Abstract
The present study aims to investigate the relationship between the neutrophil-percentage-to-albumin ratio (NPAR) and asthma using least absolute shrinkage and selection operator (LASSO) regression and Boruta algorithm. Based on the National Health and Nutrition Examination Survey database from 2001 to 2018, a total of 31,138 eligible participants were included in this study. The participants were randomly divided into a training cohort and a validation cohort in a 7:3 ratio. LASSO regression and Boruta algorithm were applied to the training cohort for assessment, selection of the optimal model, and identification of potential confounding factors. A nomogram prediction model, receiver operating characteristic curve, calibration curve, and decision curve analysis were constructed to evaluate the model’s ability to predict the risk of asthma and its stability. These analyses aim to provide a reference for clinical diagnosis and treatment. The study demonstrated that after adjusting for potential confounding factors, the NPAR was positively correlated with asthma incidence (P < 0.01). The area under the curve for the training set was 0.66 for LASSO regression and 0.64 for Boruta algorithm, indicating that LASSO regression exhibited superior performance. Through LASSO regression, 10 variables were selected, including gender, race, smoking status, hypertension, diabetes, cancer, poverty-income ratio, BMI, cardiovascular disease, and age. A nomogram prediction model was constructed based on these predictors. The calibration curve showed good fit between the two groups. A higher NPAR is significantly positively correlated with an increased risk of asthma.
Keywords: LASSO regression, Boruta algorithm, NPAR, Asthma, NHANES
Introduction
Asthma is a chronic inflammatory airway disease characterized by airway hyper responsiveness and reversible airflow obstruction. It is estimated that the number of asthma patients worldwide reached 262 million in 2019, imposing a substantial economic and health burden on patients, families, and society [1]. Asthma is generally classified into two types: extrinsic asthma and intrinsic asthma. Extrinsic asthma, also known as allergic asthma, is the main type of asthma, caused by allergens and primarily characterized by inflammation driven by abnormal T helper type 2 (Th2) responses, intrinsic asthma is triggered by various factors, such as the use of aspirin, pulmonary infections, exercise, colds, stress, obesity, genetics, and pathogen infections [2, 3]. Based on the state of Th2 inflammation, asthma is divided into two groups: Th2-high and Th2-low asthma. Th2-high asthma is characterized by eosinophilic airway inflammation, which is associated with increased blood eosinophil counts or elevated fractional exhaled nitric oxide (FENO), while Th2-low asthma includes neutrophilic asthma and paucigranulocytic asthma [4].
IL-17 is a pro-inflammatory cytokine produced by a subset of T helper cells. Studies have shown that IL-17 can activate neutrophils in the airways, induce their migration, and thereby maintain immune defense and inflammatory responses in the airways [5]. IL-17 plays a crucial role in regulating neutrophilic inflammation, airway hyper responsiveness, and pulmonary remodeling [6, 7]. Research has also demonstrated that neutrophils, as core effector cells in inflammatory responses, are significantly associated with asthma severity when their percentage is elevated [8, 9].
Serum albumin is the most abundant protein in human plasma, serving not only as an important nutritional indicator but also possessing anti-inflammatory and antioxidant properties. Studies have shown that decreased serum albumin levels, or hypoalbuminemia, are associated with an increased risk of asthma and mortality [10–12]. The NPAR, which reflects both neutrophil and nutritional inflammatory status, may have a potential association with asthma risk. Therefore, this study analyzed data from the NHANES, using LASSO regression and Boruta algorithm to investigate risk factors for asthma.
Methods
Data sources
The NHANES is a national survey program conducted by the National Center for Health Statistics (NCHS) to assess the health and nutritional status of adults and children in the United States through interviews, physical examinations, and laboratory tests. The NHANES study protocol adheres to the STROBE guidelines for observational research and has been approved by the NCHS Research Ethics Review Board. All participants have provided informed consent. Detailed NHANES data can be accessed at “https://www.cdc.gov/nchs/nhanes/”.
Definition of NPAR and asthma
NPAR Calculation: The NPAR is calculated as the percentage of neutrophils (%) divided by serum albumin (g/dL). Asthma Diagnosis: Asthma diagnosis in this study was based on two questions: (1) “Has a doctor or other health professional ever told you that you have asthma?” (2) “Do you still have asthma?” Participants who answered “yes” to either question were classified as having asthma.
