Abstract
Background
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex disorder characterized by persistent fatigue and cognitive impairments, with emerging evidence highlighting the role of gut health in its pathophysiology. The main objective of this review was to synthesize qualitative and quantitative data from research examining the gut microbiota composition, inflammatory markers, and therapeutic outcomes of interventions targeting the microbiome in the context of ME/CFS.
Methods
The data collection involved a detailed search of peer-reviewed English literature from January 1995 to January 2025, focusing on studies related to the microbiome and ME/CFS. This comprehensive search utilized databases such as PubMed, Scopus, and Web of Science, with keywords including “ME/CFS,” “Gut-Brain Axis,” “Gut Health,” “Intestinal Dysbiosis,” “Microbiome Dysbiosis,” “Pathophysiology,” and “Therapeutic Approaches.” Where possible, insights from clinical trials and observational studies were included to enrich the findings. A narrative synthesis method was also employed to effectively organize and present these findings.
Results
The study found notable changes in the gut microbiota diversity and composition in ME/CFS patients, contributing to systemic inflammation and worsening cognitive and physical impairments. As a result, various microbiome interventions like probiotics, prebiotics, specific diets, supplements, fecal microbiota transplantation, pharmacological interventions, improved sleep, and moderate exercise training are potential therapeutic strategies that merit further exploration.
Conclusions
Interventions focusing on the gut-brain axis may help reduce neuropsychiatric symptoms in ME/CFS by utilizing the benefits of the microbiome. Therefore, identifying beneficial microbiome elements and incorporating their assessments into clinical practice can enhance patient care through personalized treatments. Due to the complexity of ME/CFS, which involves genetic, environmental, and microbial factors, a multidisciplinary approach is also necessary. Since current research lacks comprehensive insights into how gut health might aid ME/CFS treatment, standardized diagnostics and longitudinal studies could foster innovative therapies, potentially improving quality of life and symptom management for those affected.
Keywords: Chronic fatigue syndrome, Health, Myalgic encephalomyelitis, Microbiota-gut-brain-axis, Microbiome-gut-brain-axis, Therapeutics
Background
The multifaceted condition known as Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) poses significant challenges to both patients and healthcare professionals. Characterized by persistent fatigue, post-exertional malaise, and cognitive dysfunction, ME/CFS affects approximately 1% of the global population, with a higher prevalence in women [1, 2]. The complexity of this disorder arises from its unclear etiology and the lack of definitive diagnostic tools, making it difficult to differentiate from other overlapping illnesses [3, 4]. Research has increasingly highlighted the relationship between gut health and the symptoms of ME/CFS, particularly chronic inflammation and dysbiosis, which may exacerbate fatigue and cognitive issues [1, 5, 6]. Hence, exploring therapeutic approaches that target gut health could provide promising avenues for alleviating ME/CFS symptoms and improving patient quality of life [7–9]. Recent investigations into the gut microbiome reveal a concerning prevalence of dysbiosis in ME/CFS patients, characterized by reduced microbial diversity and an imbalance between beneficial and harmful bacteria [1, 10–14]. This microbial dysregulation contributes to systemic inflammation and immune dysfunction, further complicating the clinical picture of ME/CFS [15–17]. Moreover, gastrointestinal symptoms often reported by individuals with ME/CFS underscore the potential gut-brain axis involvement in disease manifestation [8, 13]. As chronic inflammation can lead to increased gut permeability, also known as “leaky gut”, the interactions between gut health and neurological function become increasingly relevant [1, 10, 11, 13, 18]. These insights suggest that interventions aimed at restoring gut microbiota balance—through dietary modifications, probiotics, fecal microbiota transplantation, etc.—may offer novel therapeutic strategies to alleviate the debilitating symptoms of this complex condition [1, 8]. Furthermore, the recognition of gut health as a pivotal aspect of ME/CFS management opens the door to a range of therapeutic approaches that warrant further exploration [1, 10, 11, 13]. Prebiotics, probiotics, and dietary interventions have shown promise in preliminary studies, pointing toward the potential for restoring microbial balance and combating dysbiosis [10, 13]. Adjunct therapies focusing on digestive health may not only mitigate fatigue but could also enhance cognitive function, presenting a comprehensive approach to treatment [6, 7]. Integrating these gut health-focused strategies into clinical practice could transform the management of ME/CFS, fostering a more holistic perspective on the condition that places emphasis on underlying biological mechanisms [5, 10, 15]. Ultimately, as research progresses, the significance of gut health in ME/CFS may lead to more effective and personalized treatment modalities that better address this challenging syndrome [8, 9, 19].
