199) | 15.067 (10.853,20.541) | |
| Yes | 50.865 (43.464,58.228) | 64.234 (56.937,70.926) | 74.512 (66.801,80.943) | 84.933 (79.459,89.147) | |
| Hypertension, n (%) | < 0.001 | ||||
| No | 82.953 (77.931,87.023) | 67.959 (60.544,74.566) | 62.841 (55.432,69.692) | 42.618 (35.945,49.571) | |
| Yes | 17.047 (12.977,22.069) | 32.041 (25.434,39.456) | 37.159 (30.308,44.568) | 57.382 (50.429,64.055) | |
| Angina, n (%) | 0.022 | ||||
| No | 99.792 (99.086,99.953) | 99.043 (96.964,99.702) | 98.267 (95.459,99.350) | 97.254 (95.128,98.467) | |
| Yes | 0.208 (0.047,0.914) | 0.957 (0.298,3.036) | 1.733 (0.650,4.541) | 2.746 (1.533,4.872) | |
| CHF, n (%) | 0.003 | ||||
| No | 99.313 (98.141,99.748) | 98.760 (96.828,99.521) | 98.546 (96.210,99.450) | 95.620 (91.990,97.647) | |
| Yes | 0.687 (0.252,1.859) | 1.240 (0.479,3.172) | 1.454 (0.550,3.790) | 4.380 (2.353,8.010) | |
| COPD, n (%) | < 0.001 | ||||
| No | 99.764 (98.295,99.968) | 98.602 (95.345,99.590) | 93.418 (88.092,96.457) | 91.232 (84.464,95.218) | |
| Yes | 0.236 (0.032,1.705) | 1.398 (0.410,4.655) | 6.582 (3.543,11.908) | 8.768 (4.782,15.536) | |
| CHD, n (%) | < 0.001 | ||||
| No | 99.704 (98.827,99.926) | 97.642 (94.359,99.034) | 96.622 (93.201,98.352) | 91.510 (87.192,94.465) | |
| Yes | 0.296 (0.074,1.173) | 2.358 (0.966,5.641) | 3.378 (1.648,6.799) | 8.490 (5.535,12.808) | |
| Stroke, n (%) | < 0.001 | ||||
| No | 99.861 (98.986,99.981) | 97.765 (94.768,99.063) | 96.214 (92.718,98.067) | 91.517 (86.907,94.604) | |
| Yes | 0.139 (0.019,1.014) | 2.235 (0.937,5.232) | 3.786 (1.933,7.282) | 8.483 (5.396,13.093) | |
| Medications | |||||
| Antidiabetic drug | < 0.001 | ||||
| No | 97.318 (93.781,98.868) | 94.314 (89.884,96.872) | 91.674 (87.104,94.722) | 82.079 (75.081,87.440) | |
| Yes | 2.682 (1.132,6.219) | 5.686 (3.128,10.116) | 8.326 (5.278,12.896) | 17.921 (12.560,24.919) | |
| Antihypertensive drug | < 0.001 | ||||
| No | 90.349 (86.461,93.209) | 79.862 (73.166,85.224) | 71.053 (63.080,77.908) | 48.034 (39.423,56.764) | |
| Yes | 9.651 (6.791,13.539) | 20.138 (14.776,26.834) | 28.947 (22.092,36.920) | 51.966 (43.236,60.577) | |
| Antihyperlipidemic drug | < 0.001 | ||||
| No | 96.787 (93.828,98.352) | 88.243 (83.449,91.785) | 80.934 (74.234,86.216) | 60.925 (53.059,68.262) | |
| Yes | 3.213 (1.648,6.172) | 11.757 (8.215,16.551) | 19.066 (13.784,25.766) | 39.075 (31.738,46.941) | |
| Anti-infective drug | 0.203 | ||||
| No | 94.374 (90.180,96.839) | 92.713 (88.146,95.608) | 96.827 (94.095,98.318) | 92.586 (87.126,95.841) | |
| Yes | 5.626 (3.161,9.820) | 7.287 (4.392,11.854) | 3.173 (1.682,5.905) | 7.414 (4.159,12.874) | |
| Alternative adiposity indices | |||||
| VAI | 1.650 (1.484,1.816) | 2.367 (2.020,2.714) | 2.722 (2.301,3.143) | 3.282 (2.842,3.723) | < 0.001 |
| LAP | 39.081 (34.910,43.252) | 63.506 (54.668,72.345) | 73.508 (64.628,82.389) | 97.560 (86.130,108.990) | < 0.001 |
| RFM | 32.287 (31.060,33.514) | 34.672 (33.650,35.694) | 35.984 (34.672,37.296) | 37.361 (36.203,38.520) | < 0.001 |
| BRI | 4.082 (3.857,4.307) | 4.974 (4.715,5.233) | 5.498 (5.200,5.796) | 6.313 (5.960,6.666) | < 0.001 |
| WHtR | 0.530 (0.519,0.540) | 0.573 (0.561,0.584) | 0.597 (0.585,0.610) | 0.632 (0.618,0.646) | < 0.001 |
| Laboratory data | |||||
| eGFR, ml/min/1.73 m | 101.421 (98.957,103.885) | 98.392 (95.781,101.004) | 93.282 (90.697,95.866) | 83.186 (80.517,85.856) | < 0.001 |
| FPG, mg/dL | 95.807 (94.515,97.099) | 102.316 (97.482,107.151) | 105.744 (101.511,109.976) | 115.139 (110.807,119.472) | < 0.001 |
| HDL-C, mg/dL | 54.954 (52.902,57.005) | 54.543 (52.265,56.821) | 53.756 (49.884,57.629) | 50.597 (48.674,52.519) | 0.007 |
| LDL-C, mg/dL | 110.787 (107.814,113.759) | 116.232 (112.930,119.533) | 116.916 (112.685,121.148) | 113.892 (110.280,117.504) | 0.032 |
| TG, mg/dL | 115.507 (105.985,125.029) | 149.357 (132.819,165.896) | 163.954 (145.575,182.334) | 193.406 (171.780,215.031) | < 0.001 |
| TC, mg/dL | 187.619 (182.220,193.018) | 198.859 (194.077,203.641) | 202.463 (195.505,209.421) | 197.939 (191.590,204.288) | 0.002 |
| Alb, g/dL | 4.352 (4.301,4.403) | 4.350 (4.311,4.389) | 4.305 (4.259,4.351) | 4.202 (4.136,4.267) | 0.001 |
| Uric acid, mg/dL | 5.045 (4.860,5.229) | 5.254 (5.073,5.435) | 5.345 (5.140,5.550) | 5.543 (5.360,5.725) | 0.005 |
| SCR, mg/dL | 0.848 (0.825,0.871) | 0.838 (0.807,0.868) | 0.862 (0.833,0.892) | 0.940 (0.890,0.990) | < 0.001 |
| u-ALB, ug/mL | 12.867 (9.373,16.361) | 16.063 (9.834,22.293) | 32.724 (19.206,46.243) | 30.396 (21.031,39.761) | < 0.001 |
| Ucr, mg/dL | 125.284 (114.531,136.038) | 125.871 (114.642,137.101) | 120.127 (108.461,131.794) | 110.287 (98.923,121.651) | 0.130 |