Study variables
The data collected from the NHANES included demographic information (age, gender, race, education level, PIR, and marital status), physical examination data (BMI), and questionnaire survey data (smoking habits, alcohol consumption frequency, hypertension, diabetes, asthma, cancer, and CVD).
Statistical analysis
After using the na.omit function to remove missing values, the data were analyzed. LASSO regression and Boruta algorithm are both types of machine learning methods. LASSO regression is an improved method of linear regression, primarily used for feature selection and model regularization. For LASSO regression, linear scaling normalization was applied to the variables, with a maximum of 1,000 iterations. For positive indicators, the maximum value of the indicator Xmax was taken, and each observed value of the variable was divided by the maximum value, i.e., X′ = X/max (where X ≥ 0). For negative indicators, the minimum value of the indicator Xmin was taken, and the minimum value was divided by each observed value of the variable, i.e., X′ = Xmin/X (where X > 0). In comparison, the Boruta algorithm determines the relevance of features by comparing their importance with that of randomly permuted (noise) features. The Boruta algorithm employs the feature selection method of random forests, setting the number of trees in the random forest to 1,000 in order to enhance the stability and accuracy of the model.
The data in this study were analyzed using R version 4.4.2. Categorical variables were compared using the chi-square test, while continuous variables were compared using the t test. Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as percentages (%). The data were randomly divided into a training cohort and a validation cohort in a 7:3 ratio. In the training cohort, LASSO regression and Boruta algorithm were applied to analyze risk factors for asthma. The method with the higher AUC was selected to construct a nomogram prediction model. ROC curves, calibration curves, and DCA curves were plotted to validate the prediction model. A P value < 0.05 was considered statistically significant.
Results
Characteristics of participants
After excluding participants with missing data, a total of 31,138 participants from the NHANES 2001–2018 were included in this study. Compared with non-asthma patients, individuals who were female, younger, of other Hispanic, non-Hispanic White, non-Hispanic Black, or other races, with partial college education, divorced, separated, never married, or cohabitating, with low PIR, high BMI, current smokers, with diabetes, CVD, cancer, and high NPAR had an increased risk of asthma (P < 0.05) (Table 1).
Table 1.
Demographic Baseline Chart of NHANES Participants from 2001 to 2018
| Characteristics | Overall (N = 31,138) | Asthma (N = 4,325) | Non-asthma (N = 26,813) | P value |
|---|---|---|---|---|
| Gender | < 0.001 | |||
| Male | 16,365 (51%) | 1,904 (42%) | 14,461 (53%) | |
| Female | 14,773 (49%) | 2,421 (58%) | 12,352 (47%) | |
| Age | 46.56 ± (16.41) | 44.45 ± (16.34) | 46.92 ± (16.39) | < 0.001 |
| Race | < 0.001 | |||
| Mexican American | 4,987 (7.7%) | 397 (4.5%) | 4,590 (8.3%) | |
| Other Hispanic | 2,417 (4.8%) | 384 (5.5%) | 2,033 (4.7%) | |
| Non-Hispanic White | 15,193 (72%) | 2,219 (73%) | 12,974 (72%) | |
| Non-Hispanic Black | 6,055 (9.6%) | 976 (11%) | 5,079 (9.3%) | |
| Other Race—Including Multi-Racial | 2,486 (5.8%) | 349 (6.2%) | 2,137 (5.8%) | |
| Education Level | < 0.001 | |||
