Abstract
Baculovirus-expressed foot-and-mouth disease virus (FMDV) nonstructural protein 3AB was used as the antigen in an enzyme-linked immunosorbent assay. This assay allowed the differentiation of vaccinated from infected pigs. Serial studies were performed using sera collected from pigs in the field. Positive reactions were found in sera from fattening pigs and sows 16 weeks and 3.5 years postoutbreak, respectively. There was, however, no positive reaction in sows with at least 10 vaccinations. Maternally derived antibodies to the 3AB antigen persisted in piglets up to 13 weeks of age. A high correlation coefficient (r = 0.93) was found between the test results from sow sera and those from their offspring. Therefore, piglet serum was a good substitute for sow serum to monitor the infection status of the dam. The application of this assay to serological surveillance in an FMD eradication program in Taiwan showed that the positive reactors steadily decreased over time in both finishers and sows, indicating that the pig population risk of infection by FMDV has decreased.
Explosive epidemics of foot-and-mouth disease (FMD) occurred in Taiwan in 1997 (6, 16, 19). Field observations (16, 19) and experimental studies (1, 8) demonstrated that the causative agent, FMD virus (FMDV) O/TWN/97, was a porcine-adapted strain which caused typical lesions in swine but not in cattle. The disease spread rapidly in the pig population and resulted in 6,147 pig farms being infected, with >4 million pigs destroyed (19). This massive epidemic devastated the Taiwanese swine industry and caused direct losses of US$378.6 million for control of the outbreak (19). An additional loss of US$1.6 billion was estimated due to the cessation of pork exports to Japan.
To achieve an FMD-free country without vaccination, eradication campaigns were undertaken by the Taiwanese government and farmers during and after the disease outbreak. A compulsory control program based on an intensive vaccination regimen and a partial slaughter policy involving slaughtering only those animals that showed typical clinical signs and lesions was finally adopted by the government.
To facilitate the FMD eradication campaign, a serological surveillance program was also started. One of the purposes of this surveillance program was to evaluate and adjust the massive vaccination program to achieve control of the disease. Identifying and assessing the pig populations infected with FMDV among intensively vaccinated animals was an important part of this evaluation. The effectiveness of the vaccination program could be monitored by using a standard serum neutralization test. To identify infected pigs or pig herds under intensive vaccination, an assay that could discriminate infected from vaccinated pigs had to be established.
Recently, differentiation of FMDV-infected from vaccinated animals based on the detection of antibodies to nonstructural protein 3ABC, 3AB, 3A, or 3B by enzyme-linked immunosorbent assay (ELISA) has been reported. The antigens used were bioengineered proteins expressed in Escherichia coli (5, 7, 11, 13, 14) or baculovirus (17, 18) or obtained from chemically synthesized peptides (15). The nonstructural protein 2C has also been reported to be useful for this purpose (9, 10, 12). An ELISA using baculovirus-expressed 3AB and 3ABC as the antigens has been demonstrated to successfully differentiate vaccinated from infected cattle and sheep (18). This paper describes the adaptation of this assay, using the same reagents and methods, to pig populations and the application of the developed kits to the serological surveillance system to monitor the progress of the FMD eradication program in Taiwan.
MATERIALS AND METHODS
Serum samples for the establishment of cutoff value and preliminary sensitivity and specificity.
Samples from naïve animals included 100 sera collected from nonvaccinated and noninfected pigs before the FMD outbreak in Taiwan. No serum neutralization antibody to FMDV was detected for these sera. Postvaccination sera were collected from 106 pigs 3 weeks after the third FMD vaccine immunization on FMD-free swine farms. Positive sera were collected from 120 naturally FMDV-infected pigs with obvious vesicular lesions at various stages during the FMD outbreak in Taiwan in 1997. Infection by FMDV was confirmed by a serum neutralization test.
Sera from naturally infected pig population.
