Abstract
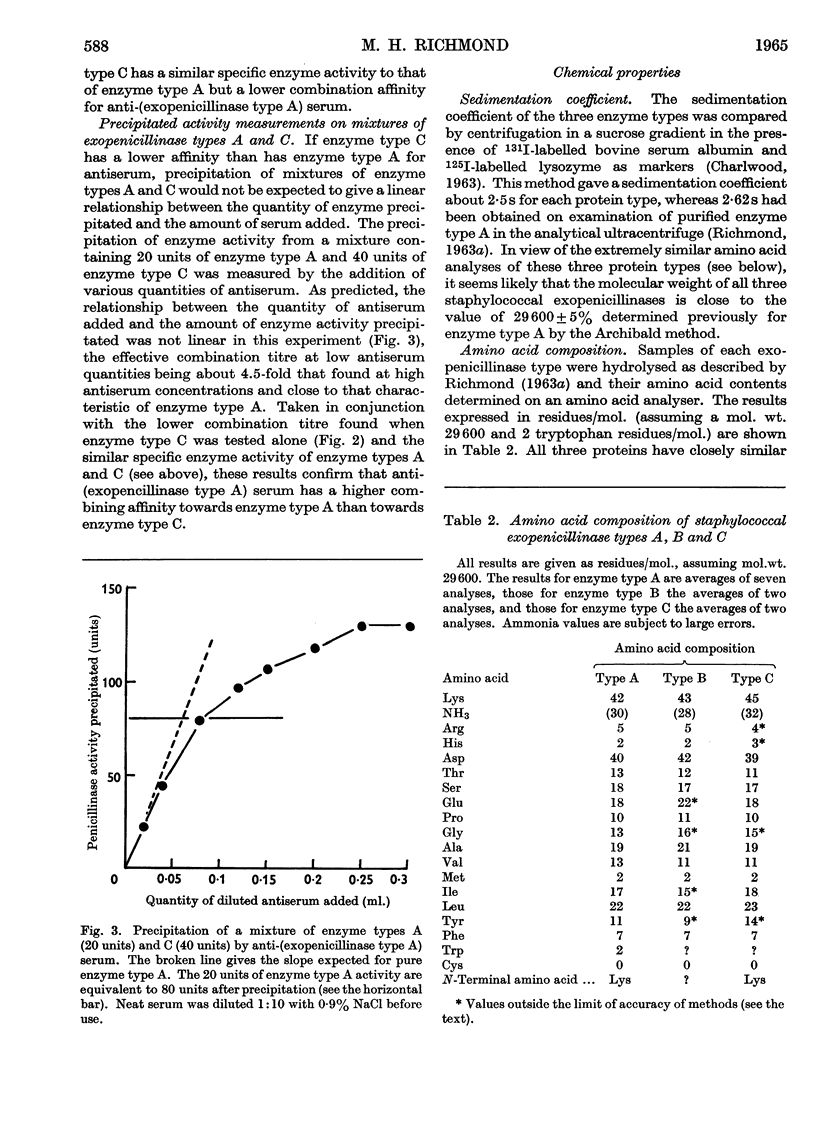
1. Three variants of staphylococcal exopenicillinase (types A, B and C) can be distinguished on chemical, enzymological and immunological grounds. 2. Enzyme type A has a higher specific activity than that of type B, but has a similar combination affinity with anti-(exopenicillinase type A) serum. 3. Enzyme types A and C have a similar specific activity, but enzyme type C has a lower combination affinity for anti-(exopenicillinase type A) serum than has enzyme type A. 4. The sedimentation coefficients and amino acid analyses of the three enzyme types are similar. 5. All three enzyme types have small but significant differences in kinetics of action when hydrolysing benzylpenicillin, methicillin, cloxacillin and cephalosporin C. 6. Peptide maps, obtained from enzyme types A and C after digestion with trypsin, show that these two variants probably differ in the nature of only a very few amino acid residues. 7. Enzyme type B seems to be confined to staphylococci that are members of staphylococcal phage group II. Enzyme types A and C are produced by staphylococci that are members either of phage group I or III, but never group II. 8. The low specific enzyme activity and affinity of enzyme type B towards all penicillins tested suggest that this enzyme type has a lower `efficiency' in hydrolysing penicillin and therefore in protecting bacteria from the action of penicillin. This could account for the low incidence among `hospital staphylococci' of penicillin-resistant staphylococci that are members of phage group II.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., WILLIAMS R. E. Bacteriophage typing of enteric pathogens and staphylococci and its use in epidemiology. J Clin Pathol. 1956 May;9(2):94–127. doi: 10.1136/jcp.9.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER M., WILDY P. A study of the antigenic specificity of staphylococcal coagulase in relation to bacteriophage group. J Gen Microbiol. 1958 Feb;18(1):92–106. doi: 10.1099/00221287-18-1-92. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Applications of radioactively labeled marker proteins in density gradient ultracentrifugation. Anal Biochem. 1963 Mar;5:226–245. doi: 10.1016/0003-2697(63)90120-9. [DOI] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJELLANDER J. O., FINLAND M. STUDIES ON THE PENICILLINASE OF STAPHYLOCOCCUS ALBUS. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:1031–1037. doi: 10.3181/00379727-113-28564. [DOI] [PubMed] [Google Scholar]
- MUNCH-PETERSEN E., BOUNDY C. Yearly incidence of penicillin-resistant staphylococci in man since 1942. Bull World Health Organ. 1962;26:241–252. [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R., TORRIANI A. M. Purification et caractéristiques physicochimiques de la pénicillinase de Bacillus cereus. C R Hebd Seances Acad Sci. 1953 Jul 20;237(3):276–278. [PubMed] [Google Scholar]
- RICHARDS H. C., HOUSLEY J. R., SPOONER D. F. QUINACILLIN: A NEW PENICILLIN WITH UNUSUAL PROPERTIES. Nature. 1963 Jul 27;199:354–356. doi: 10.1038/199354a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J. Variant populations within a hyaluronidase-producing culture of Staphylococcus aureus. J Pathol Bacteriol. 1953 Oct;66(2):545–551. doi: 10.1002/path.1700660226. [DOI] [PubMed] [Google Scholar]
- ROUNTREE P. M. Staphylococci harboured by people in western highlands of New Guinea. Lancet. 1956 May 19;270(6925):719–720. doi: 10.1016/s0140-6736(56)90747-4. [DOI] [PubMed] [Google Scholar]
- SHAW C., STITT J. M., COWAN S. T. Staphylococci and their classification. J Gen Microbiol. 1951 Nov;5(5 Suppl):1010–1023. doi: 10.1099/00221287-5-5-1010. [DOI] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- SZYBALSKI W. Sampling of virus particles and macromolecules sedimented in an equilibrium density gradient. Experientia. 1960 Apr 15;16:164–164. doi: 10.1007/BF02157737. [DOI] [PubMed] [Google Scholar]


