Abstract
Introduction
Mesothelioma is a type of lung cancer caused by asbestos exposure, and early diagnosis is crucial for improving survival chances. Artificial intelligence offers a potential solution for the timely diagnosis and staging of the disease. This study aims to review the latest research conducted in artificial intelligence applications to predict mesothelioma.
Methods
Until April 24, 2023, PubMed, Scopus, and Web of Science databases were searched comprehensively for articles on artificial intelligence in mesothelioma management. The data was gathered using a standardized extraction form, and the findings were reported in figures and tables.
Results
One hundred and seventy-three articles were identified from database searches, which were then reduced to 151 after eliminating duplicates. Finally, 19 articles were selected for inclusion in our study. The applications of artificial intelligence in these articles primarily focused on tumor diagnosis and classification (73.69%), followed by prevention and prognosis (21.05%) and tumor volumetric measurement of malignant pleural mesothelioma (5.26%). The most frequently used AI models include types of neural networks (NN), decision trees (DT), random forests (RF), logistic regression (LogR), Naïve Bayes (NB), and support vector machines (SVM). SVM, DT, and RF emerged as prominent models, achieving high accuracies ranging from 78.3% to 99.97%. Genetic algorithms, correlation-based algorithms, and Neural Networks were employed for risk factor identification and feature selection.
Conclusion
Artificial intelligence, particularly machine learning models such as neural networks, decision trees, support vector machines, and random forests, holds promise in predicting and managing mesothelioma, potentially enhancing early detection and improving patient outcomes.
Keywords: mesothelioma, lung neoplasms, artificial intelligence, machine learning, support vector machine, logistic regression
Introduction
Mesothelioma is a rare and malignant cancer that mainly originates from mesothelial cells, 1 with around 80% of cases globally attributed to asbestos exposure. 2 Due to varying levels of asbestos exposure, mesothelioma incidence significantly fluctuates worldwide. By 2025, mesothelioma in Western countries is predicted to increase by 5 to 10% annually. 3 Mesothelioma incidence was estimated at 1 to 2 million in states with low asbestos exposure and 10 to 15 million in states with high asbestos exposure. 4 Furthermore, Australia has one of the highest age-standardized rates globally, primarily due to its extensive asbestos industry and long-standing mining operations. 5
Mesothelioma progresses very slowly; sometimes, it takes more than ten years from the onset of cancer cells to the manifestation of symptoms. Nevertheless, unfortunately, when the symptoms appear, the disease has significantly progressed. 6 Moreover, diagnosing mesothelioma based on its symptoms and signs can be challenging, as many other diseases exhibit similar symptoms. However, one of the specific characteristics of mesothelioma symptoms is that they persist for more than six months. Mesothelioma patients usually present with symptoms of shortness of breath, often accompanied by chest pain, cough, and systemic disease symptoms such as weakness, atrophy, fatigue, and anorexia. 1
Additionally, due to the hidden signs and non-specific symptoms of mesothelioma (such as cough, chest pain, and shortness of breath), many patients are diagnosed in their advanced stages. Medical imaging is essential in diagnosing, staging, and managing mesothelioma patients. 7 Multimodal imaging can provide valuable information during various diagnosis, treatment, and follow-up phases. Common approaches to multimodal imaging include Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Positron Emission Tomography (PET). 8 However, these traditional methods are expensive, dangerous, and painful. 9 In addition, medical imaging requires enormous infrastructure, facilities, and skilled personnel, which makes it costly, particularly in developing countries. Since, after diagnosis, the probability of patient survival until one year later is less than 50%, the need for innovative approaches to improve the management process of this cancer seems necessary. 10
Recent advancements in artificial intelligence (AI) have significantly enhanced disease prediction and diagnosis across various medical fields, including oncology.11,12 AI, a cutting-edge field in computer science, seeks to enhance various technologies, especially in medicine, by equipping computers and machines with intelligence for improved disease diagnosis and treatment success rates. 13 AI-driven models have been successfully applied to lung and colon cancer classification using secure and transparent frameworks, such as blockchain and Microsoft Azure, ensuring reliability in medical decision-making. 14 Additionally, feature reduction techniques have been explored to improve hepatocellular carcinoma prediction, optimizing model performance through machine learning algorithms. 15
AI has also been instrumental in identifying synergistic drug combinations for FDA-approved cancer treatments, facilitating more effective therapeutic strategies. 16 In medical imaging, new edge detection techniques based on Shannon entropy have enhanced feature extraction in grayscale images, improving diagnostic accuracy. 17 Given these advancements, AI-based approaches hold great potential for mesothelioma prediction, where precise diagnosis and early detection remain critical challenges. This study aims to comprehensively review common AI models and their applications in mesothelioma disease prediction by leveraging insights from these related works. Choudhury et al 18 suggested using AI models to develop the optimal system for early diagnosis and prognosis of malignant pleural mesothelioma (MPM). In a 2020 study, Alam et al 19 used association rule extraction to identify prognostic factors of malignant mesothelioma. They concluded that the proposed algorithm successfully predicted the disease's risk factors. Courtiol et al, 20 showed that deep learning, through models such as MesoNet, improves mesothelioma outcome prediction by analyzing whole-slide images without relying on pathologist annotations. MesoNet offers higher accuracy than traditional methods and identifies key histological regions linked to patient survival, revealing potential new biomarkers for better diagnosis and treatment.
To our knowledge, no review has been conducted on predicting mesothelioma disease using AI techniques, and only a few original studies have been undertaken on mesothelioma diagnosis.21–23 Therefore, this study aims to review the current state of AI technologies, such as machine learning and deep learning, in predicting mesothelioma disease.
Materials and Methods
Study Design
This scoping review is reported according to the PRISMA extension for scoping reviews (PRISMA-ScR) checklist. 24
Search Strategy
This scoping review aimed to explore the application of AI in mesothelioma prediction. Our investigation encompassed articles published up to April 24, 2023, with no temporal restrictions. We reviewed the literature using the PubMed, Scopus, and Web of Science databases to identify relevant studies. The search strategy involved utilizing Medical Subject Headings (MeSH) terms, keywords, and synonyms related to concepts such as mesothelioma and artificial intelligence, as detailed in Table 1.