Sera were collected from 8 to 10 pigs in a naturally FMDV-infected pig population. Characteristic vesicular lesions were observed first in 7-week-old nursery piglets 5 days before the first bleeding, which was designated the first-week samples postoutbreak. Sera were collected from pigs from 1 to 16 weeks postoutbreak. Pigs continually died or were culled for humanitarian reasons during the blood-sampling period and were replaced by pigs from the same pen. Only two pigs had complete sets of sera throughout the experiment.
Sera from naturally infected pigs with failed vaccinations.
The animals on pig farms were immunized twice with FMD vaccine at the ages of 10 and 14 weeks. Vesicular lesions were observed in pigs at the age of about 22 weeks. Sera were randomly collected from 9 to 11 pigs on the first day and 3 and 9 weeks after the first observation of vesicular lesions in the pig population. Sera from uninfected pigs were also collected from pig herds belonging to the same herd but 500 m apart, at the same time and at the same ages as those collected 9 weeks postoutbreak.
Serum samples from sows at parities 9 to 12 with or without natural exposure to FMDV.
A total of 20 sera from sows at parities 9 to 12 were collected from six FMDV-infected pig farms 3.5 years postoutbreak. At the same time, 20 sera were also collected from sows at parities 9 to 12 from two non-FMDV-infected herds. By calculation, the ages of the sows at parities 9 to 12 were at least 4.5 years. Therefore, these sows were at least 1 year of age during the FMD outbreak in March 1997. No further cases were reported after the first FMD outbreak for those six infected pig farms. Sows from infected or noninfected herds received at least 6 or 10 vaccinations, respectively, before being bled.
Sera from piglets born from naturally infected sows.
Sequential sera were collected from three piglets 1 to 15 weeks of age from each of three naturally infected (2 years postinfection) sows and three vaccinated but noninfected sows. The immune status against 3AB antigen was determined for each sow before blood was collected from the piglets.
Paired sow sera and colostrum and offspring sera from naturally infected pig herds.
A total of 85 paired sow sera and colostrum and the sera of their offspring were collected from three pig farms 2 years after the outbreak. No further FMD cases were identified after the first outbreak for those three pig farms. In the preliminary surveillance, all three pig farms had at least 40% of the sows with positive reactions in the ELISA using 3AB as the antigen at the time of sample collection. Sow sera, colostrum, and offspring sera were collected within 24 h after farrowing. The colostrum samples were centrifuged at 3,000 × g for 10 min to sediment particulate matter, and the fat layer was removed. The remaining aliquots were frozen at −70°C until they were used.
Surveillance of antibodies to 3AB antigen in the FMD eradication program.
Preliminary studies were done at 10 and 15 intensive pig farms 2 and 2.5 years postoutbreak, respectively. All of the farms had a farrow-to-finish management system with a female inventory between 500 and 1,500. All pig farms with herd sizes of ≧5,000 were involved in this program thereafter. For those prefectures without pig farms with herd sizes of ≧5,000, the five largest herds in that area were selected to join the program. A total of 179 and 199 pig farms were surveyed for sows and finishers, respectively, 3 years postoutbreak. Surveillance was repeated 6 months later based on the pig populations mentioned above, with totals of 174 and 188 pig farms tested for sows and finishers, respectively. Ten sera each from sows at parities 1 to 2, 3 to 4, and ≧5 were randomly collected from each pig farm at each time point. Thirty finishers were also randomly selected for bleeding at each pig farm at each time point except 2.5 years after the outbreak, at which time only 10 pigs per farm were tested.
ELISA.
The baculovirus-expressed FMDV 3AB antigen, hyperimmune guinea pig serum immunoglobulin G (IgG) against FMDV, the necessary reagents, and a modified double-antibody sandwich ELISA were prepared and performed essentially as described previously (18). The optimal dilutions for each batch of FMDV 3AB antigen, hyperimmune guinea pig serum IgG, and biotinylated IgG were determined to give optical density (OD) values of 0.5 to 1.0. A negative control serum collected from an FMDV-naïve pig served as the negative reference serum throughout this experiment. All samples, including the positive and negative controls, were diluted 1:5 in ELISA buffer with 10% normal bovine serum, 0.05% NaN3, and 1% normal guinea pig serum. An OD reading was performed at a wavelength of 450 nm. For each serum sample tested, the result was expressed as the percentage of the OD (ODp) value of the negative reference serum run on each plate and calculated according to the following formula: ODp = (sample OD × 100)/(mean OD of negative reference serum).