Research objectives and significance
The primary objectives of this review are multifaceted; firstly, it aims to synthesize existing literature on the gut microbiome’s composition in individuals with ME/CFS, identifying whether specific dysbiotic patterns correlate with the severity of ME/CFS symptoms. Secondly, this article addresses the possible biological pathways that link dysbiosis to the symptoms of ME/CFS. Thirdly, the paper will evaluate a spectrum of interventions focused on restoring gut health. Lastly, the research seeks to generate recommendations for clinical practice, outlining how such interventions could be integrated into existing ME/CFS treatment protocols. The significance of this research extends beyond the academic realm; it holds practical implications for patients suffering from ME/CFS, who often find themselves with limited treatment options that adequately address their complex symptoms. By elucidating the connections between gut health and ME/CFS, this study not only contributes to the existing body of knowledge but also seeks to empower healthcare providers to implement evidence-based strategies that could significantly enhance patient outcomes. Moreover, understanding these relationships may catalyze future research endeavors aimed at developing more effective therapies tailored to ME/CFS, potentially reshaping the clinical landscape for this frequently overlooked condition. Consequently, this section emphasizes the urgency of addressing gut health as a viable therapeutic target, thereby optimizing patient care within the ME/CFS community and fostering a more integrative approach to chronic illness management.
Methods
In this narrative review, data collection techniques included a comprehensive search of English peer-reviewed literature from January 1995 to January 2025 to ensure that an extensive range of studies addressing the microbiome and ME/CFS were included. Databases such as PubMed, Scopus, and Web of Science were utilized, employing relevant keywords related to the “ME/CFS,” “Gut-Brain Axis,” “Gut Health,” “Intestinal Dysbiosis,” “Microbiome Dysbiosis,” “Pathophysiology,” and “Therapeutic Approaches.” The goal was to extract qualitative and quantitative data from studies that investigate gut microbiota composition, assess inflammatory markers, and evaluate the therapeutic outcomes of interventions targeting the microbiome. Additionally, when available, insights from clinical trials and observational studies were included to enrich the findings and support the overarching narrative being developed. Finally, the method of narrative synthesis was utilized to organize and convey the findings effectively, adhering to the framework suggested by MacLure [20]. This approach emphasizes the critical role of researcher engagement in various activities including reading, writing, thinking, interpretation, argumentation, and justification.
Overview of gut microbiota composition in ME/CFS patients vs. healthy individuals
Research on ME/CFS patients compared to healthy controls has identified changes in intestinal microbiota, although no specific microbial signature has been consistently found (see Table 1) [12, 21–31]. This inconsistency in results makes it difficult to establish a direct link between changes in gut microbiota and the disease mechanisms [32–34]. In this regard, a systematic review by Newberry et al. indicates conflicting study results, but the overall evidence suggests dysbiosis in ME/CFS patients [35]. A study identified microbial dysbiosis in individuals with ME/CFS, showing 83% classification accuracy using gut microbiome dysbiosis and elevated inflammatory blood markers due to microbial translocation. Despite a small sample