Table 1.
Keywords Used in the Search Strategy.
| #1 | “Mesothelioma”* OR “Malignant Mesothelioma” OR “Malignant Pleural Mesothelioma” |
| #2 | “Decision support techniques” OR “Data mining” OR “Artificial intelligence”* OR “Machine Intelligence” |
| Search | #1 AND #2 |
*Note: MeSH terms are written in bold.
Inclusion and Exclusion Criteria
The inclusion criteria encompassed original and English-language articles investigating the use of AI and its models in managing mesothelioma prevention, diagnosis, and treatment. Conversely, the exclusion criteria included studies that did not align with the study's objectives, those published in non-English language, review articles, short communications, conference abstracts, editorials, and letters to the editor.
Data Extraction Process
Initially, the titles and abstracts of the articles were independently evaluated by three researchers (EE, MR, and MSKH). Subsequently, relevant data from the selected articles were meticulously extracted using a researcher-designed form, carefully crafted to align with the study's objectives. This form collected essential information, including the article's title, publication year, and country; the first author's name; the study's aim; the type and number of algorithms used; the application of AI in disease management; and the key findings derived from the research.
Risk of Bias and Quality Assessment
The assessment of bias risk in the studies included in this scoping review was meticulously conducted by three independent evaluators (MR, AG, and AS), employing the Prediction Model Study Risk of Bias Assessment Tool (PROBAST). PROBAST, specifically developed for application in systematic reviews focusing on prediction model studies, is a comprehensive quality assessment instrument. It is structured around four critical domains: participant selection, predictors, outcome, and analysis, each crucial for ensuring the reliability and validity of the studies under review. Moreover, the tool includes 20 detailed signaling questions that guide the appraisers in thoroughly examining potential biases within each domain. 25
Synthesis of Results
To provide a structured overview of the reviewed studies, we categorized them based on key attributes, including publication year, country, algorithm type, AI applications, AI models used in mesothelioma prediction, and the contributions and limitations of each study. This systematic categorization enhances clarity, facilitates a deeper understanding of relationships among studies, and supports a comprehensive synthesis of existing knowledge. A descriptive analysis incorporating frequency and percentage parameters was also performed based on the study variables and presented through tables and figures.
Results
After searching the databases, we identified 173 relevant articles. Subsequently, we removed duplicates, narrowing the total to 151 articles. Finally, we examined the full texts of 36 articles, of which 19 were selected for the current study (Figure 1).
Figure 1.
PRISMA Extension for ScR Flow Diagram of the Screening Process.
Main Characteristics of the Selected Studies
Table 2 offers an overview of the studies included.
Table 2.
The Main Characteristics of Included Studies.
| Authors | Year | Country | Aim of the study | Algorithm Type | Number of algorithms | AI application | Main Results |
|---|---|---|---|---|---|---|---|
| Parodi et al 26 | 2015 | Italy | Assessment of LLM classification accuracy and comparison with three alternative methods (Decision Tree (DT), K-Nearest Neighbors (KNN), and Artificial Neural Network (ANN)) using leave-one-out cross-validation | Logic Learning Machine (LLM) | 1 | Diagnosis | _ The LLM model outperformed other models _ Provide helpful classification rules |
| Podolsky et al 27 | 2016 | USA | Assessing the efficiency of machine learning (ML) algorithms in categorizing lung cancer using gene expression levels | KNN, Naïve Bayes (NB), Support Vector Machine (SVM), C4.5 DT | 4 | Tumor Segmentation, Diagnosis | SVM achieved better accuracy than other models in classification |
| Gudmundsson et al 28 | 2018 | USA | Exploring the use of U-Net deep CNN architecture in deep convolutional neural networks (DCNNs) for the automated segmentation of MPM tumors in CT scan images | DCNN (U-Net architecture) | 1 | Tumor Segmentation | The DCNN-based approach yielded notably higher dice similarity coefficient (DSC) scores |
| Mukherjee 29 | 2018 | India | _ Discovering the optimal classifier _ Identifying crucial input variables for the detection of malignant mesothelioma disease |
SVM, Multi-Layer Perceptron (MLP) neural network | 2 | Diagnosis | SVM obtained better classification accuracy |
| Hu and Yu 21 | 2018 | China | Creating a deep learning approach to diagnose malignant mesothelioma automatically | Back Propagation (BP), Extreme Learning Machine (ELM), Stacked Sparse Auto-Encoders (SSAE), Genetic algorithm (GA), Relief-F | 5 | Diagnosis | SSAE and GA + SSAE models obtained the best classification accuracy |