Statistical analysis.
Correlation coefficients were calculated to evaluate the relationship between the test results from the paired sow sera and colostrum and the offspring sera. Comparisons of the proportions of animals with positive reactions among different parity groups of sows or at different times postoutbreak were made by chi-square analysis. Significance was indicated by a probability (P) of <0.05.
RESULTS
Establishing cutoff value and preliminary sensitivity and specificity.
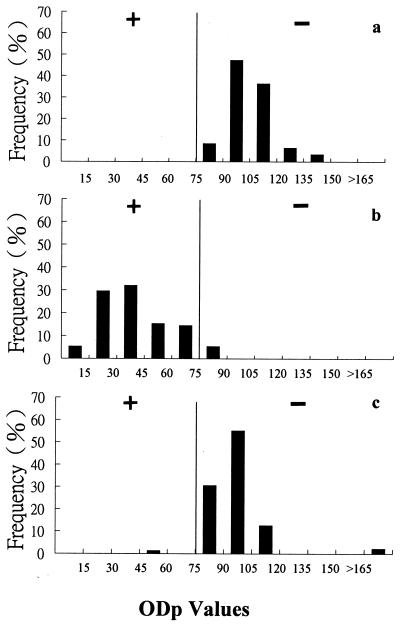
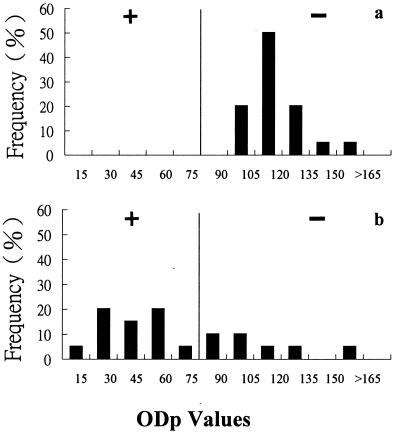
The distributions of ODp values of naïve, vaccinated, and infected pigs are shown in Fig. 1. The mean ODp value (± standard deviation [SD]) for the naïve pigs was 104 ± 12. The sera from the infected pigs gave a mean ODp value of 40 ± 19. A cutoff value was then set at an ODp value of 75, with values of ≤75 designated test positive for antibody against FMDV 3AB antigen and values of >75 designated test negative, as indicated in Fig. 1. This cutoff value was equal to the mean ODp value for naïve pigs with 2.4 standard deviations subtracted. The sera from the vaccinated but noninfected pigs gave a mean ODp value of 97 ± 23. The choice of the aforementioned cutoff value resulted in an assay with a preliminary sensitivity of 95.8% (115 of 120) and preliminary specificities of 99.1 (105 of 106) and 100% (100 of 100) for vaccinated and naïve pigs, respectively.
FIG. 1.
Percent frequency distribution of ODp values of sera from naïve, vaccinated, and infected pigs. (a) Sera from 100 nonvaccinated and noninfected pigs; (b) sera from 120 infected pigs; (c) sera from 106 vaccinated pigs. Pigs with ODp values of ≤75 are test positive (+) for antibody against FMDV 3AB antigen, and pigs with ODp values of >75 are test negative (−).
Kinetics of the antibody response to 3AB antigen in a naturally infected pig population.