| HbA1c, % | 5.296 (5.241,5.352) | 5.503 (5.413,5.593) | 5.620 (5.499,5.740) | 5.850 (5.738,5.963) | < 0.001 |
| Neutrophil, % | 57.198 (56.069,58.328) | 58.618 (57.406,59.830) | 58.787 (57.449,60.124) | 61.538 (60.346,62.730) | < 0.001 |
| Monocyte, % | 8.145 (7.842,8.449) | 7.549 (7.251,7.848) | 7.907 (7.616,8.199) | 8.101 (7.802,8.400) | 0.016 |
| Lymphocyte, % | 31.189 (30.189,32.189) | 30.531 (29.528,31.535) | 29.668 (28.409,30.926) | 26.677 (25.580,27.774) | < 0.001 |
| All-cause mortality, (%) | < 0.001 | ||||
| No | 97.615 (95.168,98.838) | 93.885 (90.297,96.202) | 89.093 (84.190,92.610) | 74.510 (68.064,80.037) | |
| Yes | 2.385 (1.162,4.832) | 6.115 (3.798,9.703) | 10.907 (7.390,15.810) | 25.490 (19.963,31.936) | |
| CVD mortality, n(%) | < 0.001 | ||||
| No | 99.931 (99.498,99.991) | 98.040 (95.028,99.242) | 96.314 (93.039,98.080) | 92.448 (89.139,94.807) | |
| Yes | 0.069 (0.009,0.502) | 1.960 (0.758,4.972) | 3.686 (1.920,6.961) | 7.552 (5.193,10.861) | |
| Cancer mortality, n(%) | < 0.001 | ||||
| No | 99.565 (98.398,99.883) | 99.612 (98.282,99.913) | 96.388 (93.108,98.138) | 92.523 (88.729,95.110) | |
| Yes | 0.435 (0.117,1.602) | 0.388 (0.087,1.718) | 3.612 (1.862,6.892) | 7.477 (4.890,11.271) | |
Significant differences are indicated by bold P values (P < 0.05)
Abbreviations: Q1–Q4 quartile 1–4 Q1(6.677–7.808), Q2(7.808–8.160), Q3(8.160–8.504), Q4(8.504–9.895), NHANES National Health and Nutrition Examination Survey, WHtR Waist height ratio, PIR the ratio of family income to poverty, PA physical activity, DM Diabetes mellitus, BMI body mass index, CKD chronic kidney disease, CHF Congestive heart failure, ABSI a body shape index, VAI visceral adiposity index, BRI Body roundness index, WC waist circumference, HDL-C high-density lipoprotein-cholesterol, TG triglyceride, TC cholesterol, RFM Relative fat mass, CHD Coronary heart disease, Alb albumin, SCR serum creatinine, u-ALB urinary albumin, Ucr Urine Creatinine, HbA1c glycosylated hemoglobin, Neutrophil Neutrophil percentage, Monocyte Monocyte percentage, FPG fasting plasma glucose, Lymphocyte Lymphocyte percentage, CVD cardiovascular disease, COPD chronic obstructive pulmonary disease, LDL-C low-density lipoprotein-cholesterol, eGFR estimated glomerular filtration rate, LAP lipid accumulation product
aFor continuous variables: survey-weighted mean (95% CI); categorical variables: Percentages (weighted %) (95%CI); the p-value was determined by survey-weighted linear regression (svyglm)
Among the alternative adiposity indices, the ABSI, WWI, C-index, and RFM significantly influenced the all-cause mortality, with higher values observed in individuals who died. For specific causes of mortality, including cancer and CVD, the ABSI, WWI, and C-index also demonstrated significant associations, with higher values observed in the mortality groups. Additionally, CVD mortality was significantly associated with the WHtR and BRI, which were elevated in individuals who died from cardiovascular events.
Feature selection and univariate analysis
Figure 2 illustrates the outcomes of feature selection using Boruta’s algorithm. After 500 iterations, the six elements most strongly linked to overall mortality, ranked by the z-value, were age, eGFR, ABSI, U-ALB, lymphocyte percentage, and CVD. The six factors most strongly linked to CVD mortality were age, CVD, eGFR, CKD, CHF, and WWI. Finally, for cancer mortality, the six most closely correlated variables were age, ABSI, U-ALB, eGFR, COPD, and CVD. Although several key variables—including smoking, PIR, hyperlipidemia, ethnicity, sex, education, neutrophil percentage, marital status, DM, alcohol use, and the usage of antihyperlipidemic medications—were excluded from the initial analysis due to lower z-values relative to the most significant associations, they were nevertheless retained for further analysis.
Fig. 2.
Feature selection process for mortality based on Boruta’s algorithm
A weighted univariate Cox repercussion was performed to estimate the relationships among various factors and mortality outcomes (Tables S4–S6). The results demonstrated that body composition indicators, such as ABSI, WWI, C-index, WHtR, BRI, WC, and RFM, were strongly positively correlated with overall and CVD deaths (P < 0.05), whereas BMI, VAI, and LAP were not. Age was identified as a major contributor to various forms of mortality (HR = 1.1, P < 0.001).
Conversely, a higher PIR (> 3.5) and an education level exceeding high school were correlated with a lower risk of mortality (P < 0.05).