| Win et al 30 | 2018 | Japan | Performance assessment of different supervised ML models for the detection of malignant mesothelioma | Linear Discriminant Analysis (LDA), NB, SVM, DT, KNN, Logistic Regression (LogR), Random Forest (RF) | 7 | Tumor Segmentation, Diagnosis | The SVM, DT, LogR, and RF techniques obtained 100% accuracy, sensitivity, and specificity in diagnosing the disease |
| Chicco and Rovelli 31 | 2019 | Canada | Predicting the diagnosis and selecting features in the medical records of a patient with mesothelioma using computational models | Probabilistic Neural Network (PNN), perceptron-based NN, DT, RF, One Rule | 5 | Diagnosis | RFs outperformed the other models |
| Gudmundsson et al 32 | 2020 | USA | Exploring the application of deep learning in segmenting MPM tumors in CT scans | DCNN | 1 | Tumor Segmentation | The suggested approach obtained an average DSC of 0.690 in the tumor and effusion test set |
| Latif et al 33 | 2021 | Pakistan | Identification of factors that increase the risk of developing malignant mesothelioma | Apriori algorithm | 1 | Prevention | The algorithm has predicted the risk factors associated with malignant mesothelioma |
| Li et al 34 | 2021 | China | Developing a diagnostic approach involving the use of plasma-based metabolomics combined with ML algorithms to detect malignant mesothelioma | RF | 1 | Diagnosis | The RF algorithm achieved 100% specificity |
| Kitajima et al 22 | 2021 | Japan | Analysis of AI deep learning model using 3D DCNN for differentiating MPM from benign pleural disease based on FDG-PET/CT results | VGG12, 3D DCNN | 2 | Diagnosis | PET/CT, SUVmax, gender, and age combination outperformed other protocols with an accuracy of 0.82 |
| Zauderer et al 35 | 2021 | USA | Development and validation of OncoCast MPM | OncoCast algorithm | 1 | Prognosis | OncoCast-MPM provided precise risk assessment scores for patients with MPM |
| Naso et al 36 | 2021 | Canada | _ Investigating whether deep learning can accurately distinguish between benign and malignant spindle cell mesothelial proliferation _ Analyzing the features used for prediction by a trained NN |
Convolutional Neural Network (CNN) (called “SpindleMesoNET”) | 1 | Diagnosis | SpindleMesoNET accurately distinguishes sarcomatoid mesothelioma from benign spindle cell mesothelioma |
| Alam et al 19 | 2021 | Australia | Examining prognostic factors in malignant mesothelioma across clinical, radiological, and histopathological aspects | Growth algorithm, Apriori model | 2 | Diagnosis | Improved early detection and more effective management of malignant mesothelioma |
| Choudhury et al 18 | 2021 | USA | Predicting mesothelioma cancer using supervised ML | Stochastic Gradient Descent (SGD), Adaptive Boosting M1, Kernel Logistic Regression (KLR), MLP, Voted Perceptron (VP), Hoeffding Tree, Clojure Classifier (CC), Primal Estimated sub-Gradient Solver for SVM | 8 | Diagnosis | _ In Phase 1, SGD, Adaptive Boosting (AdaBoost), M1, KLR, MLP, and Very Fast Decision Trees (VFDT) achieved optimal results with 100% classification accuracy _ AdaBoost performed the best in Phase 2, achieving a classification accuracy of 71.29% |
| Xiao et al 37 | 2022 | China | Investigating the link between glycolytic pathway genes and the prognosis of MPM through the construction of prognostic risk models using bioinformatics and ML | SVM, RF | 2 | Prognosis | A risk model based on six glycolytic genes (with Area Under Curve (AUC) = 0.830) was developed to predict the prognosis of MPM patients |
| Massafra et al 38 | 2022 | Italy | Identifying clinical characteristics of advanced pleural mesothelioma patients and exploring the potential link between prognosis and treatment results | The sequential forward selection algorithm | 1 | Prognosis | A positive correlation exists between advanced pleural mesothelioma patients and their response to innovative therapies |
| Li et al 39 | 2022 | China | Developing a ML model to differentiate MPM from other malignant pleural diseases using early CT signs | multivariate LogR | 1 | Diagnosis | The proposed model can differentiate between MPM and malignant pleural disease, serving as a diagnostic tool |
| Kidd et al 40 | 2022 | Scotland | Comparison of tumor volume responses using manual human assessment and fully automated AI algorithm on CT scans of 30 patients before and after chemotherapy | CNN | 1 | Tumor volumetric measurement of MPM | Agreement between human and AI volume responses was observed in 20 out of 30 cases (67%) with a kappa value of 0.439 (0.178 to 0.700) |
The Geographical and Temporal Distribution of Included Studies
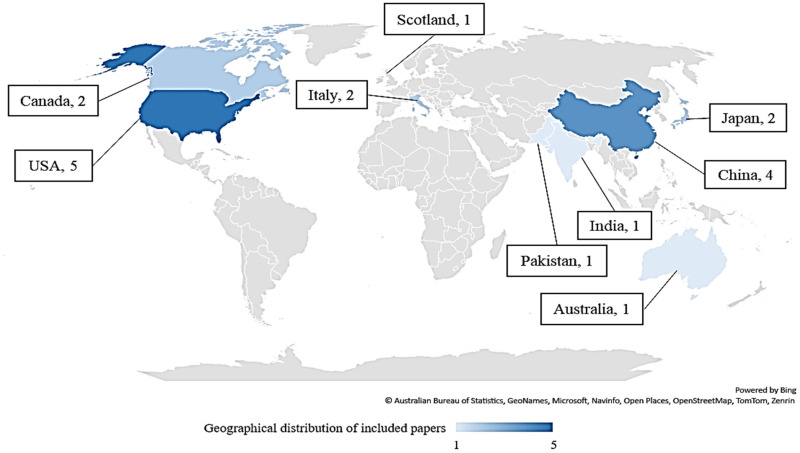
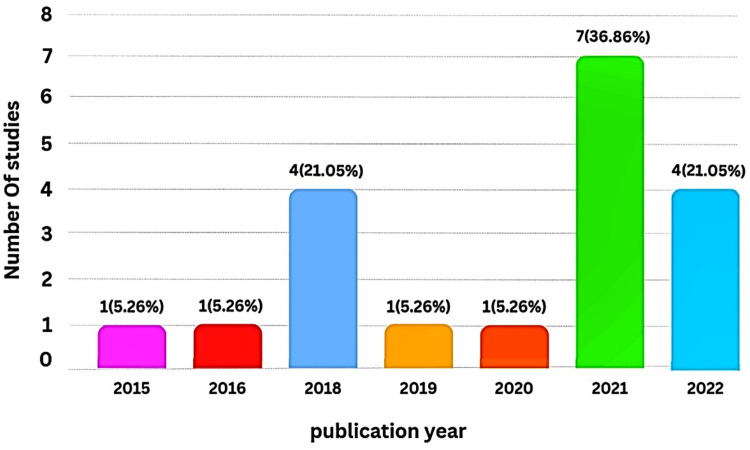
The highest frequency of articles was related to the USA (n = 5, 26.32%),18,27,28,32,35 followed by China (n = 4, 21.05%)21,34,37,39 (Figure 2). The year of publication of articles varied from 2015 to 2022; no relevant articles were found that were published in 2017. The most frequent publication of articles was in 2021 (n = 7, 36.86%),18,19,22,33–36 followed by 202237–40 and 201821,28–30 (n = 4, 21.05%) (Figure 3).
Figure 2.
The Geographical Distribution of Included Studies.
Figure 3.
Temporal Distribution of Included Studies.