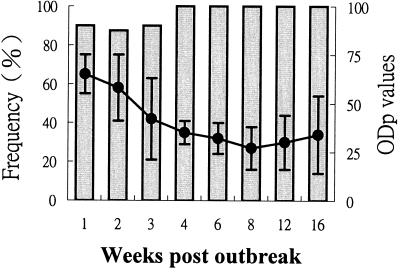
Positive reactions could be found for 9 of 10 pigs with a mean ODp value of 65 ± 10 5 days after the first observation of vesicular lesions in 7-week-old pigs (Fig. 2, week 1 postoutbreak) in the naturally infected pig herd. For pigs at 2 and 3 weeks postoutbreak, the percentages of pigs with positive reactions was similar to those in the first week postoutbreak but with lower mean ODp values of 58 ± 17 and 42 ± 21, respectively. All sera collected from pigs at 4 weeks postoutbreak had positive reactions, with a mean ODp value of 35 ± 6. The ODp value reached the lowest level of 27 ± 11 at 8 weeks postoutbreak and then gradually increased but remained at the quite low mean ODp value of 34 ± 20 for animals at 16 weeks postoutbreak.
FIG. 2.
Percent frequency distribution of pigs with positive results in ELISA following natural exposure to FMDV. Vesicular lesions were observed first in 7-week-old nursery pigs on one pig farm 5 days before the first bleeding (designated week 1 postoutbreak). Eight to 10 sera were collected from pigs in the same pen with the first case. Shaded bars, percentage of pigs with ODp values of ≤75 (test positive); solid circles, means ± SD of ODp values.
Antibody response to 3AB antigen in naturally infected pigs with failed vaccinations.
No positive reaction could be found from uninfected pigs and pigs with vesicular lesions on the first day of observation. All pigs in infected populations had positive reactions 3 and 9 weeks after the observation of the first lesion, with mean ODp values of 48 ± 16 and 37 ± 10, respectively.
Persistence of antibodies to 3AB antigen in multiply vaccinated sows from infected and noninfected herds.
The antibody profiles for sera from multiply vaccinated sows were different in FMDV-infected and noninfected herds (Fig. 3). Positive reactions could not be found for sows from noninfected farms, with ODp values ranging from 95 to 159. Of the 20 sera collected from sows on infected farms 3.5 years postoutbreak, 13 (65%) had positive reactions, with ODp values ranging from 14 to 68.
FIG. 3.
Percent frequency distribution of ODp values of sera from sows at parities 9 to 12 with or without prior FMDV infection. (a) Sera collected from pig farms without outbreak of FMD. Each sow had received at least 10 vaccinations; n = 20. (b) Sera collected from pig farms 3.5 years after outbreak of FMD. Each sow had received at least six vaccinations; n = 20. Pigs with ODp values of ≤75 are test positive (+), and pigs with ODp values of >75 are test negative (−).
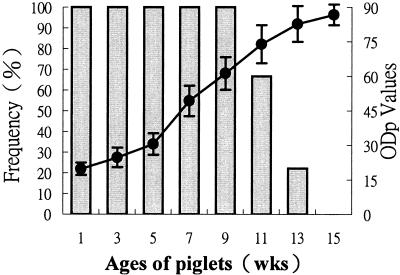
Persistence of maternal antibodies to the 3AB antigen in piglets born from infected sows.
The persistence of maternally derived antibodies to the 3AB antigen in piglets born from naturally infected sows is shown in Fig. 4. Three infected sows had low ODp values between 19 and 23. All piglets had positive reactions before the age of 9 weeks, with the ODp values gradually increasing as the pigs grew older. At the ages of 11 and 13 weeks, 67 and 22% of the pigs had positive reactions. There were no positive reactions for any pigs at the age of 15 weeks. The mean ODp values for pigs at the ages of 1 and 15 weeks were 20 ± 3 and 87 ± 5, respectively. None of the sera collected from piglets born from vaccinated but noninfected sows had positive reactions throughout this experiment.
FIG. 4.
Persistence of maternally derived antibodies against FMDV 3AB antigen in piglets born from naturally infected sows. Sequential sera were collected from three piglets of each of 3 sows. Shaded bars, percentage of piglets with ODp values of ≤75 (test positive). Solid circle, mean ± SD of ODp values.
The correlation of test results among sow sera, colostrum, and offspring sera.