Among the sociodemographic characteristics, non-Hispanic White individuals and those who had never been married exhibited significantly higher mortality risks, whereas females had significantly lower mortality risks than males (P < 0.05). Mortality was also significantly linked to smoking, alcohol use, and levels of physical activity, as individuals who had previously smoked and those who did not participate in physical activity exhibited a greater mortality risk (P < 0.05). Disease-related factors, such as hypertension, CHD, COPD, stroke, CHF, and CKD, markedly elevated the likelihood of overall CVD mortality (HR > 3.0, P < 0.001). Analysis of metabolic parameters revealed divergent correlations with mortality outcomes. Elevated FPG, HbA1c, SCR, and u-ALB levels exhibited positive relationships with fatal risk, while increased eGFR and higher concentrations of blood albumin demonstrated protective effects (P < 0.05). By a combination of methodological approaches, including Boruta feature selection and weighted univariate Cox regression analyses along with clinical significance, the covariates for adjustment were confirmed.
The capacity of alternative adiposity indices to predict mortality among individuals with RA
Time-dependent ROC evaluation revealed that the area under the curve (AUC) of ABSI in forecasting 5-year, 10-year, and 20-year overall mortality rates was 0.823, 0.801, and 0.752, respectively (Fig. 3A, B, and C). Similarly, the AUCs of ABSI for CVD deaths at 5, 10, and 20 years were 0.827, 0.793, and 0.681, respectively (Fig. 3D, E, and F). The AUCs for cancer mortality were 0.869, 0.806, and 0.794 at 5, 10, and 20 years of age, respectively (Fig. 3G, H, and I). The results indicate that the ABSI has a reliable and efficient predictive capability for mortality over various periods. Moreover, we assessed the predictive potential of other alternative adiposity indices for all-cause and specific mortalities related to CVD and cancer in patients with RA. The results indicated that, regardless of the time frame (5, 10, or 20 years), the predictive performance of the other adiposity indices was inferior to that of the ABSI (Fig. 3).
Fig. 3.
The ROC curves dependent on time and the AUC values for the alternative adiposity indices were utilized to forecast mortality
In addition, we determined Harrell’s C-index for the ABSI and WWI, demonstrating significant associations with overall CVD and cancer mortality. We found that the ABSI had the best predictive performance (Fig. 4). Ultimately, we selected these three relatively well-performing indicators for inclusion in the Cox regression analysis.
Fig. 4.
Time-dependent predictive capacity of ABSI, WWI, and C-index for all-cause, CVD, and cancer mortality
Associations of ABSI, WWI, and C-index with all-cause, cardiovascular, and cancer mortality
In assessing the mortality risk among clients with RA, HRs were computed for overall and specific mortality through three distinct models: Model 1 (univariate Cox regression), Model 2 (modified for age, sex, ethnicity, education, and marital status), and Model 3 (further modified for additional characteristics, including PIR, smoking, alcohol use, comorbidities, medication use, and laboratory parameters) (Table 2).
Table 2.
Associations of ABSI, WWI, and C-index with all-cause, cardiovascular, and cancer mortality
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | ||
| All-cause mortality | All-cause mortality | ||||||||||
|
ABSI (per 0.01 units) |
6.726 (4.788,9.447) | <0.001 | 2.060 (1.439,2.948) | <0.001 | 2.074 (1.413,3.044) | <0.001 | WWI | 2.818 (2.275,3.491) | <0.001 | 1.596 (1.271,2.005) | <0.001 |
| ABSI quartiles | WWI quartiles | ||||||||||
| Q1 | ref | ref | ref | Q1 | ref | ref | |||||
| Q2 | 2.800 (1.188,6.603) | 0.019 | 1.944 (0.794,4.761) | 0.146 | 2.359(0.874,6.370) | 0.090 | Q2 | 2.732 (1.270,5.879) | 0.010 | 1.334(0.652,2.731) | 0.429 |
| Q3 | 5.847 (2.576,13.272) | <0.001 | 3.040 (1.322,6.990) | 0.009 | 3.541(1.481,8.463) | 0.004 | Q3 | 4.842(2.262,10.362) | <0.001 | 1.751(0.916,3.348) | 0.09 |
| Q4 | 15.998 (8.164,31.348) | <0.001 | 3.710 (1.939,7.099) | <0.001 | 4.025(1.945,8.330) | <0.001 | Q4 | 11.667(5.775,23.570) | <0.001 | 2.326(1.234,4.384) | 0.009 |
| P for trend | <0.001 | <0.001 | <0.001 | P for trend | <0.001 | ||||||
| CVD mortality | CVD mortality | 0.007 | |||||||||
|
ABSI (per 0.01 units) |
7.842 (4.561,13.484) | <0.001 | 2 .241(1.218,4.123) | 0.009 | 2.248(1.048,4.821) | 0.038 | WWI | 3.991 (2.936,5.425) | <0.001 | 2.437 (1.581,3.756) | <0.001 |
| ABSI quartiles | WWI quartiles | ||||||||||
| Q1 | ref | ref | ref | Q1 | ref | ref | |||||
| Q2 | 32.118 (3.669,281.140) | 0.002 | 16.199(1.838,142.785) | 0.012 | 33.626 (1.982,570.559) | 0.015 | Q2 | 7.682 (1.693,34.868) | 0.008 | 3.318(0.630,17.472) | 0.157 |
| Q3 | 72.662 (9.100,580.192) | <0.001 | 28.477(3.490,232.338) | 0.001 | 52.837(3.055,913.893) | 0.006 | Q3 | 18.101 (5.025,65.210) | <0.001 | 5.070(1.275,20.156) | 0.021 |
| Q4 | 178.517 (23.826,11337.548) | <0.001 | 28.655(3.560,230.631) | 0.001 | 49.183(2.587,934.922) | 0.009 | Q4 | 53.987(17.186,169.595) | <0.001 | 8.491(2.427,29.708) | <0.001 |