AI Application for Mesothelioma
Figure 4 illustrates that the most common application of AI in mesothelioma management was tumor diagnosis and classification. As shown in this figure, AI has been used for tumor diagnosis and classification (n = 14, 73.69%),18,19,21,22,26–32,34,36,39 prevention and prognosis (n = 4, 21.05%),33,35,37,38 and tumor volumetric measurement of MPM (n = 1, 5.26%). 40
Figure 4.
AI Application for Mesothelioma.
AI Models Used in the Prediction of Mesothelioma
According to Figure 5, the most commonly used AI models include various neural networks (NN) (n = 9), decision trees (DT) and random forests (RF) (n = 8), statistical models such as logistic regression (LogR) and Naïve Bayes (NB) (n = 7), and support vector machines (SVM) (n = 4). Furthermore, GAs, correlation-based algorithms, and NN were used to determine risk factors and feature selection.
Figure 5.
Frequency of Different AI Models Used in the Management of Mesothelioma.
Comparison of Included Studies
The comparison of various AI models for diagnosing mesothelioma reveals significant advancements in accuracy and efficacy across different studies. For instance, Mukherjee 29 utilized SVM on a dataset of 324 patients, achieving an impressive accuracy of 99.87% through metrics such as sensitivity and AUC. Similarly, Hu and Yu 21 employed a SSAE with 97 patients and 227 healthy controls, achieving 100% accuracy. Win et al 30 demonstrated the effectiveness of multiple models, including DT and RF, on the same patient cohort, securing a perfect accuracy rate. In contrast, Chicco and Rovelli 31 reported a lower accuracy of 75% using RF on clinical data from 192 patients. Choudhury et al 18 utilized AdaBoost on 324 patients, achieving an accuracy of 71.29%. Notably, this study reported the lowest accuracy among the reviewed studies. These studies collectively highlight the potential of machine learning techniques, particularly deep learning models, to enhance diagnostic performance, identify critical biomarkers, and improve patient outcomes in mesothelioma detection (Table 3).
Table 3.
Comparison of Included Studies.
| Authors | Dataset | Application | Data type | Best proposed model | Accuracy of the proposed model | Model evaluation methods |
|---|---|---|---|---|---|---|
| Mukherjee 29 | 324 patients | Diagnosis | Biomarkers | SVM | 99.87 | Sensitivity, Accuracy, AUC, F-score |
| Hu and Yu 21 | 97 patients with MM and 227 healthy controls | Diagnosis | Biomarkers | SSAE | 100 | Sensitivity, Accuracy, AUC, F-score |
| Win et al 30 | 324 patients | Diagnosis | Biomarkers | DT, SVM, LogR, and RF | 100 | Sensitivity, Specificity, Accuracy, Recall, F-score, Precision |
| Chicco and Rovelli 31 | 192 patients | Diagnosis | Clinical Data, Biomarkers | RF | 75 | MCC, Sensitivity, Specificity, Accuracy, F-score |
| Choudhury et al 18 | 324 patients | Diagnosis, Prognosis | Clinical Data, Biomarkers | AdaBoost | 71.29 | Sensitivity, Accuracy, Specificity, AUC |
| Li et al 39 | 297 patients | Diagnosis | Clinical data and CT signs | multivariate LogR | Not Mentioned | AUC, F-score |
| Kitajima et al 22 | Training: 525 patients Test: 176 patients |
Diagnosis | PET/CT scan, clinical features | 3D DCNN | 82.4 | Sensitivity, Accuracy, Specificity, AUC |
| Gudmundsson et al 28 | Training: 2663 CT scan sections from 76 scans from 61 patients Test: 2567 CT scan sections from 78 scans from 65 patients |
Classification | CT scan | DCNN (U-Net) | Not Mentioned | DSC |
| Gudmundsson et al 32 | Training: 154 CT scans of 126 patients Test: 224 CT scans of 77 patients |
Classification | CT scan | CNN | Not Mentioned | Bland-Altman Method, DSC |
| Kidd et al 40 | Training: 108 patients Test: 30 patients |
Classification and tumor volume measurement | Tumor morphological features, CT scan | DCNN (U-Net) | Not Mentioned | Bland-Altman Method, DSC |
| Li et al 34 | 25 patients with MM and 32 healthy controls | Prediction | Plasma samples | RF | 100 | Accuracy, AUC |
| Zauderer et al 35 | 194 patients | Prognosis, Prediction | Clinical, pathological and genomic data | Oncocast-MPM | Not Mentioned | OncoPrint charts |
| Xiao et al 37 | 84 patients | Prognosis | Gene expression data | RF | Not Mentioned | AUC |
| Massafra et al 38 | 46 patients | Prediction | Clinical Data, Biomarkers | The sequential forward selection algorithm | 75 | Sensitivity, Accuracy, Specificity, AUC |
| Latif et al 33 | 234 participants (96 patients and 128 healthy controls) | Discovering the association rules of risk factors | Clinical Data | Apriori | Not Mentioned | Support, Confidence, and Lift |
| Alam et al 19 | 96 patients with MM and 228 healthy controls | Discovering the association rules of risk factors | Critical risk factors | Apriori | Not Mentioned | Support, Confidence, and Lift |
Moreover, Table 3 reveals that in disease classification using CT scans, the most widely used models and algorithms were DCNNs, and these models were mainly evaluated using the Bland-Altman and DSC methods.28,32,40 RF and SVM were the most widely used machine learning models and algorithms for disease diagnosis using clinical data and vital biomarkers,29–31 outperforming other methods and algorithms. Furthermore, the RF was the most widely used model in disease prediction and prognosis using gene expression data and clinical parameters.34,37
Comparison of Accuracies Obtained from Different AI Models
Table 4 presents the accuracy of various models used to diagnose and classify mesothelioma. Among the most used models in this field, the SVM model was used four times with an accuracy of 99.97%; the RF model was used four times with an accuracy of 93.75%; five times the DT model with an accuracy of 88.36%; and three times the NB model with an accuracy of 78.3%. In addition, in the two studies that used the LogR model, the accuracy was 100%. In the two articles that used the KNN model, the accuracy was 91.68%.
Table 4.