| Less than 9th grade | 2,771 (4.2%) | 276 (3.2%) | 2,495 (4.4%) | |
| 9-11th grade | 4,317 (10%) | 582 (9.7%) | 3,735 (10%) | |
| High school graduate/GED or equivalent | 7,249 (24%) | 943 (22%) | 6,306 (24%) | |
| Some college or AA degree | 9,461 (32%) | 1,526 (36%) | 7,935 (31%) | |
| College graduate or above | 7,340 (30%) | 998 (29%) | 6,342 (30%) | |
| Marital status | < 0.001 | |||
| Married | 16,593 (57%) | 2,030 (52%) | 14,563 (58%) | |
| Spouse Deceased | 2,173 (4.9%) | 285 (4.7%) | 1,888 (5.0%) | |
| Divorced | 3,435 (11%) | 567 (12%) | 2,868 (10%) | |
| Separated | 996 (2.3%) | 161 (2.8%) | 835 (2.3%) | |
| Never married | 5,373 (17%) | 900 (20%) | 4,473 (17%) | |
| Living with partner | 2,568 (8.2%) | 382 (8.4%) | 2,186 (8.2%) | |
| Ratio of family | 3.12 ± (1.62) | 2.94 ± (1.68) | 3.15 ± (1.61) | < 0.001 |
| BMI(kg/m2) | 28.82 ± (6.72) | 29.99 ± (7.79) | 28.63 ± (6.50) | < 0.001 |
| Smoke | 0.023 | |||
| Never | 15,222 (50%) | 2,002 (48%) | 13,220 (50%) | |
| Former | 8,630 (27%) | 1,192 (27%) | 7,438 (27%) | |
| Current smoker | 7,286 (23%) | 1,131 (25%) | 6,155 (23%) | |
| Alcohol Frequency | 4.47 ± (18.55) | 4.16 ± (15.10) | 4.52 ± (19.07) | 0.782 |
| Diabetes | < 0.001 | |||
| Yes | 9,717 (27%) | 1,571 (31%) | 8,146 (27%) | |
| No | 21,421 (73%) | 2,754 (69%) | 18,667 (73%) | |
| Borden | 3,807 (9.0%) | 650 (11%) | 3,157 (8.7%) | |
| CVD | < 0.001 | |||
| Yes | 2,898 (7.3%) | 559 (11%) | 2,339 (6.7%) | |
| No | 28,240 (93%) | 3,766 (89%) | 24,474 (93%) | |
| Cancer | 0.005 | |||
| Yes | 2,939 (9.7%) | 461 (11%) | 2,478 (9.4%) | |
| No | 28,199 (90%) | 3,864 (89%) | 24,335 (91%) | |
| NPAR | 1,383.10 ± (257.54) | 1,400.11 ± (271.13) | 1,380.26 ± (255.10) | 0.005 |
LASSO regression analysis and Boruta algorithm
Through LASSO regression analysis, 10 variables were selected, including gender, race, smoking, hypertension, diabetes, cancer, PIR, BMI, CVD, and age. The LASSO coefficient curve (Fig. 1) illustrates the changes in coefficients of each predictor variable as lambda varies. The x-axis represents the logarithmic transformation of lambda, while the y-axis represents the coefficients. Each line corresponds to a variable, and as lambda increases, the coefficients gradually shrink to zero. The cross-validation error plot (Fig. 2) displays the cross-validated mean-square error (MSE) for different values of lambda. The x-axis indicates the logarithmic transformation of lambda, and the y-axis shows the cross-validated MSE, with error bars representing the standard error of the MSE. The vertical line indicates the lambda value that minimizes the MSE, representing the optimal balance between model complexity and predictive accuracy.
Fig. 1.
LASSO regression coefficient plot
Fig. 2.
Cross-validation error plot
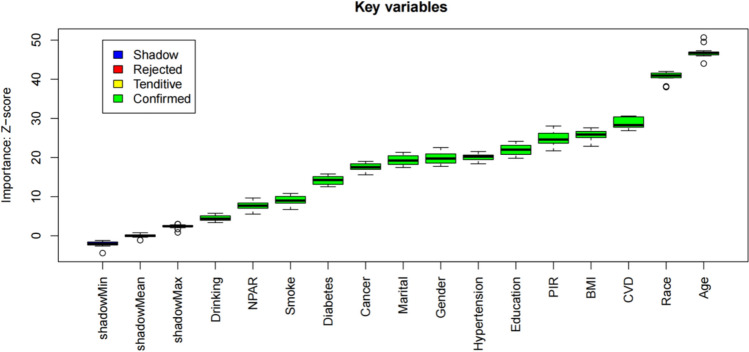
Through analysis using the Boruta algorithm, 14 variables were identified: gender, race, smoking, hypertension, diabetes, cancer, PIR, BMI, CVD, age, NPAR, alcohol consumption, education, and marital status. The feature selection results of the Boruta algorithm are shown in Fig. 3, where variables in the green area are marked as important features. The score variation plot of the Boruta algorithm is presented in Fig. 4, with the x-axis representing the number of iterations and the y-axis representing the feature importance score (Z-score). Green indicates features that have been confirmed as important, while blue represents shadow features, which are random features used for comparison.