| P for trend | <0.001 | 0.008 | 0.024 | P for trend | <0.001 | ||||||
| Cancer mortality | Cancer mortality | 0.001 | |||||||||
|
ABSI (per 0.01 units) |
8.904 (5.232,15.150) | <0.001 | 3.420 (1.754,6.669) | <0.001 | 4.237 (2.092,8.580) | <0.001 | WWI | 2.356 (1.746,3.179) | <0.001 | 1.362 (0.879,2.110) | 0.167 |
| ABSI quartiles | WWI quartiles | ||||||||||
| Q1 | ref | ref | ref | Q1 | ref | ref | |||||
| Q2 | 0.971 (0.138,6.812) | 0.976 | 0.555(0.084,3.693) | 0.543 | 0.573(0.089,3.680) | 0.557 | Q2 | 4.976(1.284,19.280) | 0.020 | 2.124(0.477,9.459) | 0.323 |
| Q3 | 10.213 (2.546,40.975) | 0.001 | 4.613(1.120,19.014) | 0.034 | 4.607(1.144,18.557) | 0.032 | Q3 | 6.828(1.953,23.867) | 0.002 | 2.292(0.660,7.967) | 0.192 |
| Q4 | 24.584 (6.706,90.126) | <0.001 | 5.751(1.631,20.273) | 0.006 | 6.783(2.069,22.236) | 0.002 | Q4 | 11.571(3.767,35.541) | <0.001 | 2.327(0.689,7.858) | 0.174 |
| P for trend | <0.001 | <0.001 | <0.001 | P for trend | <0.001 | 0.343 | |||||
| Model 3 | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | ||
| All-cause mortality | All-cause mortality | ||||||||
|
ABSI (per 0.01 units) |
1.436 (1.096,1.882) | 0.008 |
C_index ( per 0.01 units) |
1.095 (1.075,1.115) |
<0.001 | 1.596 (1.271,2.005) | <0.001 | 1.025 (1.002,1.048) | 0.035 |
| ABSI quartiles | C_index quartiles | ||||||||
| Q1 | ref | Q1 | ref | ref | ref | ||||
| Q2 | 1.370(0.565,3.321) | 0.486 | Q2 | 2.732 (1.270,5.879) | 0.010 | 1.633(0.794,3.362) | 0.183 | 1.835(0.824,4.087) | 0.137 |
| Q3 | 1.738(0.834,3.620) | 0.140 | Q3 | 4.842(2.262,10.362) | <0.001 | 1.617(0.844,3.098) | 0.147 | 1.442(0.667,3.119) | 0.352 |
| Q4 | 2.259(1.053,4.843) | 0.036 | Q4 | 11.667(5.775,23.570) | <0.001 | 2.053(1.182,3.565) | 0.011 | 1.939(1.000,3.759) | 0.049 |
| P for trend | <0.001 | P for trend | <0.001 | 0.017 | 0.122 | ||||
| CVD mortality | CVD mortality | ||||||||
|
ABSI (per 0.01 units) |
2.431(1446,4.087) | <0.001 |
C_index ( per 0.01 units) |
1.114 (1.081,1.148) | <0.001 | 2.437 (1.581,3.756) | <0.001 | 1.047(1.005,1.090) | 0.028 |
| ABSI quartiles | C_index quartiles | ||||||||
| Q1 | ref | Q1 | ref | ref | ref | ||||
| Q2 | 4.297 (0.616,29.974) | 0.141 | Q2 | 7.682 (1.693,34.868) | 0.008 | 1.620(0.341,7.698) | 0.544 | 2.147(0.370,12.475) | 0.395 |
| Q3 | 7.937(1.438,43.790) | 0.017 | Q3 | 18.101 (5.025,65.210) | <0.001 | 1.703(0.331,8.757) | 0.524 | 1.381(0.190,10.061) | 0.750 |
| Q4 | 13.129(2.522,68.364) | 0.002 | Q4 | 53.987(17.186,169.595) | <0.001 | 2.603(0.543,12.476) | 0.231 | 3.006(0.489,18.473) | 0.235 |
| P for trend | 0.017 | P for trend | <0.001 | 0.106 | 0.186 | ||||
| Cancer mortality | Cancer mortality | ||||||||
|
ABSI (per 0.01 units) |
1.541 (0.960,2.473) | 0.074 |
C_index ( per 0.01 units) |
1.100 (1.068,1.134) | <0.001 | 1.362 (0.879,2.110) | 0.167 | 1.055 (1.015,1.097) | 0.007 |
| ABSI quartiles | C_index quartiles | ||||||||
| Q1 | ref | Q1 | ref | ref | ref | ||||
| Q2 | 2.188(0.529,9.049) | 0.280 | Q2 | 4.976(1.284,19.280) | 0.020 | 2.278(0.438,11.843) | 0.327 | 2.474(0.503,12.170) | 0.265 |
| Q3 | 2.409(0.648,8.951) | 0.189 | Q3 | 6.828(1.953,23.867) | 0.002 | 2.398(0.580,9.908) | 0.227 | 2.464(0.516,11.764) | 0.258 |
| Q4 | 3.184(0.737,13.743) | 0.121 | Q4 | 11.571(3.767,35.541) | <0.001 | 3.090(0.754,12.663) | 0.117 | 4.246(0.878,20.529) | 0.072 |
| P for trend | 0.160 | P for trend | <0.001 | 0.102 | 0.037 | ||||
Results: HR (95% CI) P -value
Outcome: All-cause mortality, CVD mortality, and Cancer mortality
Exposure: ABSI(per 0.01 units); Stratified by ABSI quartiles; WWI; WWI quartiles; C_index(per 0.01 units); C_index quartiles; ABSI Q1–Q4, quartile 1–4; Q1(6.677–7.808), Q2(7.808–8.160), Q3(8.160–8.504), Q4(8.504–9.895);
WWI Q1–Q4, quartile 1–4; Q1(8.551–10.432), Q2(10.432–11.052), Q3(11.052–11.622), Q4(11.622–14.381); C-index Q1–Q4, quartile 1–4; Q1(103.597–124.418), Q2(124.418–130.861), Q3(136.861–137.215), Q4(137.215–159.212); p for trend
Model 1: None
Model 2: adjusted for age, sex, ethnicity, education level, and marital status
Model 3: adjusted for age, sex, ethnicity, education level, marital status; PIR, smoking status, alcohol consumption, DM, hyperlipidemia, hypertension, CKD, COPD, stroke, antihypertensive drug, antihyperlipidemic drug, lymphocyte percentage, neutrophil percentage, HbA1c, and eGFR
Abbreviations : CI confidence interval, CVD cardiovascular disease, HR hazard ratioCox proportional hazard models were used to estimate HRs and 95% CIs
In Model 1, ABSI × 100 exhibited a notable positive correlation with overall mortality (HR: 6.726, 95% CI: 4.788–9.447). Following adjustments for potential confounding variables, the relationship remained significant in Models 2 (HR: 2.060; 95% CI: 1.439–2.948) and 3 (HR: 2.074; 95% CI: 1.413–3.044). A similar trend was observed for the WWI and C-index × 100. Moreover, a significant relationship was found between ABSI × 100 and mortality rates from CVD and cancer in Model 1 and the revised models (Models 2 and 3). However, WWI showed no significant association for cancer mortality in Models 2 and 3, while the C-index × 100 was not significantly associated in Model 2.