Comparison of Accuracies Obtained from Different AI Models.
| First author | Model type | Model accuracy (%) |
|---|---|---|
| Chicco 31 | RF | 75 |
| DT | 69 | |
| MLP | 52 | |
| PNN | 57 | |
| Mukherjee 29 | SVM | 99.87 |
| MPE | 99.56 | |
| Artificial Immune Systems (AIS) | 97.7 | |
| PNN | 96.3 | |
| MLP | 94.41 | |
| NN | 91.3 | |
| Learning Vector Quantization (LVQ) | 91.14 | |
| Li 34 | RF | 100 |
| ELM | 93.9 | |
| BP | 94.9 | |
| GA + BP | 98 | |
| GA + ELM | 98 | |
| SSAE | 100 | |
| GA + SSAE | 100 | |
| Li 39 | Multivariate LogR | 83 |
| Kitajima 22 | 3D DCNN | 77.3% |
| Naso 36 | SpindleMesoNET | 92.5% |
| Parodi 26 | LLM | 77.5 |
| DT | 72.8 | |
| ANN | 63.9 | |
| LLM | 54.4 | |
| Win 30 | LDA | 61.73 |
| NB | 67.90 | |
| KNN | 91.36 | |
| SVM | 100 | |
| DT | 100 | |
| LogR | 100 | |
| RF | 100 |
Discussion
This study investigated the state of AI research on mesothelioma prediction. Researchers have utilized AI in tumor diagnosis, classification, prevention, prognosis, and mesothelioma treatment. The SVM model achieved an impressive average accuracy of 99.97%. In comparison, the RF model had an average accuracy of 93.75%, while the DT model averaged 88.36%. Additionally, the NB model recorded an average accuracy of 78.3%. Notably, the two studies that employed the LogR model both reported an average accuracy of 100%.
Furthermore, the average accuracy in the two articles that utilized the KNN model was 91.68%. The most common uses of AI applications for mesothelioma were tumor diagnosis and classification, prevention and prognosis, and tumor volumetric measurement of MPM. These models and applications are discussed below.
Application of AI in Tumor Classification and Diagnosis
In a study by Koul et al, 41 AI methods have shown superior predictive abilities for airway disorders compared to pulmonologists. They suggested that linear regression, SVM, RF, DT, NB, LogR, and fuzzy particles, the most commonly used machine learning models in this field, can identify airway conditions like pulmonary edema, COVID-19, lung cancer, asthma, and mesothelioma. The results of this study align with our findings.
Win et al 30 conducted a study that utilized different ML techniques under the proper supervision of LDA, NB, SVM, KNN, DT, LogR, and RF to diagnose malignant mesothelioma. In this study, the performance of these techniques was evaluated in diagnosing mesothelioma, and the researchers found that RF, LogR, SVM, and DT models can diagnose the disease with an accuracy, sensitivity, and specificity of 100%. These models can be used as decision support systems for physicians to diagnose malignant mesothelioma. In Khan et al's study, 42 the efficiency of the SVM model for mesothelioma diagnosis was 98%. In addition, the study of Mukherjee et al, 29 which was conducted to diagnose malignant mesothelioma using data mining techniques, showed that the SVM algorithm achieves the best classification with an accuracy of 99.87% through ten cross-validation in 5 times runs compared to the MLP neural network, which has been obtained with 99.56% classification accuracy. In another study, Li et al 34 conducted a study to improve the prognosis of malignant mesothelioma by optimizing clinical diagnosis. This study employed a combined untargeted metabolomic strategy and ML models to investigate the differential metabolites in plasma samples from malignant mesothelioma patients compared to age- and sex-matched healthy controls (HCs). Both metabolite-based ROC models and multiple metabolite-based RF models distinguished between malignant mesothelioma and HC to better clarify the diagnostic significance of these metabolites. They found that the RF algorithm used in this study could diagnose malignant mesothelioma with 100% sensitivity. In addition, the combination of metabolomics and ML revealed outstanding diagnostic value for malignant mesothelioma and indicated a new and effective strategy for diagnosing this disease.
Chicco et al, 31 in a study with the aim of computational prediction of diagnosis and feature selection in the health records of patients with mesothelioma, analyzed the health records of 324 Turkish patients with symptoms of mesothelioma who were previously exposed to asbestos and showed symptoms consistent with mesothelioma. They compared PNNs, RFs, and DTs to predict the diagnosis of patient records. This study demonstrated that ML can predict the diagnosis of mesothelioma patients with high accuracy, sensitivity, and specificity in a few minutes. In addition, RF can efficiently identify the most critical features of this clinical dataset (lung side and platelet count) in just a few seconds. Pleural plaques and blood platelets are significant in diagnosing mesothelioma, so doctors should concentrate on these two features when reviewing the records of patients with mesothelioma symptoms. As AI techniques evolve, there is a growing trend towards combining different AI models to improve classification accuracy, such as hybrid models combining deep learning and traditional machine learning algorithms. Furthermore, a new classification method based on histological features and imaging data could emerge, potentially providing more personalized predictions for patients. These findings emphasize the transformative potential of AI in healthcare, particularly in mesothelioma diagnosis, and underscore the significance of leveraging advanced computational techniques for improved patient outcomes.
Application of AI in Tumor Volumetric Measurement
Tumor volume is relevant for prognostic assessment, treatment response assessment, and MPM staging. In a study, Koul et al 41 stated that CNNs are often used as deep-learning models for disease diagnosis and tumor volume assessment. Two of the 19 studies we reviewed also identified this issue. In another study, Gudmundsson et al 32 implemented a deep learning-based method for automatically segmenting mesothelioma tumors in CT scans to improve segmentation performance in scans of patients who presented with pleural effusion. Deep CNNs were developed using a two-dimensional U-Net architecture to segment left and right hemithorax tumors. These networks utilized layers that were pre-trained on the ImageNet dataset. The training was conducted using a dataset of 5230 axial sections taken from 154 CT scans of 126 mesothelioma patients. This study demonstrated improved segmentation performance for deep learning-based mesothelioma segmentation using an extensive validation method and a more diverse set of training segments representing both tumor and pleural effusion. They agreed strongly with supervised tumor lines regarding segmentation overlap and the average distance between computerized and manual tumor lines.