Of 85 paired sow sera and colostrum and offspring sera, 76 (89.4%) had consistent results which were either all positive or all negative in ELISA. The correlation coefficients between the sow sera and colostrum, sow sera and offspring sera, and colostrum and offspring sera were 0.86, 0.93, and 0.81, respectively (Table 1). Among the three farms, samples from farm CL had the lowest correlation coefficients of 0.36, 0.89, and 0.48 between sow sera and colostrum, sow sera and offspring sera, and colostrum and offspring sera, respectively. Only 19 (76%) of 25 paired sow sera and colostrum and offspring sera had consistent test results for farm CL.
TABLE 1.
Correlation coefficients between the paired sow serum and colostrum and offspring serum test results for antibodies to the FMDV 3AB antigen
| Farma | No. of sows tested | Correlation coefficient between:
|
||
|---|---|---|---|---|
| Sow sera and colostrum | Sow sera and offspring sera | Colostrum and offspring sera | ||
| CL | 25 | 0.36 | 0.89 | 0.48 |
| SW | 17 | 1.0 | 0.89 | 0.89 |
| HS | 43 | 0.95 | 0.95 | 0.90 |
| Total | 85 | 0.86 | 0.93 | 0.81 |
Sow sera, colostrums, and offspring sera were collected from three pig farms 2 years after the outbreak of FMD. At least half of the sows selected had been naturally exposed to FMDV for each pig farm.
Antibody profiles to the 3AB antigen in pig populations undergoing the eradication program.
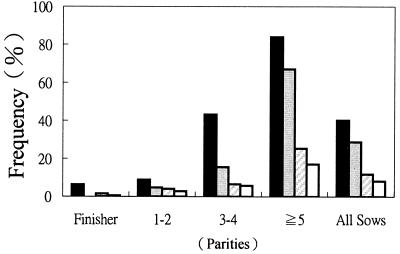
The percentages of finishers with positive reactions steadily decreased from 6.3 (17 of 270) to 0 (0 of 153), 1.5 (89 of 5,754), and 0.4% (22 of 5,649) at 2, 2.5, 3, and 3.5 years postoutbreak, respectively (Fig. 5). The proportions of sows with positive results significantly (P < 0.05) decreased from 40.7 (122 of 300) to 28.7 (129 of 450), 11.7 (605 of 5,161), and 8.3% (419 of 5,061) at 2, 2.5, 3, and 3.5 years postoutbreak, respectively. There were significant (P < 0.05) differences in the percentages of sows with positive reactions among different parity groups at 3.5 years postoutbreak. Only 2.7 (47 of 1,766), 5.7 (95 of 1,666), and 17% (277 of 1,629) of sera collected from sows at parities 1 to 2, 3 to 4, and ≧5, respectively, were positive in ELISA.
FIG. 5.
Percentages of sows and finishers with positive results in ELISA at various intervals after the outbreak of FMD in Taiwan. A total of 10, 15, 179, and 174 pig farms were surveyed for sows 2 (solid bars), 2.5 (shaded bars), 3 (hatched bars), and 3.5 (open bars) years postoutbreak, respectively. Ten sera each from sows at parities 1 to 2, 3 to 4, and ≧5 were randomly collected from each pig farm. A total of 9, 15, 199, and 188 farms were surveyed for finishers 2, 2.5, 3, and 3.5 years postoutbreak, respectively. Thirty finishers were randomly selected from each pig farm for bleeding, except for 2.5 years postoutbreak, when only 10 pigs per farm were tested.
DISCUSSION
In a preliminary study, the baculovirus-expressed FMDV nonstructural proteins 3AB, 3ABC, and 3D were used as antigens in ELISAs. As expected (2, 11, 18), unlike 3D, the 3AB and 3ABC antigens used in ELISA were able to differentiate FMDV-infected from vaccinated pigs. The initial estimates of analytical sensitivity were 73 (22 of 30) and 90% (27 of 30) for ELISA using 3ABC and 3AB as antigens, respectively. Therefore, ELISA using 3AB as the antigen was further refined, standardized, and applied to field studies, including the FMD eradication campaigns in Taiwan. In contrast, the E. coli-expressed 3ABC antigen has been reported to be better than 3AB as the antigen in ELISA for differentiation between infected and vaccinated pigs (14). The discrepancies might be due mainly to the conformational differences among the polypeptides produced in different expression systems.