Fig. 3.
Feature selection results of the Boruta algorithm
Fig. 4.
Score variation plot of the Boruta algorithm
As shown in Table 2, a comparison of LASSO regression and Boruta algorithm is presented.
Table 2.
Comparison of LASSO Regression and Boruta Algorithm
| Characteristics | LASSO Selection | Boruta Selection | LASSO Coef | LASSO Wald Z | LASSO P-value | Boruta Z-Score |
|---|---|---|---|---|---|---|
| Gender | Confirmed | 19.8 | ||||
| Male | Yes | 0.33 | 8.62 | < 0.0001 | ||
| Age | Yes | Confirmed | -0.02 | -12.47 | < 0.0001 | 46.9 |
| Race | Confirmed | 40.7 | ||||
| Mexican American | NA | NA | NA | NA | ||
| Other Hispanic | Yes | 0.78 | 9.21 | < 0.0001 | ||
| Non-Hispanic White | Yes | 0.71 | 10.61 | < 0.0001 | ||
| Non-Hispanic Black | Yes | 0.69 | 9.41 | < 0.0001 | ||
| Other Race—Including Multi-Racial | Yes | 0.66 | 7.41 | < 0.0001 | ||
| Education Level | Confirmed | 22.1 | ||||
| Less than 9th grade | NA | NA | NA | NA | ||
| 9-11th grade | No | 0.02 | 0.27 | 0.79 | ||
| High school graduate/GED or equivalent | No | 0.01 | 0.17 | 0.87 | ||
| Some college or AA degree | Yes | 0.25 | 3.01 | 0.0027 | ||
| College graduate or above | Yes | 0.29 | 3.16 | 0.0016 | ||
| Marital status | Confirmed | 19.4 | ||||
| Married | NA | NA | NA | NA | ||
| Spouse Deceased | No | 0.14 | 1.75 | 0.08 | ||
| Divorced | Yes | 0.21 | 3.51 | 0.0004 | ||
| Separated | No | 0.18 | 1.84 | 0.07 | ||
| Never married | Yes | 0.12 | 2.18 | 0.03 | ||
| Living with partner | No | 0.08 | 1.11 | 0.27 | ||
| Ratio of family | Yes | Confirmed | -0.07 | -5.59 | < 0.0001 | 24.9 |
| BMI(kg/m2) | Yes | Confirmed | 0.02 | 9.66 | < 0.0001 | 25.7 |
| Smoke | Confirmed | 9.1 | ||||
| Never | Yes | 0.16 | 3.54 | 0.0004 | ||
| Former | Yes | 0.16 | 3.43 | 0.0006 | ||
| Current smoker | NA | NA | NA | NA | ||
| Alcohol Frequency | No | Confirmed | 0.0004 | 0.35 | 0.7264 | 17.6 |
| Diabetes | Yes | Confirmed | 0.24 | 4.20 | < 0.0001 | 14.1 |
| CVD | Yes | Confirmed | 0.49 | 7.95 | < 0.0001 | 28.7 |
| Cancer | Yes | Confirmed | 0.25 | 3.96 | < 0.0001 | 17.6 |
| NPAR | No | Confirmed | 0.00 | -0.05 | 0.96 | 7.6 |
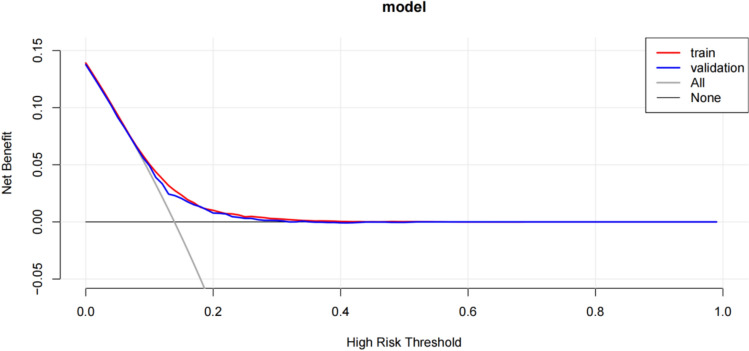
Construction of the nomogram prediction model and plotting of the ROC curve, calibration curve, and DCA curve
The AUC for the LASSO regression model was 0.66, while that for the Boruta algorithm was 0.64, indicating superior performance of the LASSO regression method. A nomogram prediction model was constructed based on the variables identified by LASSO regression (Fig. 5), and the ROC curve (Fig. 6), calibration curve (Fig. 7), and DCA curve (Fig. 8) of the prediction model were plotted.