size of 48, these findings need further validation. The 16S rRNA stool analysis revealed reduced bacterial diversity and richness, lower anti-inflammatory species, and higher pro-inflammatory species like Enterobacteriaceae in ME/CFS patients [27]. Typically, high microbial diversity correlates with good health and fitness, as cardiorespiratory fitness accounts for roughly 20% of gut diversity variation [36]. This suggests that reduced gut diversity in ME/CFS might relate to different physical fitness levels; notably, ME/CFS patients show lower VO2 peaks [37]. While one study reported a decrease in anaerobic bacteria in ME/CFS patients [25], another observed an increase, indicating varied findings regarding intestinal bacteria changes in this condition [22]. Recent studies have also identified alterations in gut microbiota among individuals with ME/CFS. These changes include higher levels of Enterococcus and Streptococcus species and increased Enterobacteriaceae, while beneficial Bifidobacteria and anti-inflammatory Firmicutes are decreased [21–23, 27]. Metagenomic analysis reveals that specific bacterial taxa, including the Firmicutes phylum and genera such as Faecalibacterium, Roseburia, and Clostridium, show associations with ME/CFS. Notably, increased levels of Alistipes and reduced butyrate-producing Faecalibacterium are emerging as potential diagnostic biomarkers [28]. However, findings about Clostridium genus abundance remain inconsistent [27]. In a case study involving ME/CFS discordant identical twins, the affected twin showed reduced abundance of Bifidobacterium and Faecalibacterium compared to the non-affected twin, coupled with decreased gut alpha diversity [26]. Furthermore, high-throughput 16S rRNA sequencing from stool samples identified increased gut inflammation markers, such as elevated levels of Lactonifactor and Alistipes [23]. A study involving 48 ME/CFS patients and 52 controls identified 26 distinct bacterial markers between the two groups. The abundances of Coprobacillus, Eggerthella, and Blautia were key differentiators. Additionally, a decrease in Faecalibacterium and an increase in Coprobacillus were observed [30], which aligns with previous studies’ findings [27, 28]. Another recent study with 35 patients and 70 controls identified a distinct microbial pattern, showing decreased anti-inflammatory Firmicutes [12], supporting previous findings [23, 27]. The research compared patients with internal vs. external controls and found overlaps between patients and their relatives, highlighting the importance of considering lifestyle and genetic factors [12]. Although these findings of dysbiosis in ME/CFS are evident [12, 21–31], their exact role in the disease mechanism remains unclear [10]. The studies vary significantly in sample sizes, recruitment strategies, and microbiota characterization methods, with most using culturing or 16S amplicon sequencing and only one employing whole genome shotgun sequencing. However, the hypothesis that restoring gut health could play a key role in managing ME/CFS emphasizes the importance of integrating microbiome research into current therapeutic frameworks, thereby advancing the understanding and treatment of this complex and multifaceted condition [1].
Table 1.
Overview of microbial alterations in ME/CFS patients relative to healthy controls
| Enhanced microbiota | Reduced microbiota | Intestinal dysbiosis | Subjects | Country | Authors’ names (year) |
|---|---|---|---|---|---|
| Enterobacteriaceae (e.g. Proteus mirabilis) | None | Yes | 29 cases and 11 healthy controls (Fukuda criteria) | Belgium | Maes et al. (2007) [21] |