Kidd et al 40 measured and compared the volumetric responses of the primary tumor using a fully automatic algorithm of AI, manually by humans, and on CT scans of 30 patients before and after the chemotherapy according to modified RECIST criteria by an MPM-certified radiologist. In a multicenter retrospective cohort study, 183 CT datasets were divided into training internal validation and external validation. Comprehensive manual annotations were utilized to train the CNN using a two-dimensional U-Net architecture. CNN's performance was assessed using correlation, Bland-Altman analysis, and Dice agreement. These results were compared with the mRecist using Cohen's Kappa. Survival was also evaluated using the Kaplan-Meier method, as a result of which human and AI volumetric responses agreed in 30/20 (67%) validation cases, κ = 0.439 (0.178 to 0.700). AI and mRecist agreed in 16/30 (55%) validation cases, κ = 0.284 (0.026 to 0.543). A higher baseline tumor volume was associated with shorter survival. In the independent validation set comprising 60 CT datasets, the mean difference between tumor volumes measured by AI and humans was not significantly different from zero.
The application of AI in tumor volumetric measurement, particularly in the context of MPM, holds significant promise for enhancing diagnostic accuracy and treatment assessment. Studies such as those by Koul et al, 41 Gudmundsson et al, 32 and Kidd et al 40 demonstrate the efficacy of deep learning models, particularly CNNs, in accurately segmenting tumors and measuring their volumes from CT scans. A notable trend in AI for tumor volumetric measurement is the increased focus on multi-modality imaging, where AI models combine CT and MRI scans to enhance measurement accuracy. These advancements streamline the assessment process and offer insights into treatment response and prognostic evaluation. Moreover, the agreement between AI-generated volumetric measurements and manually obtained ones highlights the reliability and potential clinical utility of AI-driven approaches in oncology. As a result, AI could lead to the development of real-time volumetric monitoring systems that can assist clinicians in making timely treatment decisions. As research in this field continues to evolve, integrating AI into routine clinical practice can significantly improve patient care and outcomes in managing MPM and other cancers.
Application of AI in Disease Prevention and Prognosis
Choudhury et al 18 illustrated that the diagnostic approach (including biopsy and imaging findings), C-reactive protein, duration of symptoms, gender, pleural protein, platelet count, and pleural protein are crucial for diagnosing pleural mesothelioma. However, effective diagnostic procedures such as thoracoscopy, laparoscopy, thoracotomy, laparotomy, and imaging tests (CT scan and MRI) are expensive. This study recommends AdaBoost models for pleural mesothelioma prognosis. Alam et al 19 proposed a new framework for identifying prognostic indicators using non-invasive and cost-effective methods based on different techniques. It assists doctors, patients, physicians, and other healthcare professionals in early diagnosis and better treatment of malignant mesothelioma, considering critical prognostic factors. In a study, Latif et al 33 identified the risk factors associated with malignant mesothelioma. They found that the algorithm obtained 75% support, and the proposed algorithm successfully predicted the risk factors for malignant mesothelioma. LLM is an innovative method for analyzing supervised data to build classifiers with understandable rules, including simple conditions on their priors. It efficiently implements the switching NN model, achieving excellent classification accuracy while decreasing computational demand.
Parodi et al, 26 in their study aimed at the differential diagnosis of malignant mesothelioma using three tumor markers (CEA, CYFRA 21-1, and SMRP), concluded that LLM is an innovative method that can offer helpful classification rules from the complex multivariate correlation between the different analyzed features and provide classification rules in excellent agreement with the previous knowledge about the biological role of mesothelial proliferation. Accurate differentiation of benign and malignant spindle cells can be an essential diagnostic challenge for pathologists. Naso et al 36 used the SpindleMesoNET NN in their study to distinguish between sarcomatoid mesothelioma and benign spindle cell mesothelial proliferation. The SpindleMesoNET NN predicted cases’ benign or malignant status with AUCs of 0.932, 0.925, and 0.989 in cross-validation, reference, and external validation sets, respectively. The accuracy of this trained NN in the reference set cases was 92.5%, higher than the average accuracy of 91.7% of three experienced pathologists in a slide set. As a result, SpindleMesoNET can accurately distinguish sarcomatoid mesothelioma from benign spindle cell mesothelial proliferation. In disease prevention and prognosis, AI models are beginning to explore integrating genomic and histopathological data to refine prognostic predictions. Furthermore, novel algorithms for dynamic prediction modeling are emerging, which allow real-time updates based on patient data, improving the accuracy of prognosis over time.
Therefore, in disease prevention and prognosis, the integration of AI demonstrates promising potential across various aspects of diagnosing and managing malignant mesothelioma. Studies by Choudhury et al, 18 Alam et al, 19 and Latif et al 33 underscore ML's importance in predicting cancer and finding critical prognostic indicators. This approach will help with early diagnosis and better treatment plans. Remarkably, the implementation of AdaBoost models and innovative frameworks highlights AI's role in harnessing non-invasive and cost-effective methods for prognostic assessment. Also, improvements in AI algorithms like the LLM were studied by Parodi et al, 26 help with differential diagnosis by giving clinicians easy-to-understand classification rules that help them correctly tell the difference between diseases. Furthermore, the development of specialized NNs such as SpindleMesoNET, as demonstrated by Naso et al, 36 showcases AI's potential in accurately distinguishing between malignant and benign mesothelial proliferations, potentially surpassing the accuracy of human pathologists. These findings collectively underscore the transformative impact of AI in enhancing disease prevention, prognosis, and, ultimately, patient care in the context of malignant mesothelioma.
Study Limitations and Future Research Directions
The current study has a few notable limitations that must be addressed in future research. Firstly, we considered only studies written in English, potentially excluding valuable research conducted in other languages. Secondly, our review was limited to three databases (PubMed, Scopus, and Web of Science), which may have resulted in overlooking relevant literature available from additional reputable sources. Future research should address these limitations by including studies published in other languages and expanding the search to encompass a broader range of databases, thereby enhancing the overall comprehensiveness of the review.