Fig. 5.
Nomogram prediction model
Fig. 6.
ROC curve of the nomogram
Fig. 7.
Calibration curve of the nomogram
Fig. 8.
DCA curve of the nomogram
Discussion
This study employed LASSO regression and the Boruta algorithm to investigate the relationship between NPAR and asthma. Analysis of data from the NHANES from 2001 to 2018 revealed that higher NPAR is associated with an increased risk of asthma (P < 0.05). Compared with non-asthma patients, females, younger age, race (other Hispanic, non-Hispanic White, non-Hispanic Black, other races), partial college education, marital status (divorced, separated, never married, living with partner), lower PIR, higher BMI, current smoking, diabetes, CVD, cancer, and higher NPAR are associated with an increased risk of asthma (P < 0.05).
Female patients are at an increased risk of developing asthma. Studies have found that women with premenstrual asthma are usually older, have more severe asthma, higher BMI, and longer duration of asthma. In addition, they are more likely to be aspirin-sensitive and often experience dysmenorrhea, premenstrual syndrome, shorter menstrual cycles, and longer menstrual bleeding duration [13]. Another study investigated the relationship between BMI, diabetes, and asthma risk, revealing a strong association among asthma, type 2 diabetes, and increased BMI in adults, especially in females [14]. These findings suggest that asthma and metabolic syndrome may share common etiological factors.
Patients with diabetes have an increased risk of developing asthma. Studies have shown that individuals with diabetes have a significantly higher risk of developing asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and pneumonia, while the risk of lung cancer does not show a significant increase [15]. This may be related to the decline in lung function observed in patients with diabetes. Another study suggested that the increased risk of pulmonary diseases in patients with diabetes could be due to the systemic inflammatory state caused by diabetes, which may adversely affect respiratory health [16].
The relationship between CVD and asthma has garnered increasing attention in recent studies. Multiple investigations have demonstrated a significant association between asthma and the incidence of CVD. For instance, individuals with asthma have a notably higher risk of developing cardiovascular events, particularly heart failure and myocardial infarction [17]. Moreover, the coexistence of asthma and CVD may lead to higher mortality, especially in obese individuals [18]. Several potential mechanisms underlie the association between asthma and CVD. Both asthma and CVD involve chronic inflammatory processes, which may represent a crucial link between the two conditions [19]. In asthma patients, airway and systemic inflammation could exacerbate the burden on the cardiovascular system, thereby increasing the risk of cardiovascular events. Additionally, the use of asthma medications may also impact cardiovascular health. For example, certain asthma treatments might either exacerbate cardiovascular symptoms or potentially offer some benefits for cardiovascular health [20].
Patients with cancer may face an increased risk of various complications, including asthma. Research has shown that cerebrovascular events and related mortality among cancer patients exhibit seasonal variations, suggesting that certain seasons may predispose these patients to specific health issues [21]. This seasonal variation may be related to the immune system status of cancer patients, and changes in the immune system could also increase the risk of asthma. Another study investigated the relationship between asthma severity and cancer incidence, finding that asthma patients had a significantly higher cancer incidence compared to controls without respiratory diseases [22]. This finding further supports the notion that asthma may increase the risk of cancer in patients, as asthma severity is associated with immune system changes and inflammatory responses that could also impact the health status of cancer patients.
In children and adolescents, the neutrophil-to-lymphocyte ratio (NLR) has been identified as a significant systemic inflammatory marker associated with the occurrence of asthma [23]. Although NLR and NPAR are distinct indicators, both involve the proportion of neutrophils, suggesting that NPAR may play a similar role in asthma risk. In adults, elevated NPAR is significantly associated with an increased risk of metabolic syndrome, which in turn is linked to various chronic inflammatory diseases, indicating that it may influence asthma occurrence through inflammatory pathways [24]. Another study has shown that NPAR, as an inflammatory marker, is positively correlated with the incidence of depression, which is also associated with asthma [25]. This suggests that NPAR may indirectly increase the risk of asthma. To our knowledge, we are the first to investigate the relationship between NPAR and asthma, and our findings indicate that elevated NPAR may increase the risk of asthma.