| Aerobic bacteria, D-lactic acid producing: E. faecalis, S. sanguinis | E. coli, Gram-positive/Gram-negative bacteria ratio | Yes | 108 cases and 177 healthy controls (Holmes, Fukuda and Canadian criteria) | Australia | Sheedy et al. (2009) [22] |
| Lactonifactor and Alistipes | Firmicutes | Yes | 35 cases, 36 healthy controls (Fukuda criteria) | Belgium | Frémont et al. (2013) [23] |
| Bacteroidetes (non-significant) | Actinobacteria; Firmicutes (non-significant) | Yes | 10 cases, 10 healthy controls (Fukuda criteria) | Italy | Shukla et al. (2015) [24] |
| Aerobic bacteria (non-significant), Clostridium spp. | Anaerobic bacteria, Bacteroides spp. | Yes | 34 cases and 25 healthy controls (Canadian Criteria) | Australia | Armstrong et al. (2016) [25] |
| None | Faecalibacterium, Bifidobacterium | Yes | 1 case, 1 healthy control (34-year-old monozytogenic male twins) (Fukuda Criteria) | United States | Giloteaux et al. (2016) [26] |
| Pro-inflammatory species, Proteaobacteria (e.g. Enterobacteriaceae) | Firmicutes (non-significant), anti-inflammatory species (non-significant) | Yes | 48 cases, 39 healthy controls (Fukuda Criteria) | United States | Giloteaux et al. (2016) [27] |
| Alistipes (with IBS), Bacteroides (without IBS) | Faecalibacterium (with IBS), Bacteroides vulgatus (without IBS) | Yes | 50 cases, 50 healthy controls (Fukuda, Canadian Criteria) | United States | Nagy-Szakal et al. (2017) [28] |
| None | None | No | 17 cases und 17 healthy controls (Fukuda Criteria) | United States | Mandarano et al. (2018) [29] |
| Blautia, Coprobacillus, Eggerthella | None | Yes | 48 cases, 52 healthy controls (Fukuda und International Consensus Criteria) | Japan | Kitami et al. (2020) [30] |
| Bacteroidetes | Firmicutes | Yes | 35 cases, 70 healthy controls (Fukuda Criteria) | Italy | Lupo et al. (2021) [12] |
| Anaerobic bacteria (e.g., Paraprevotella, Ruminococcaceae UCG_014) | None | Yes | 2076 cases, 460,857 healthy controls | 11 countries | He et al. (2023) [31] |
Hypothetical theoretical frameworks linking dysbiosis and ME/CFS
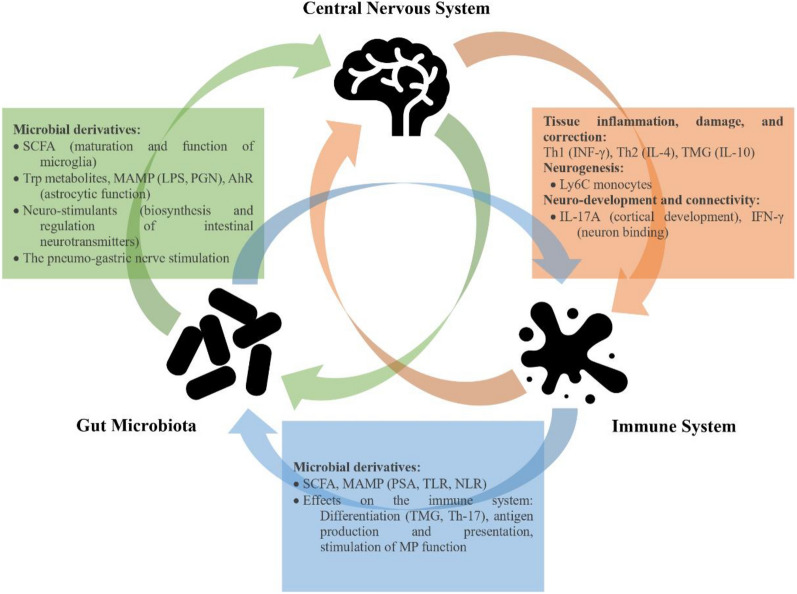
Research shows that gut microbiota can affect individuals’ clinical conditions by influencing the central nervous system (CNS) and the immune system, causing physical or mental symptoms. Although it seems this bidirectional connection is guided by various mechanisms, there is no definitive epidemiological evidence linking gut dysbiosis, the CNS, and the peripheral immune system (see Fig. 1). This highlights the necessity for further research into the microbiome-gut-brain axis to fully understand its role and implications [38]. In this context, the exploration of ME/CFS pathogenesis has increasingly focused on the microbiome-gut-brain axis, revealing a complex interplay between intestinal dysbiosis and the multifaceted symptomatology of this debilitating condition [10]. The convergence of these influences suggests that disruptions in microbial homeostasis could contribute significantly to the development and perpetuation of ME/CFS [10, 33]. Despite the lack of sufficient and solid information on this topic, the following sections attempt to discuss the current evidence supporting hypothetical theoretical frameworks that connect dysbiosis with ME/CFS (see Fig. 2).
Fig. 1.
The impact of the bidirectional relationship between the gut microbiota and the brain on the peripheral immune system and metabolome. SCFA: Short-Chain Fatty Acids; Trp: L-tryptophan; LPS: Lipopolysaccharides; AhR: Aryl hydrocarbon receptor; MAMP: Microbe-Associated Molecular Patterns; PGN: Peptidoglycan; PSA: Polysaccharide; TLR: Toll-Like Receptor; NLR: NOD-Like Receptor; TMG: Thymoglobulin; Th-17: T helper 17; MP: Mononuclear Phagocyte; INF-γ: Interferon-Gamma; IL: Interleukin.
(Adapted from Montagnani et al. [38])
Fig. 2.