When ABSI × 100, WWI, and C-index × 100 were divided into quartiles, analyses from Model 1 and the adjusted models predominantly demonstrated a linear correlation between these quartiles and overall deaths (P trend < 0.05). Among the adjusted models (Model 2 and Model 3), participants in Q3 (HR: 5.847, 95% CI: 2.576–13.272) and Q4 (HR: 15.998, 95% CI: 8.164–31.348) demonstrated a substantially increased risk of overall death relative to those in Q1, whereas no meaningful relationship was found for Q2 (HR: 2.800, 95% CI: 1.188–6.603). Similarly, in Model 1 and the adjusted models, the quartiles of ABSI × 100 were significantly associated with CVD but only partially associated with cancer mortality. In Model 1, WWI and C-index × 100 quartiles were significantly linked to CVD and cancer mortality; however, these associations were only partially significant in Models 2 and 3.
In contrast, ABSI × 100, evaluated as a continuous variable, demonstrated a significant relationship with overall and specific deaths in Model 1 and the adjusted model (P trend < 0.05). However, continuous WWI and a C-index × 100 demonstrated only partially significant associations.
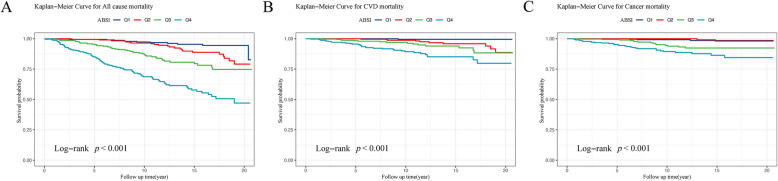
Among the 1,266 individuals, 299 deaths occurred during follow-up (190 all-cause, 59 cardiovascular, 50 cancer). Analysis using the Kaplan–Meier curve method indicated that across all three models, the survival rates for total mortality, CVD, and cancer-related deaths in Q4 of ABSI were markedly lower than those in Q1 (P < 0.001). (Fig. 5A, B, C). Similarly, the mortality rates for WWI and the C-index were notably lower in Q4 compared to Q1 for all-cause, CVD, and cancer-related deaths (P < 0.001). (Fig. S1 A, B; Fig. S2), except for WWI in Model 2, where the survival rate difference was not statistically significant (Fig. S1 C).
Fig. 5.
The survival curves using the K-M method for all-cause, CVD, and cancer mortality categorized by the ABSI groups (A, B, C) are shown
This result reveals a pronounced difference in the survival status in the Q4 and Q1, emphasizing the degree to which various factors influence different populations. Whether assessed as continuous variables or by quartiles, the ABSI, WWI, and C-index were consistently and independently correlated with a heightened risk of overall and specific deaths among individuals with RA. Even after accounting for multiple confounders, these associations remained significant, highlighting the potential significance of ABSI, WWI, and the C-index as prognostic indicators in RA.
Analysis of Nonlinear Trends and Threshold Effects of Alternative adiposity indices in Relation to Mortality Among Patients with RA
We explored the complex relationship between mortality rate, ABSI × 100, WWI, and C-index × 100. RCS demonstrated a nonlinear association between unadjusted ABSI and overall-specific deaths among subjects with RA (Fig. 6A, D, G, P < 0.001, nonlinear P < 0.05). In the adjusted models, the RCS analysis showed an upward trend in mortality with increasing ABSI × 100, along with evidence of nonlinearity. However, in Models 2 and 3, although all-cause mortality continued to increase with ABSI × 100 (Fig. 6B, C), no statistical evidence supported a nonlinear relationship.
Fig. 6.
Curves of restricted cubic splines for the associations between ABSI multiplied by 100 and mortality from all causes (A, B, C), CVD (D, E, F), and cancer (G, H, I)
Additionally, we analyzed the WWI and C-index. RCS analysis for both indices showed an upward trend in mortality in all models. However, no statistical evidence of nonlinearity was observed (Fig. S3, Fig. S4).
To investigate these connections more deeply, we applied a segmented Cox proportional hazards model, which indicated statistically significant findings in the likelihood ratio tests (Table S7). Below the inflection point, ABSI × 100 was strongly and positively linked to overall mortality in Model 1, with each unit increase in ABSI × 100 correlating with a markedly higher risk (HR: 9.104; 95% CI: 6.158–13.459). The significance of this association persisted even after adapted for potential confounding variables in Models 2 and 3. Conversely, between Model 1 and the modified models (Models 2 and 3), there was no notable relationship between ABSI × 100 and death due to CVD or cancer. Beyond the critical threshold, no meaningful relationship was found between ABSI × 100 and overall mortality in either Model 1 or the adjusted models. However, in Model 1, a one-unit increase in ABSI × 100 was significantly attached to a greater likelihood of CVD deaths (HR: 3.849, 95% CI: 2.170–6.825), although this association lost significance after adjusting for confounders in Models 2 and 3. Similarly, in Model 1, an increase in ABSI × 100 was significantly and positively linked to cancer mortality, with each unit increase corresponding to higher risk (HR, 7.900; 95% CI, 4.586–13.610). This relationship was maintained after factored in potentially confounding elements in Models 2 and 3.
Subgroup analysis of mortality (all-cause, CVD, and cancer) in patients with RA
An additional subgroup analysis categorized by age, sex, educational attainment, marital status, ethnicity, tobacco use, alcohol intake, PIR, hyperlipidemia, hypertension, CKD, use of antihypertensive medications, DM, and antihyperlipidemic medications was performed for participants with RA. When considering the overall mortality, significant interactions were detected for age, sex, and DM (P = 0.024, 0.002, and 0.021) (Fig. 7A). Regarding cardiovascular mortality, CKD (P for interaction = 0.041) was identified as having a significant interaction, whereas no significant interactions were detected for other factors (Fig. 7B). For cancer mortality, sex (P for interaction = 0.027) was identified as having a significant interaction, whereas no significant interactions were detected for other factors (Fig. 7C).
Fig. 7.