In this study, the LASSO regression method demonstrated superior performance. Through LASSO regression analysis, we identified 10 variables: gender, race, smoking, hypertension, diabetes, cancer, PIR, BMI, CVD, and age. Based on these predictive variables, we constructed a nomogram model and developed calibration, ROC, and DCA curves to assess the potential for predicting the risk in asthma patients. Although LASSO regression did not explicitly reveal the relationship between NPAR and asthma, analysis of the NHANES indicated a significant association between elevated NPAR and increased asthma risk. Regarding the direct relationship between NPAR and asthma, a study examining the association between NPAR and all-cause mortality and cancer-specific mortality in cancer patients found that higher NPAR is related to increased mortality risk. This finding indirectly supports the potential impact of NPAR as an inflammatory marker in diseases like asthma [26]. In asthma, the increase in neutrophils is commonly associated with more severe disease manifestations, airway obstruction, and resistance to corticosteroid therapy [27]. Moreover, neutrophils are closely related to the heterogeneity of asthma, particularly in refractory severe asthma, which may be associated with potential pathogens in the airways. These pathogens may activate the innate immune response, thereby leading to the recruitment and activation of neutrophils [28]. The increase in NPAR may reflect the activated state of neutrophils in patients with asthma, which may be related to the inflammatory phenotypes of asthma. Studies have found that neutrophils in patients with asthma can release a variety of mediators, including neutrophil extracellular traps (NETs), which may play an important role in the pathophysiology of asthma [29]. The formation of NETs may be associated with airway remodeling and excessive mucus secretion in patients with asthma, both of which are important features of asthma pathophysiology. Additionally, changes in NPAR may be related to the systemic inflammatory status of patients with asthma. Studies have shown that systemic inflammation in patients with asthma may be associated with the activation and release of neutrophils, which can further exacerbate the pathological process of asthma [30]. Therefore, NPAR may be a potential biomarker of neutrophil activity and systemic inflammatory status in patients with asthma, helping to identify and manage patients with different inflammatory phenotypes of asthma.
In summary, although LASSO regression did not directly reveal the relationship between NPAR and asthma, analysis of the NHANES further supported the association between NPAR, as an inflammatory marker, and increased asthma risk. This is mainly due to the differences in the model principles, feature selection methods, data processing, and optimization objectives of LASSO regression and logistic regression. Future research can continue to explore the specific mechanisms and roles of NPAR in asthma and other inflammation-related diseases.
This study has several distinct advantages. First, it utilized a large, nationally representative database and implemented appropriate covariate adjustments, which enhanced the reliability of the study findings. Second, the study comprehensively assessed the impact of NPAR on asthma risk, filling a gap in previous research and providing a novel theoretical basis for the early prediction of asthma risk. Finally, the use of NPAR as a validation marker in this study offers the benefits of ease of operation and low cost. It is an easily accessible and cost-effective biomarker with potential for clinical application.
This study also has several limitations. First, data collection primarily relied on questionnaires, which may be subject to recall bias from participants, potentially affecting the accuracy of the data and, in turn, the reliability of the study conclusions. Second, despite efforts to control for variables during the study, there may still be unmeasured or uncontrolled confounding factors that could interfere with the study results. Third, the NPAR used in this study was calculated based on the percentage of neutrophils and serum albumin levels. However, these measurements may vary depending on factors such as the time of day, dietary intake, and physical activity levels of participants at the time of data collection. Fourth, the AUC values of the two models are 0.66 for LASSO and 0.64 for Boruta, indicating a moderate difference between the models. LASSO achieves feature selection through L1 regularization. However, when there is high correlation (multicollinearity) among features, it may randomly select one of the correlated features and ignore the others. Boruta is based on random forests and determines important features by comparing them with shadow features. However, its performance may be affected by randomness. In future, the following methods could be employed to improve the models. Introducing More Variables: Other clinical or biochemical variables, such as inflammatory markers and immune-related indicators, could be considered to enhance the predictive power of the model. Feature Engineering: A more in-depth analysis and processing of existing features, such as interaction features and polynomial features, could be conducted to improve the model’s fitting ability. Model Integration: The feature selection results of the LASSO and Boruta models could be integrated, or ensemble learning methods (such as random forests, XGBoost) could be used to improve model performance. Hyperparameter Optimization: More meticulous hyperparameter tuning (such as the regularization parameter of LASSO, the number of iterations of Boruta) could be carried out to optimize model performance.