Different hypothetical theoretical frameworks connect dysbiosis with ME/CFS: a Increased Lipopolysaccharides (LPS) Theory: Endotoxins, or LPS, from Gram-negative bacteria can enter mesenteric lymph nodes and the bloodstream by disrupting tight-junction proteins, leading to an immune response and systemic inflammation. This inflammation can result in a leaky gut, with cytokines such as IL-1β, IL-6, TNF-α, and IFN-γ further compromising gut permeability and allowing bacterial translocation. Reduced ceramide production also harms epithelial cells by decreasing LPS hydrolysis and increasing gut permeability. Additionally, translocated commensals activate IgA and IgM against bacterial LPS—these act as superantigens for T lymphocytes, possibly causing autoimmune conditions against neuronal structures like gangliosides. Gut-derived inflammation also activates signaling pathways that coordinate inflammatory gene expression; b Decreased short-chain fatty acids (SCFA) theory: A reduction in SCFA-producing bacteria weakens the intestinal barrier by decreasing butyrate production; c Increased D-lactic acid theory: Overgrowth of Gram-positive bacteria raise D-lactic acid production, lowering intestinal pH and increasing gut permeability; d Kynurenine production insufficiency theory: When proinflammatory cytokines induce the activation of the enzyme indoleamine-2,3-dioxygenase (IDO), it results in the production of kynurenine, which binds to the aryl hydrocarbon receptor (AhR) and plays a vital role in immune regulation by promoting regulatory T-cell generation and preventing excessive inflammation. Additionally, microbial metabolites such as SCFA and tryptophan derivatives can activate AhR, influencing the expression of genes like IL-6, IL-22, prostaglandin G/H synthase 2 (PTGS2), vascular endothelial growth factor A (VEGFA), and cytochrome P450 1 A1 (CYP1 A1) in the intestine. This AhR signaling can modify gut microbial composition, serving as a key regulator of host-microbiota communication, affecting metabolism, and modulating immune responses; e Past antibiotic intake hypothesis: Using antibiotics can disrupt the microbiome, impair anti-inflammatory metabolite production, and increase D-lactate-producing bacteria, potentially leading to conditions like D-lactic acidosis and intestinal barrier dysfunction; f The stress crash theory: Physiological stress may alter the gut microbiota by decreasing beneficial bacteria, potentially contributing to ME/CFS. This imbalance can increase harmful bacteria and LPS production, triggering inflammation through IL-22, affecting the HPA axis, and reducing cortisol levels [10, 21, 22, 24, 25, 28, 36, 39–80]. ROS: Reactive oxygen species; RNS: Reactive nitrogen species
Increased lipopolysaccharides (LPS) theory
In a healthy gut environment, bacterial translocation across the intestinal barrier is typically prevented [39]. However, numerous studies indicate that individuals with ME/CFS experience increased intestinal permeability, supporting the theory that Gram-negative bacterial endotoxins, also known as LPS, may enter the mesenteric lymph nodes and the blood stream through the loosening of tight-junction proteins (e.g., Zonulin), triggering an immune response and resulting in systemic inflammation [10, 21, 24, 39–42]. Leaky gut may also stem from systemic inflammation, where cytokines like interleukin (IL)−1β, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ disrupt gut permeability and promote bacterial translocation by weakening tight junctions [21]. Additionally, decreased production of ceramides may potentially harm gut epithelial cells by reducing LPS hydrolysis and increase gut permeability [28]. Consequently, two pathways may be triggered by commensals entering from the gut. The first involves binding with the Toll-Like Receptors (TLR) complex, which activates nuclear factor κB and leads to the production of pro-inflammatory cytokines, including IL-1 and TNF-α, as well as reactive oxygen species (ROS) and reactive nitrogen species (RNS). This can potentially cause neuroinflammation and symptoms of depression and fatigue. The second pathway involves the translocation of gram-negative commensals, resulting in immunoglobulin (Ig)A and IgM responses against bacterial LPS. This can act as a superantigen for T lymphocytes, inducing autoimmunity against neuronal lipid structures like gangliosides, potentially causing autoimmune conditions. This phenomenon helps explain the unusually high levels of IgA and IgM antibodies against LPS found in the blood of these patients, with 67% and 40% of ME/CFS patients respectively showing elevated antibody levels, compared to none in the control group [21]. Regardless of these events, gut-derived inflammation may also activate TLR and intracellular signaling pathways like NFκB, p38, and MAP kinase, coordinating the expression of inflammatory genes [21].