Subgroup analysis of mortality outcomes (all-cause (A), CVD (B), and cancer (C)) in RA patients based on ABSI
These outcomes demonstrate that the relationship between ABSI × 100 and a higher probability of mortality persists within the subgroups of potential confounding factors.
Analysis of inflammatory markers as mediators for overall mortality in individuals with RA
The mediation analysis results, as depicted in Fig. 8, indicate that ABSI remains consistently attached to a greater likelihood of overall mortality in participants with RA despite controlling for covariates. Additionally, the outcomes revealed that the partial relationship between ABSI and overall survival in individuals with RA was influenced by MHR, NLR, ALI, and SII. The mediation percentages were 4.9% (P = 0.008), 5.1% (P = 0.002), 8.5% (P = 0.016), and 4.5% (P = 0.024), in that order. However, no mediation was detected for NPAR, PLR, SIRI, CLR, or LHR (Table S8). Mediation analyses were conducted for CVD and cancer survival; however, no significant mediation effects were identified.
Fig. 8.
Mediation Analysis of Inflammatory Markers on All-Cause Mortality in Patients with RA
Sensitivity analyses and XGBoost
The sensitivity analysis used a weighted multivariable Cox regression, excluding individuals who died during the initial 24 months of follow-up (n = 27) (Table S9). The all-cause mortality analysis demonstrated dose-dependent associations with ABSI increments. A 100-unit elevation in the ABSI conferred escalating risks across progressive adjustment stages: crude adjustment (HR = 5.18, 95% CI 2.93–9.17), partial covariate control (HR = 1.80, 1.24–2.61), and full adjustment (HR = 1.74, 1.13–2.66), all attaining P < 0.01 significance. Quartile stratification revealed a pronounced risk amplification in the highest quartile (Q4), particularly in the fully adjusted specification (HR = 3.43, 1.45–8.14; P = 0.005), supported by significant linear progression (P < 0.05).
CVD mortality patterns mirrored this relationship, with 100-unit ABSI increases showing unadjusted HR = 7.69 (4.38–13.48), intermediate adjustment HR = 2.28 (1.24–4.21), and comprehensive adjustment HR = 2.43 (1.13–5.24), each surpassing P < 0.05 thresholds. The uppermost quartile exhibited an extraordinary risk elevation in the final model (HR = 55.85, 2.85–1092.79; P = 0.008), with progressive trend confirmation (P < 0.05). Notably, cancer mortality demonstrated the strongest ABSI association. Incremental 100-unit exposure produced HRs of 7.61 (4.39–13.19) in baseline specifications, progressing to 4.02 (2.01–8.04) in the maximally adjusted model (all P < 0.001). The fourth quartile demonstrated a remarkable risk magnitude in refined analyses (HR = 6.79, 1.86–24.77; P = 0.003), complemented by highly significant trend validation (P < 0.001). Additionally, after excluding participants with baseline CVD (n = 146) and cancer deaths (n = 110), we conducted additional sensitivity analyses on the ABSI and all-cause mortality and found that ABSI remained positively correlated with all-cause mortality (Table S10, Table S11).
Ultimately, the study’s findings were reinforced using the XGBoost algorithm to assess the importance of various factors in predicting the overall and specific mortality rates of RA, including those linked to CVD and cancer (Fig. 9). The results of the XGBoost model revealed that the 10 most important factors for all-cause mortality were age, ABSI, eGFR, PIR, weight, LAP, height, VAI, BMI, and antihypertensive drug use. The top 10 risk factors for CVD mortality were Age, CKD, PIR, ABSI, WHtR, RFM, LAP, smoking status, WWI, and weight. The top 10 factors associated with cancer mortality were ABSI, age, eGFR, height, LAP, PIR, VAI, weight, BMI, and ethnicity. ABSI is considered the best obesity indicator for predicting the prognosis of patients with RA, having greater relative significance than other new obesity indices, such as WWI and RFM. These findings are consistent with the outcomes of the multivariate regression analysis, highlighting ABSI as a significant predictive biomarker.
Fig. 9.
XGBoost modeling was used to evaluate the relative importance of variables for RA mortality
Discussion
In our study, we rigorously scrutinized the connection between several alternative adiposity indices (ABSI, BRI, WWI, C-index, WHTR, VAI, LAP, and RFM) and overall and specific mortality (CVD and cancer) among patients with RA, employing a substantial, population-centered sample for the first time. The findings from this research indicated that ABSI exhibited considerable predictive capability and surpassed conventional metrics (BMI) in assessing the likelihood of overall, cardiovascular, and cancer-related mortality among individuals with RA. In addition, the WWI and C-index were also associated with mortality, but their predictive ability was relatively weak and inconsistent. Kaplan–Meier curve assessment further validated the findings, revealing that patients in the top quartile of ABSI (Q4) exhibited significantly lower survival rates than those in the bottom quartile Q1 for overall-specific mortality. These findings suggest that ABSI may be a vital predictive indicator of mortality risk among individuals with RA.
The results of this research align with the existing literature and contribute to the advancement of research in assessing the mortality risks in patients with RA. Numerous studies have highlighted the limitations of traditional metrics, such as BMI, in accurately reflecting health risks in this population. England et al. demonstrated that BMI often underestimates risks related to fat distribution and metabolic dysfunction in patients with RA, potentially misclassifying their health status [28]. Similarly, Dubovyk et al. found that BMI failed to capture central adiposity and metabolic irregularities, which are critical determinants of RA mortality [29]. Considering these limitations, alternative anthropometric metrics have been developed to offer a more nuanced understanding of health risks. ABSI has been widely recognized for its ability to predict conditions such as DM and hypertension [30]. This study builds on previous research by confirming that ABSI is a major parameter of mortality risk, specifically in patients with RA, a group for which such data have been limited. ABSI is vital for enhancing clinical risk assessments by accurately reflecting central obesity and its related hazards.
Moreover, compared to previous studies, this research is the first to systematically compare the associations of various alternative metrics, such as the BRI, WWI, and C-index, with mortality in patients with RA. While previous research has explored these metrics in other populations, their relevance to RA remains unexplored [20, 31, 32]. This study validates these metrics by addressing this gap and offers comprehensive insights into their comparative predictive performance. This study contributes significantly to the field by providing practical recommendations for incorporating these indices into standard clinical protocols, enhancing the categorization and treatment approaches for patients with RA.