Conclusions
Based on the NHANES large-sample data, this study investigated the correlation between the NPAR and the risk of asthma. The results showed a significant positive correlation between NPAR and the risk of asthma, suggesting that NPAR could serve as a potential biomarker for predicting asthma risk and provide a reference for early prevention and intervention of asthma.
Acknowledgements
Relevant data were obtained from the NHANES database. The authors thank the patients who contributed to this study. The authors extend their gratitude to all contributing investigators for their invaluable data contributions.
Abbreviations
- AUC
Area under the curve
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CVD
Cardiovascular Disease
- DCA
Decision curve analysis
- FENO
Fractional exhaled nitric oxide
- LASSO
Least Absolute Shrinkage and Selection Operator
- MSE
Mean-square error
- NCHS
National Center for Health Statistics
- NETs
Neutrophil extracellular traps
- NHANES
National Health and Nutrition Examination Survey
- NLR
Neutrophil-to-Lymphocyte Ratio
- NPAR
Neutrophil-percentage-to-albumin ratio
- PIR
Poverty-to-income ratio
- ROC
Receiver operating characteristic
- Th2
T helper type 2
Author contributions
Yumin Fu conceived and designed the project, steering its conceptual framework and execution, and authored this article. Author Yunpeng Wang meticulously collected and processed the data. Jijing Zhao contributed significantly by providing linguistic support, offering writing assistance, and meticulously proofreading the manuscript. Yumin Fu and Yunpeng Wang meticulously reviewed the revised manuscript, ensuring its accuracy and coherence. All authors collectively approved the final version of the manuscript, endorsing its content and presentation.
Funding
None.
Data availability
The datasets analyzed in this study are publicly available summary statistics (https://www.cdc.gov/nchs/nhanes/).
Declarations
Conflict of interests
The authors declare no competing interests.
Consent for publication
Not Applicable.
Ethics approval and consent to participate
The NHANES study protocol was approved by the NCHS Research Ethics Review Board, and informed written consent was obtained from all participants in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shin YH, Hwang J, Kwon R, Lee SW, Kim MS, Shin JI, Yon DK. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy. 2023;78(8):2232–54. 10.1111/all.15807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib N, Pasha MA, Tang DD. Current understanding of asthma pathogenesis and biomarkers. Cells. 2022;11(17):2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maspero J, Adir Y, Al-Ahmad M, Celis-Preciado CA, Colodenco FD, Giavina-Bianchi P, Lababidi H, Ledanois O, Mahoub B, Perng DW, Vazquez JC, Yorgancioglu A. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3):00576–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu T, Woodruff PG, Zhou X. Advances in non-type 2 severe asthma: from molecular insights to novel treatment strategies. Eur Respir J. 2024;64(2):2300826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurczynski SJ, Moore BB. IL-17 in the lung: the good, the bad, and the ugly. Am J Physiol Lung Cell Mol Physiol. 2018;314(1):L6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evasovic JM, Singer CA. Regulation of IL-17A and implications for TGF-β1 comodulation of airway smooth muscle remodeling in severe asthma. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L843–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo LDN, Righetti RF, Aristóteles LRCRB, Dos Santos TM, De Souza FCR, Fukuzaki S, Cruz MM, Alonso-Vale MIC, Saraiva-Romanholo BM, Prado CM, Martins MA, Leick EA, Tibério IFLC. Effects of Anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol. 2018;5(8):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Zhang Y, Yao C, Li B, Li S, Liu W, Chen R, Shi F. Increased levels of serum IL-17 and induced sputum neutrophil percentage are associated with severe early-onset asthma in adults. Allergy Asthma Clin Immunol. 2021;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Jiang YQ, Wang WX, Zhou ZX, Wang YG, Yang L, Ji YL. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum Immunol. 2012;73(11):1171–4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XY, Simpson JL, Powell H, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Lin JT, Gibson PG. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy. 2014;44(9):1137–45. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang R, Liao J, Giri M, Wen J, Guo S. Relationship between dietary protein, serum albumin, and mortality in asthmatic populations: a cohort study. Front Immunol. 2024;4(15):1396740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song B, Jin C, Li N. Association between serum albumin and asthma in the general population of the United States: a retrospective study based on NHANES 2003–2018. J Asthma. 2025;10:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Ramos JL, Pereira-Vega AR, Alvarado-Gómez F, Maldonado-Pérez JA, Svanes C, Gómez-Real F. Risk factors for premenstrual asthma: a systematic review and meta-analysis. Expert Rev Respir Med. 2017;11(1):57–72. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen SF, Duffy DL, Kyvik KO, Skytthe A, Backer V. Risk of asthma in adult twins with type 2 diabetes and increased body mass index. Allergy. 2011;66(4):562–8. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SC, Gau SY, Huang JY, Wu WJ, Wei JC. Increased risk of hypothyroidism in people with asthma: evidence from a real-world population-based study. J Clin Med. 2022;11(10):2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua ML, Li L, Diao LL. Bronchial asthma and risk of 4 specific cardiovascular diseases and cardiovascular mortality: a meta-analysis of cohort studies. Eur Rev Med Pharmacol Sci. 2022;26(14):5081–91. [DOI] [PubMed] [Google Scholar]
- 18.Lin P, Zhao Y, Shi Y, Liang Z. Associations of asthma control with hypertension, cardiovascular disease, and mortality in obese individuals. Intern Emerg Med. 2024;19(4):951–7. [DOI] [PubMed] [Google Scholar]
- 19.Cazzola M, Hanania NA, Rogliani P, Matera MG. Cardiovascular disease in asthma patients: from mechanisms to therapeutic implications. Kardiol Pol. 2023;81(3):232–41. [DOI] [PubMed] [Google Scholar]
- 20.Cazzola M, Page CP, Hanania NA, Calzetta L, Matera MG, Rogliani P. Asthma and cardiovascular diseases: navigating mutual pharmacological interferences. Drugs. 2024;84(10):1251–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivarov V, Shivarov H, Yordanov A. Seasonal pattern of cerebrovascular fatalities in cancer patients. Healthcare (Basel). 2023;11(4):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salameh L, Mahboub B, Khamis A, Alsharhan M, Tirmazy SH, Dairi Y, Hamid Q, Hamoudi R, Al HS. Asthma severity as a contributing factor to cancer incidence: a cohort study. PLoS ONE. 2021;16(5):e0250430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng C, Zhang L (2025) Nonlinear association between neutrophil-to-lymphocyte ratio and asthma in children and adolescents in the USA: a cross-sectional study. Clin Exp Pediatr. [DOI] [PMC free article] [PubMed]
- 24.Ji W, Li H, Qi Y, Zhou W, Chang Y, Xu D, Wei Y. Association between neutrophil-percentage-to-albumin ratio (NPAR) and metabolic syndrome risk: insights from a large US population-based study. Sci Rep. 2024;14(1):26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Liu L, Liu X, Yang L. The association between neutrophil percentage-to-albumin ratio (NPAR) and depression among US adults: a cross-sectional study. Sci Rep. 2024;14(1):21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Wu M, Chen M, Liu R, Tao Q, Hu Y, Yu J, Chen D. The association between neutrophil-percentage-to-albumin ratio (NPAR) and mortality among individuals with cancer: insights from national health and nutrition examination survey. Cancer Med. 2025;14(2):e70527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crisford H, Sapey E, Rogers GB, Taylor S, Nagakumar P, Lokwani R, Simpson JL. Neutrophils in asthma: the good, the bad and the bacteria. Thorax. 2021;76(8):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE. 2014;9(6):e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varricchi G, Modestino L, Poto R, Cristinziano L, Gentile L, Postiglione L, Spadaro G, Galdiero MR. Neutrophil extracellular traps and neutrophil-derived mediators as possible biomarkers in bronchial asthma. Clin Exp Med. 2022;22(2):285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Systemic upregulation of neutrophil α-defensins and serine proteases in neutrophilic asthma. Thorax. 2011;66(11):942–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in this study are publicly available summary statistics (https://www.cdc.gov/nchs/nhanes/).