In addition to these limitations, our review highlights several open research problems that offer fertile ground for further investigations. The relatively few studies and limited sample sizes underscore the need for larger, multicenter investigations to validate and refine AI-driven diagnostic and prognostic models for mesothelioma. While SVM, DT, and RF models have demonstrated high accuracy, exploring novel deep learning architectures and ensemble methods could improve predictive performance and clinical applicability. Moreover, standardizing evaluation metrics, datasets, and feature selection techniques is essential to enhance the comparability and reproducibility of research outcomes. Finally, integrating multimodal data, including imaging, genetic, and clinical parameters, may pave the way for more comprehensive and personalized approaches to mesothelioma management. Addressing these challenges will advance the field and foster innovations that improve patient outcomes.
Conclusions
This scoping review highlights the application of AI in diagnosing and managing mesothelioma. The primary applications of AI in these studies included tumor diagnosis and classification, prevention and prognosis, and tumor volumetric measurement of malignant pleural mesothelioma. The most commonly used AI models were neural networks, decision trees, random forests, logistic regression, Naïve Bayes, and SVM. Among these, SVM, DT, and RF showed high accuracy.
The findings suggest that AI, particularly machine learning models such as neural networks, decision trees, support vector machines, and random forests, has significant potential in improving mesothelioma prediction and management. These models offer promising accuracy for early detection, which could lead to improved patient outcomes. Future research should focus on refining these models, improving their generalizability across diverse patient populations, and integrating them into clinical practice to enhance diagnostic precision. Furthermore, the use of genetic and correlation-based algorithms for risk factor identification and feature selection could be explored further to optimize the performance of AI systems in mesothelioma prediction.
Abbreviations
- AI
Artificial Intelligence
- MPM
Malignant Pleural Mesothelioma
- NN
Neural Network
- DT
Decision Tree
- RF
Random Forest
- LogR
Logistic Regression
- NB
Naïve Bayes
- GA
Genetic Algorithm
- ML
Machine Learning
- SVM
Support Vector Machine
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews
- MeSH
Medical Subject Headings
- PROBAST
Prediction Model Study Risk of Bias Assessment Tool
- KNN
K-Nearest Neighbors
- ANN
Artificial Neural Network
- LLM
Logic Learning Machine
- CE
Cytology Examination
- BE
Benign Disease
- MLPE
Multilayer Perceptron Ensemble
- ALP
Alkaline Phosphatase
- HU
Hounsfield Unit
- SUVMax
Standardized Uptake Value
- MSK-IMPACT
Memorial Slean Kettering
- DCNN
Deep Convolutional Neural Networks
- DSC
Dice Similarity Coefficient
- MLP
Multi-Layer Perceptron
- BP
Back Propagation
- ELM
Extreme Learning Machine
- SSAE
Stacked Sparse Auto-Encoders
- LDA
Linear Discriminant Analysis
- PNN
Probabilistic Neural Network
- CNN
Convolutional Neural Network
- SGD
Stochastic Gradient Descent
- KLR
Kernel Logistic Regression
- VP
Voted Perceptron
- CC
Clojure Classifier
- AUC
Area Under Curve
- AIS
Artificial Immune Systems
- LVQ
Learning Vector Quantization
- HC
Healthy Control
- ROC
Receiver Operating Characteristic
- mRECIST
Modified Response Evaluation Criteria in Solid Tumors
- ESR
Erythrocyte Sedimentation Rate
- LDH
Lactic Dehydrogenase
- BMI
Body Mass Index
- CPP
Calcified Pleural Plaque
- CEA
Carcinoembryonic Antigen
- TCGA
The Cancer Genome Atlas
Footnotes
ORCID iD: Erfan Esmaeeli https://orcid.org/0009-0004-8229-5232
Authors’ Contributions: MR, MRA, AG, AS: Conceptualization, Methodology, Software. MR, EE, MSKH, KM, PA, and AS: Data curation, Writing- Original draft preparation. MRA, KM, MSKH, EE, and AG: Visualization, Investigation. KM, and AS: Supervision.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
References
- 1.Tipu SA, Ahmed I, Ishtiaq S. Malignant mesothelioma. Pak J Med Sci. 2013;29(6):1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis. 2013;5(6):E254–E307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SC, Dowell JE. Modern management of malignant pleural mesothelioma. Lung Cancer: Targets Ther. 2016;7:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Mazurek JM, Li Y, et al. Industry, occupation, and exposure history of mesothelioma patients in the US national mesothelioma virtual bank, 2006-2022. Environ Res. 2023;230:115085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Klerk N, Brims F, Reid A, et al. Epidemiology of malignant pleural mesothelioma in Australia. Malignant Pleural Mesothelioma: Present Status and Furture Directions. 2016;45(Suppl 1):83–95. [Google Scholar]
- 6.Sahu RK, Ruhi S, Jeppu AK, et al. Malignant mesothelioma tumours: Molecular pathogenesis, diagnosis, and therapies accompanying clinical studies. Front Oncol. 2023;13:1204722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remon J, Reguart N, Corral J, Lianes P. Malignant pleural mesothelioma: New hope in the horizon with novel therapeutic strategies. Cancer Treat Rev. 2015;41(1):27–34. [DOI] [PubMed] [Google Scholar]
- 8.Abd-Elmawla MA, Mageed SSA, Al-Noshokaty TM, et al. Melodic maestros: Unraveling the role of miRNAs in the diagnosis, progression, and drug resistance of malignant pleural mesothelioma. Pathol-Res Pract. 2023;250:154817. [DOI] [PubMed] [Google Scholar]
- 9.Strange CD, Marom EM, Ahuja J, et al. Imaging of malignant pleural, pericardial, and peritoneal mesothelioma. Adv Anat Pathol. 2023;30(4):280–291. [DOI] [PubMed] [Google Scholar]
- 10.Bibby AC, Tsim S, Kanellakis N, et al. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25(142):472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Ma K, Chen Z, et al. A nomogram to predict prognosis in malignant pleural mesothelioma. World J Surg. 2018;42:2134–2142. [DOI] [PubMed] [Google Scholar]
- 12.Hassan E, Abd El-Hafeez T, Shams MY. Optimizing classification of diseases through language model analysis of symptoms. Sci Rep. 2024;14(1):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunette ES, Flemmer RC, Flemmer CL. A review of artificial intelligence. In: 2009 4th international conference on autonomous robots and agents: 2009. Ieee, 2009, pp.385–392. [Google Scholar]
- 14.Eliwa EHI, El Koshiry AM, Abd El-Hafeez T, Omar A. Secure and transparent lung and colon cancer classification using blockchain and microsoft azure. Adv Respir Med. 2024;92(5):395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mostafa G, Mahmoud H, Abd El-Hafeez T, ElAraby ME. Feature reduction for hepatocellular carcinoma prediction using machine learning algorithms. J Big Data. 2024;11(1):88. [Google Scholar]
- 16.Abd El-Hafeez T, Shams MY, Elshaier YA, Farghaly HM, Hassanien AE. Harnessing machine learning to find synergistic combinations for FDA-approved cancer drugs. Sci Rep. 2024;14(1):2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sayed MA, Hafeez TA-E. New edge detection technique based on the shannon entropy in gray level images. arXiv preprint arXiv:12112502 2012.