Patients with RA often experience a persistent inflammatory state, marked by prolonged increases in pro-inflammatory mediators. This ongoing inflammation can result in irregular fat distribution and reduced muscle mass due to various mechanisms [33]. This phenomenon is termed rheumatoid cachexia [34]. Chronic inflammation promotes the breakdown of adipose tissue while inhibiting muscle protein synthesis, resulting in muscle atrophy and fat redistribution, particularly visceral fat [35]. These changes in body composition cannot be adequately captured by traditional metrics, such as BMI, which is merely a ratio of weight to height and does not separate adipose tissue and muscle fabric nor indicate the distribution of body fat [36].
In contrast, alternative adiposity indices, such as the ABSI, which incorporate parameters including WC, height, and weight, are better suited to capture fat distribution and body shape characteristics, particularly visceral fat accumulation [19]. This enables a more precise evaluation of mortality risk. The build-up of abdominal fat is strongly involved with metabolic syndrome disorders and intensifies chronic inflammation by releasing inflammatory mediators, such as TNF-α, thereby perpetuating a harmful cycle [37]. Additionally, WWI indices can reflect patterns of body fat distribution and demonstrate high sensitivity and specificity in predicting cardiovascular disease and cancer risk [9].
Furthermore, the mediation analysis in this study demonstrated that inflammatory indicators, such as NPAR, NLR, and PLR, may serve as key factors in the association between ABSI and mortality. These inflammatory markers not only directly reflect the chronic inflammatory state in patients with RA but may also indirectly increase the mortality risk by influencing fat distribution and metabolic functions. For instance, NPAR, an emerging composite marker of inflammation and nutritional status, may reflect patients’ overall inflammatory burden and malnutrition [25]. Similarly, NLR and PLR are closely associated with inflammatory responses and immune functions [38]. Elevated levels of these markers suggest a central role for inflammatory processes in the mortality risk among individuals with RA and further support the utility of alternative indices, such as ABSI, in capturing inflammation-related risks.
Strengths and Limitations
In clinical practice, there are limitations to the measurement methods used for different populations. Currently, most studies use BMI, an obesity index that does not account for regional differences in fat distribution [39]. Alternative measures have been proposed to account for central abdominal adiposity more effectively, including ABSI, WWI, and the C-index [16, 40]. In this analysis, ABSI demonstrated superior prognostic efficacy compared to WWI, and the C-index highlights its potential value in clinical practice. As demonstrated by the RCS analysis, the upward trend in the mortality rate associated with ABSI further corroborates the need to include this measurement in risk-stratification frameworks.
Although the results are promising, this research had certain constraints. First, although comprehensive, the NHANES database is inherently cross-sectional and may not account for temporal changes in the ABSI or its association with mortality. Moreover, reliance on self-reported information for certain covariates, including medical history and medication usage, may introduce reporting bias, affecting the precision of our effects estimates. In addition, the research sample mainly comprised individuals from specific ethnic backgrounds, thus limiting our findings’ applicability to a wider variety of populations. In conclusion, although the study considered several confounding factors, unidentified or excluded residual confounding variables may have influenced the observed relationships.
Conclusion
This study has revealed new obesity indices, specifically the ABSI, which may have clinical value in predicting the survival rates among individuals with RA compared to traditional obesity indices such as BMI and WC. Notably, maintaining a relatively low level of ABSI may help reduce the mortality of RA. Furthermore, inflammatory markers appear to play a partial mediating role in this relationship.
Supplementary Information
Acknowledgements
The researchers extend their appreciation to the contributors of the NHANES database and all personnel engaged in the research.
Authors’ contributions
The study was designed by FL and JJG. Data extraction and analysis were conducted by FL, JJG, XMY, and HZ. The initial draft of the manuscript was prepared by FL. QYW, XMY, CMC, and WKM provided revisions to the manuscript. The authors collectively approved the final version.
Funding
The research was funded by the National Natural Science Foundation of China: 82160869, 82374494, 82274678, 8247155344; Guizhou Provincial Basic Research Program (Natural Science): Qian Ke He Ji Chu-ZK [2023] No. 412; Guizhou University of Traditional Chinese Medicine - Academic Seedling Project: Gui Ke He Xue Shu Xin Miao [2023] No. 36; National and Provincial Science and Technology Innovation Talent Team Training Program of Guizhou University of Traditional Chinese Medicine: Gui Zhong Yi TD He Zi [2022] No. 004; Youth Science and Technology Talent Development Project of Guizhou Provincial Department of Education (Qianjiaoji [2024] No. 122); "Sanhang" Talent Training Program of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine: SHRC-KY2024005.
Data availability
This research examined publicly available datasets. The utilized data is accessible through NHANES at (https://wwwn.cdc.gov/Nchs/Nhanes/).
Declarations
Ethics approval and consent to participate
This research employed summary data that are publicly available, eliminating the need for ethics approval.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xue-ming Yao, Email: yxming19@foxmail.com.
Wu-kai Ma, Email: walker55@163.com.