As a result of the compulsory vaccination program implemented in the pig population, all pigs might have not only high titers of neutralization antibodies but also some degree of response to the 3AB antigen due to the impurity of the inactivated vaccine. To apply the differentiating diagnostic tool to the aforementioned situation, the sera from vaccinated pigs would be preferred for evaluating the usefulness of these kits. A preliminary specificity of 99.1% was obtained by testing sera collected from vaccinated pigs (Fig. 1). More impressively, in contrast to one report (11), which demonstrated that cattle receiving >10 vaccinations expressed antibodies to the 3ABC antigen, all sera collected from sows with at least 10 vaccinations from FMD-free pig farms were negative in ELISA (Fig. 3). The above information not only indicates the high specificity of the assay but also demonstrates the high quality of the FMD vaccine used at these pig farms.
The immune response to nonstructural proteins has been reported to develop later than that to structural proteins in the course of infection (7, 18). Following experimental infection, antibodies to the 3ABC antigen could not be detected earlier than 8, 10, and 14 days in cattle, sheep (18), and pigs (14), respectively. Since all positive sera described in this report were collected from pigs with typical lesions in naturally infected herds, the actual time of the first FMDV contact was unknown. Therefore, the earliest time after infection that the assay could detect an immune response to the 3AB antigen in pigs was undetermined. Nevertheless, the fact that three sera collected from pigs 1 to 3 weeks postoutbreak (Fig. 2) had low-to-moderate titers of serum neutralization antibodies (data not shown) but with ODp values ranging from 78 to 86 supports the finding described above. Other evidence showed that one pig farm, which had an FMD outbreak due to failed vaccinations, exhibited low-to-moderate levels of serum neutralization antibodies (data not shown), but no positive reaction was found on the first day of observed vesicular lesions. Both serum neutralization and 3AB antibodies were detected 3 and 9 weeks after the lesion observation. However, this would be a particular case, since the pigs were immunized with vaccine, although not enough to protect them.
Pigs naturally infected with FMDV at the age of 7 weeks still had strong positive reactions 16 weeks postoutbreak (Fig. 2). Out of 20 sera collected from multiply vaccinated sows in infected herds, 13 had positive reactions 3.5 years postoutbreak (Fig. 3). As mentioned above, sows vaccinated at least 10 times remained negative in ELISA. The positive reactions for those 13 sows, therefore, presumably originated from the FMDV infection, not from the vaccination. Since we did not have the individual history of each sow, the negative reactions for the remaining seven sows might be due either to the complete decay of the antibody over time or the lack of infection. Judging from the facts that FMDV is an extremely contagious agent and no animals had antibodies before the epidemic in 1997, the former would be the preferred answer. Anti-3B antibodies could be detected 301 days after experimental infection of pigs with FMDV (15). All of the information described above indicates that antibodies to the 3AB antigen can be detected in pigs by ELISA for a considerable period after infection.
Maternally derived antibodies to 3AB antigen persisted for up to 13 weeks after birth (Fig. 4). Positive reactions were found for all piglets at the age of 9 weeks if the dams had very low ODp values, as described in this report. This result gave the important message that the interpretation of the serological surveillance report must be made with caution if the tests were done in young pigs, even up to the age of 13 weeks. On the other hand, maternally derived 3AB antibodies could be a very good indicator of infection of the dam. The correlation analysis for the test results between paired sow sera and colostrum and the sera of their offspring revealed that the best correlation coefficient of 0.93 was found between sow sera and their offspring's sera. In farm CL (Table 1), the correlation coefficients between the test results from sow sera and colostrum and between colostrum and offspring sera were only moderate. However, the test results gave a high correlation coefficient of 0.89 between sow and offspring sera. The vaccine used and the experimental procedures conducted were the same for all three farms. The cause of the lack of high correlation between colostrum and sow or offspring sera for farm CL was therefore unclear. The aforementioned results indicate that piglet serum could be used to determine the infection status of sows with high accuracy. Because sow bleeding is labor-intensive, time-consuming, and dangerous, using piglet sera instead of sow sera for serological surveillance would offer a great advantage for the FMD eradication program.