- 18.Choudhury A. Predicting cancer using supervised machine learning: Mesothelioma. Technol Health Care. 2021;29(1):45–58. [DOI] [PubMed] [Google Scholar]
- 19.Alam TM, Shaukat K, Hameed IA, et al. A novel framework for prognostic factors identification of malignant mesothelioma through association rule mining. Biomed Signal Process Control. 2021;68:102726. [Google Scholar]
- 20.Courtiol P, Maussion C, Moarii M, et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med. 2019;25(10):1519–1525. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Yu Z. Diagnosis of mesothelioma with deep learning. Oncol Lett. 2019;17(2):1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitajima K, Matsuo H, Kono A, et al. Deep learning with deep convolutional neural network using FDG-PET/CT for malignant pleural mesothelioma diagnosis. Oncotarget. 2021;12(12):1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salle F, Le Stang N, Tirode F, et al. Comprehensive molecular and pathologic evaluation of transitional mesothelioma assisted by deep learning approach: A multi-institutional study of the international mesothelioma panel from the MESOPATH reference center. J Thorac Oncol. 2020;15(6):1037–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 25.Moons KG, Wolff RF, Riley RD, et al. PROBAST: A tool to assess risk of bias and applicability of prediction model studies: Explanation and elaboration. Ann Intern Med. 2019;170(1):W1–W33. [DOI] [PubMed] [Google Scholar]
- 26.Parodi S, Filiberti R, Marroni P, et al. Differential diagnosis of pleural mesothelioma using logic learning machine. BMC Bioinformatics. 2015;16(9):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podolsky MD, Barchuk AA, Kuznetcov VI, Gusarova NF, Gaidukov VS, Tarakanov SA. Evaluation of machine learning algorithm utilization for lung cancer classification based on gene expression levels. Asian Pac J Cancer Prev. 2016;17(2):835–838. [DOI] [PubMed] [Google Scholar]
- 28.Gudmundsson E, Straus CM, Armato SG, III. Deep convolutional neural networks for the automated segmentation of malignant pleural mesothelioma on computed tomography scans. J Med Imaging 2018;5(3):034503–034503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee S. Malignant mesothelioma disease diagnosis using data mining techniques. Appl Artif Intell. 2018;32(3):293–308. [Google Scholar]
- 30.Win KY, Maneerat N, Choomchuay S, Sreng S, Hamamoto K. Suitable supervised machine learning techniques for malignant mesothelioma diagnosis. In: 2018 11th biomedical engineering international conference (BMEiCON): 2018. IEEE, 2018, pp.1–5. [Google Scholar]
- 31.Chicco D, Rovelli C. Computational prediction of diagnosis and feature selection on mesothelioma patient health records. PloS One. 2019;14(1):e0208737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudmundsson E, Straus CM, Li F, Armato SG, III. Deep learning-based segmentation of malignant pleural mesothelioma tumor on computed tomography scans: Application to scans demonstrating pleural effusion. J Med Imaging 2020;7(1):012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latif MZ, Shaukat K, Luo S, Hameed IA, Iqbal F, Alam TM. Risk factors identification of malignant mesothelioma: a data mining based approach. In: 2020 international conference on electrical, communication, and computer engineering (ICECCE): 2021. IEEE, 2021, pp.1–6. [Google Scholar]
- 34.Li N, Yang C, Zhou S, et al. Combination of plasma-based metabolomics and machine learning algorithm provides a novel diagnostic strategy for malignant mesothelioma. Diagnostics. 2021;11(7):1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zauderer MG, Martin A, Egger J, et al. The use of a next-generation sequencing-derived machine-learning risk-prediction model (OncoCast-MPM) for malignant pleural mesothelioma: A retrospective study. Lancet Digit Health. 2021;3(9):e565–e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naso JR, Levine AB, Farahani H, et al. Deep-learning based classification distinguishes sarcomatoid malignant mesotheliomas from benign spindle cell mesothelial proliferations. Mod Pathol. 2021;34(11):2028–2035. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y, Huang W, Zhang L, Wang H. Identification of glycolysis genes signature for predicting prognosis in malignant pleural mesothelioma by bioinformatics and machine learning. Front Endocrinol (Lausanne). 2022;13:1056152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massafra R, Catino A, Perrotti PMS, et al. Informative power evaluation of clinical parameters to predict initial therapeutic response in patients with advanced pleural mesothelioma: A machine learning approach. J Clin Med. 2022;11(6):1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Cai B, Wang B, et al. Differentiating malignant pleural mesothelioma and metastatic pleural disease based on a machine learning model with primary CT signs: A multicentre study. Heliyon. 2022;8(11):e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidd AC, Anderson O, Cowell GW, et al. Fully automated volumetric measurement of malignant pleural mesothelioma by deep learning AI: Validation and comparison with modified RECIST response criteria. Thorax. 2022;77(12):1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koul A, Bawa RK, Kumar Y. Artificial intelligence techniques to predict the airway disorders illness: A systematic review. Arch Comput Methods Eng. 2023;30(2):831–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan SN, Sikander G, Anwar S, Khan MT. Classification of malignant mesothelioma cancer using support vector machine. In: 2018 international conference on computing, mathematics and engineering technologies (iCoMET): 2018. IEEE, 2018, pp.1–5. [Google Scholar]