References
- 1.Ahlers MJ, Lowery BD, Farber-Eger E, Wang TJ, Bradham W, Ormseth MJ, et al. Heart failure risk associated with rheumatoid arthritis-related chronic inflammation. J Am Heart Assoc. 2020;9(10): e014661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet (London, England). 2023;402(10416):2019–33. [DOI] [PubMed] [Google Scholar]
- 3.Curtis JR, Yamaoka K, Chen YH, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL surveillance trial. Ann Rheum Dis. 2023;82(3):331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles-Schoeman C, Buch MH, Dougados M, Bhatt DL, Giles JT, Ytterberg SR, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: A post hoc analysis from ORAL surveillance. Ann Rheum Dis. 2023;82(1):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougados M, Charles-Schoeman C, Szekanecz Z, Giles JT, Ytterberg SR, Bhatt DL, et al. Impact of cardiovascular risk enrichment on incidence of major adverse cardiovascular events in the tofacitinib rheumatoid arthritis clinical programme. Ann Rheum Dis. 2023;82(4):575–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin G, Zhan F, Ren W, Pan Y, Wei W. Association between novel anthropometric indices and prevalence of kidney stones in US adults. World J Urol. 2023;41(11):3105–11. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Ma N, Lin Q, Chen K, Zheng F, Wu J, et al. Body roundness index and all-cause mortality among US adults. JAMA Netw Open. 2024;7(6): e2415051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeClercq V, Cui Y, Forbes C, Grandy SA, Keats M, Parker L, et al. Adiposity measures and plasma adipokines in females with rheumatoid and osteoarthritis. Mediators Inflamm. 2017;2017:4302412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang TY, Zhang ZM, Wang XN, Kuang HY, Xu Q, Li HX, et al. Relationship between weight-adjusted-waist index and all-cause and cardiovascular mortality in individuals with type 2 diabetes. Diabetes Obes Metab. 2024;26(12):5621–9. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Cao L, Liu Y, Huang W, Pei C, Wang X, et al. Sex- and age-specific differences in associations of a body shape index with all-cause and cardiovascular death risks among US adults with diabetes. Nutr Metab Cardiovasc Dis. 2023;33(3):551–9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Hou Y, Ren X, Cai Y, Wang J, Chen O. Association of a body shape index with femur bone mineral density among older adults: NHANES 2007–2018. Arch Osteoporos. 2024;19(1):63. [DOI] [PubMed] [Google Scholar]
- 12.Ding Z, Zhuang Z, Tang R, Qu X, Huang Z, Sun M, et al. Negative association between body roundness index and bone mineral density: Insights from NHANES. Front Nutr. 2024;11:1448938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasko MC, Kay J, Hsia EC, Rahman MU. Diabetes mellitus and insulin resistance in patients with rheumatoid arthritis: Risk reduction in a chronic inflammatory disease. Arthritis Care Res. 2011;63(4):512–21. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann Rheum Dis. 2014;73(11):1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DC, Brellenthin AG, Lanningham-Foster LM, Kohut ML, Li Y. Aerobic, resistance, or combined exercise training and cardiovascular risk profile in overweight or obese adults: The CardioRACE trial. Eur Heart J. 2024;45(13):1127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X, Min Y, Song G, Ye X, Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. 2024;23(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou E, Wu J, Zhou X, Yin Y. The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: results from NHANES 1999–2020. Front Immunol. 2023;14:1309835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku HC, Cheng E, Cheng CF. A body shape index (ABSI) but not body mass index (BMI) is associated with prostate cancer-specific mortality: Evidence from the US NHANES database. Prostate. 2024;84(9):797–806. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Chen Z, Wu Y, Ren J, Shen D, Hu G, et al. Association between two novel anthropometric measures and type 2 diabetes in a Chinese population. Diabetes Obes Metab. 2024;26(8):3238–47. [DOI] [PubMed] [Google Scholar]
- 21.Zhou T, Chen S, Mao J, Zhu P, Yu X, Lin R. Association between obstructive sleep apnea and visceral adiposity index and lipid accumulation product: NHANES 2015–2018. Lipids Health Dis. 2024;23(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Cao R, Zhang S. Association between relative fat mass and periodontitis: Results from NHANES 2009–2014. Sci Rep. 2024;14(1):18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu B, Li M, Yu Z, Zheng T, Feng X, Gao A, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999–2018. BMC Med. 2024;22(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Hu J, Li J, Li Q, Lan L. Association between triglyceride-glucose index and femoral bone mineral density in community-dwelling, nondiabetic men and women: A NHANES analysis of 1,928 US individuals. Menopause (New York, NY). 2024;31(7):626–33. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Wu CH, Lee CH, Cheng CI. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: Evidence from US NHANES 2005–2016. BMC Cardiovasc Disord. 2023;23(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324(12):1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med Res Methodol. 2017;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.England BR, Baker JF, Sayles H, Michaud K, Caplan L, Davis LA, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res. 2018;70(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubovyk V, Vasileiadis GK, Fatima T, Zhang Y, Kapetanovic MC, Kastbom A, et al. Obesity is a risk factor for poor response to treatment in early rheumatoid arthritis: A NORD-STAR study. RMD Open. 2024;10(2): e004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X, Luo S, Sun J, Jin F, Chen Z, Song J. Association between “a body shape index” (ABSI) with periodontitis in a hypertension population from the NHANES 2009–2014. Sci Rep. 2024;14(1):23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Liang J, Luo H, Zhang H, Xiang J, Guo L, et al. The association between body roundness index and osteoporosis in American adults: Analysis from NHANES dataset. Front Nutr. 2024;11:1461540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Huang P, Wang H, Hu Z, Zheng S, Yang J, et al. Relationship between weight-adjusted waist index (WWI) and osteoarthritis: A cross-sectional study using NHANES data. Sci Rep. 2024;14(1):28554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravallese EM, Firestein GS. Rheumatoid arthritis - common origins, divergent mechanisms. N Engl J Med. 2023;388(6):529–42. [DOI] [PubMed] [Google Scholar]
- 34.Moradi K, Mohajer B, Guermazi A, Kwoh CK, Bingham CO, Mohammadi S, et al. Cachexia in preclinical rheumatoid arthritis: Longitudinal observational study of thigh magnetic resonance imaging from osteoarthritis initiative cohort. J Cachexia Sarcopenia Muscle. 2024;15(5):1823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee TM, Choi J, Lee H, Merino J, Park JB, Kwak SH. Discrepancy between genetically predicted and observed BMI predicts incident type 2 diabetes. Diabetes Care. 2024;47(10):1826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Moorthy MV, Cook NR, Rifai N, Lee IM, Buring JE. Inflammation, cholesterol, lipoprotein(a), and 30-year cardiovascular outcomes in women. N Engl J Med. 2024;391(22):2087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Yan Z, Li K, Liu L. The association between systemic immune-inflammation index and chronic obstructive pulmonary disease in adults aged 40 years and above in the united states: A cross-sectional study based on the NHANES 2013–2020. Front Med. 2023;10:1270368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianchettin RG, Lavie CJ, Lopez-Jimenez F. Challenges in cardiovascular evaluation and management of obese patients: JACC state-of-the-art review. J Am Coll Cardiol. 2023;81(5):490–504. [DOI] [PubMed] [Google Scholar]
- 40.Wei D, González-Marrachelli V, Melgarejo JD, Liao CT, Hu A, Janssens S, et al. Cardiovascular risk of metabolically healthy obesity in two european populations: Prevention potential from a metabolomic study. Cardiovasc Diabetol. 2023;22(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research examined publicly available datasets. The utilized data is accessible through NHANES at (https://wwwn.cdc.gov/Nchs/Nhanes/).