As described above, the 3AB ELISA has promising sensitivity and specificity to distinguish FMDV-infected pigs from vaccinated pigs. This kit has also been demonstrated to be useful for monitoring the progress of the FMD eradication program in Taiwan. The percentages of sows and finishers with positive reactions steadily decreased over time, as shown in Fig. 5, indicating that the pig population risk of FMDV infection has decreased. The low prevalence of FMD was also demonstrated by the fact that only one case of FMD each year was reported in pigs in 2000 and 2001 (Office International des Epizooties [http://www.oie.int/hs2/report.asp]). However, on the basis of the estimated preliminary sensitivity, approximately 4% of infected pigs will be missed by the ELISA. Parallel testing can be conducted by using two or more tests on sera at the same time. Animals are considered to be infected if any of the tests gives a positive reaction. This approach can enhance the sensitivity but reduce the specificity. However, as the prevalence of FMD in the pig population reaches a very low level, the predictive value for positive test results will also be very low. For example, only 22 (0.4%) of 5,649 finishers had positive reactions 3.5 years postoutbreak. Based on the evaluated preliminary specificity (99.1%; Fig. 1) and the low prevalence (probably <0.4%), a sample size of 5,649 would give approximately 51 false-positive diagnoses. In other words, most of the 22 reactors mentioned above would very possibly be false positive. Judging these results on a herd basis can partially resolve the problem. Alternatively, serial testing can be done to maximize the specificity and the predictive value of a positive test result. For example, animals with a positive reaction in screening tests can be subjected to further tests to confirm their infection status. ELISA (11) and enzyme-linked immunoelectrotransfer blot assay (3, 4, 5) using multiple nonstructural antigens have been recommended as confirmatory tests. Studies are under way to evaluate the usefulness of these paired screening and confirmation tests in our serological surveillance program.
Acknowledgments
This work was supported by grant 89BT-2.2-BQ-62 (1) and the Hog Cholera and FMD Eradication Program from the Council of Agriculture, Republic of China.
We appreciate the contributions of all the veterinarians who participated in the surveillance program.
REFERENCES
- 1.Beard, C. W., and P. W. Mason. 2000. Genetic determinants of altered virulence of Taiwanese foot-and-mouth disease virus. J. Virol. 74:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, H. G., O. C. Straub, R. Ahl, M. Tesar, and O. Marquardt. 1990. Identification of foot-and-mouth disease virus replication in vaccinated cattle by antibodies to non-structural virus proteins. Vaccine 8:213-216. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, I. E., P. Auge de Mello, E. Neitzert, E. Beck, and I. Gomes. 1993. Diagnosis of persistent aphthovirus infection and its differentiation from vaccination response in cattle by use of enzyme-linked immunoelectrotransfer blot analysis with bioengineered nonstructural viral antigens. Am. J. Vet. Res. 54:825-831. [PubMed] [Google Scholar]
- 4.Bergmann, I. E., V. Malirat, L. Eduardo Dias, and R. Dilandro. 1996. Identification of foot-and-mouth disease virus-free regions by use of a standardized enzyme-linked immunoelectrotransfer blot assay. Am. J. Vet. Res. 57:972-974. [PubMed] [Google Scholar]
- 5.Bergmann, I. E., V. Malirat, E. Neitzert, E. Beck, N. Panizzutti, C. Sanchez, and A. Falczuk. 2000. Improvement of a serodiagnostic strategy for foot-and-mouth disease virus surveillance in cattle under systematic vaccination: a combined system of an indirect ELISA-3ABC with an enzyme-linked immunoelectrotransfer blot assay. Arch. Virol. 145:473-489. [DOI] [PubMed] [Google Scholar]
- 6.Chang, T. C., C. C. Chang, S. S. Tsai, G. N. Chang, M. Kuo, and W. B. Chung. 1997. An outbreak of foot-and-mouth disease in pigs in southern Taiwan. J. Chin. Soc. Vet. Sci. 23:269-273. [Google Scholar]
- 7.De Diego, M., E. Brocchi, D. Mackay, and F. De Simone. 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch. Virol. 142:2021-2033. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, C. S., and A. I. Donaldson. 1997. Natural adaptation to pigs of a Taiwanese isolate of foot-and-mouth disease virus. Vet. Rec. 141:174-175. [DOI] [PubMed] [Google Scholar]
- 9.Lubroth, J., and F. Brown. 1995. Identification of native foot-and-mouth disease virus non-structural protein 2C as a serological indicator to differentiate infected from vaccinated livestock. Res. Vet. Sci. 59:70-78. [DOI] [PubMed] [Google Scholar]
- 10.Lubroth, J., M. J. Grubman, T. G. Burrage, J. F. E. Newman, and F. Brown. 1996. Absence of protein 2C from clarified foot-and-mouth disease virus vaccines provides the basis for distinguishing convalescent from vaccinated livestock. Vaccine 14:419-427. [DOI] [PubMed] [Google Scholar]
- 11.Mackay, D. K. J., M. A. Forsyth, P. R. Davies, A. Berlinzani, G. J. Belsham, M. Flint, and M. D. Ryan. 1997. Differentiating infection from vaccination in foot-and-mouth disease using a panel of recombinant, non-structural proteins in ELISA. Vaccine 16:446-459. [DOI] [PubMed] [Google Scholar]
- 12.Meyer, R. F., G. D. Babcock, J. F. E. Newman, T. G. Burrage, K. Toohey, J. Lubroth, and F. Brown. 1997. Baculovirus expressed 2C of foot-and-mouth disease virus has the potential for differentiating convalescent from vaccinated animals. J. Virol. Methods 65:33-43. [DOI] [PubMed] [Google Scholar]
- 13.Neitzert, E., E. Beck, P. Auge De Mello, I. Gomes, and I. E. Bergmann. 1991. Expression of the aphthovirus RNA polymerase gene in Escherichia coli and its use together with other bioengineered nonstructural antigens in detection of late persistent infections. Virology 184:799-804. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez, A., J. Dopazo, J. C. Saiz, and F. Sobrino. 1994. Immunogenicity of non-structural proteins of foot-and-mouth disease virus: differences between infected and vaccinated swine. Arch. Virol. 136:123-131. [DOI] [PubMed] [Google Scholar]
- 15.Shen, F., P. D. Chen, A. M. Walfield, J. Ye, J. House, F. Brown, and C. Y. Wang. 1999. Differentiation of convalescent animals from those vaccinated against foot-and-mouth disease by a peptide ELISA. Vaccine 17:3039-3049. [DOI] [PubMed] [Google Scholar]
- 16.Shieh, H. K. 1997. The FMD situation in Taiwan. J. Chin. Soc. Vet. Sci. 23:395-402. [Google Scholar]
- 17.Silberstein, E., G. Kaplan, O. Taboga, S. Duffy, and E. Palma. 1997. Foot-and-mouth disease virus-infected but not vaccinated cattle develop antibodies against recombinant 3AB1 nonstructural protein. Arch. Virol. 142:795-805. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen, K. J., K. G. Madsen, E. S. Madsen, J. S. Salt, J. Nqindi, and D. K. J. Mackay. 1998. Differentiation of infection from vaccination in foot-and-mouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB and 3ABC in ELISA using antigens expressed in baculovirus. Arch. Virol. 143:1-16. [DOI] [PubMed] [Google Scholar]
- 19.Yang, P. C., R. M. Chu, W. B. Chung, and H. T. Sung. 1999. Epidemiological characteristics and financial costs of the 1997 foot-and-mouth disease epidemic in Taiwan. Vet. Rec. 145:731-734. [DOI] [PubMed] [Google Scholar]