Decreased short-chain fatty acids (SCFA) theory
Gut hyperpermeability may also be linked to the by-products of amino acid fermentation, which produce SCFA [25]. A decrease in SCFA-producing bacteria, especially butyrate producers like Faecalibacterium and Roseburia from the Firmicutes phylum, along with certain Bacteroides species, has been observed in ME/CFS patients [10, 25, 28]. Butyrate essentially fuels colon cells and is crucial for the neuroimmunoendocrine system, promoting gut health through its anti-inflammatory properties and strengthening the intestinal barrier [43]. This molecular pathway may be influenced by the level of daily physical activity, as a study using high-throughput sequencing found that physically fit individuals had higher fecal butyrate levels, better VO2 max values, more diverse gut microbiota, lower LPS biosynthesis, and consequently, less systemic inflammation [22, 44]. In ME/CFS patients, the scarcity of butyrate-producing bacteria could be due to low levels of SCFA, potentially causing post-exertional malaise and a cycle of inactivity. This inactivity might increase LPS levels, exacerbating the condition. Alternatively, chronic inactivity from ME/CFS could itself reduce SCFA production over time. Additionally, the depletion of SCFA might result from an overgrowth of D-lactate producing bacteria, which are found at elevated levels in ME/CFS patients and have been studied previously [36]. However, a study challenged the butyrate deficiency theory by identifying higher fecal concentrations of butyrate, isovalerate, and valerate in ME/CFS specimens, linked to increased bacterial fermentation. Researchers suggest that elevated gut pH, gut dysbiosis (i.e., a higher Firmicutes:Bacteroidetes ratio), obesity, or malabsorption might increase fecal SCFA, such as butyrate, which could have potential neurotoxic effects [25, 45, 46]. Accordingly, it is crucial to understand that fecal SCFA excretion does not entirely reflect their concentration and production within the gut. A comprehensive analysis of SCFA serum levels is required to gain a more accurate understanding of their dynamics in the body [10, 45–48].
Increased d-lactic acid theory
Another theory regarding the reduced intestinal pH and increased gut permeability in ME/CFS patients is the increased production of D-lactic acid due to the overgrowth of Gram-positive bacteria such as Enterococci and Streptococci, which leads to systemic inflammation, immune activation, and oxidative stress [22, 44]. However, targeting bacterial overgrowth with antibiotics and probiotics hasn’t improved fatigue symptoms, questioning d-lactate’s role. Additionally, differences between cerebrospinal fluid and fecal lactate levels suggest an incomplete understanding of intestinal lactate metabolism’s impact on ME/CFS patients [25, 44, 49].
Kynurenine production insufficiency theory
Recent research has identified the aryl hydrocarbon receptor (AhR) and the enzyme indoleamine-2,3-dioxygenase (IDO) as key players in the increased gut permeability and bacterial endotoxin translocation seen in ME/CFS patients. These components are crucial in linking microbial l-tryptophan (Trp) catabolism and host endogenous Trp metabolites to regulatory T-cell function, particularly in maintaining AhR-dependent T-cell immune homeostasis at mucosal surfaces. When induced by proinflammatory cytokines, the enzyme IDO is activated, leading to the production of kynurenine [50]. These kynurenines act as ligands for the AhR, playing a crucial role in regulating immune homeostasis by fostering the generation of regulatory T-cells, thereby protecting against hyper-inflammatory responses. This interaction is part of a coevolutionary relationship between hosts and microbes, with particular relevance to tryptophan-derived AhR ligands [50, 51]. Several factors, such as diet, microbial metabolism of tryptophan, and endogenous enzymatic activity, can provide critical signals to the host, aiding in resisting colonization and defending against mucosal inflammation [51]. Specifically, Lactobacilli species, as probiotics, can convert tryptophan into AhR ligands like indole-3-aldehyde, which activate innate lymphoid cells. These cells subsequently induce the production of IL-22, which regulates the release of antimicrobial peptides and enhances their expression in the gut epithelium, thereby reducing pathogen infectivity by sequestering metal ions [52]. This intricate interplay underscores the importance of microbial metabolites in immune regulation and host defense mechanisms [52, 53]. In this regard, previous studies emphasize the bidirectional interaction between the AhR and the microbiome, indicating that this microbiome-AhR axis influences host metabolism. Microbial metabolites like SCFA and Trp derivatives can activate AhR and its target genes, including IL-6, IL-22, prostaglandin G/H synthase 2 (PTGS2), vascular endothelial growth factor A (VEGFA), and cytochrome P450 1 A1 (CYP1 A1) in the intestine or liver. Additionally, AhR signaling can alter microbial composition in the small intestine, acting as a crucial regulator of host-microbiota communication, thereby affecting host metabolism and modulating the immune system [54–58]. However, the potential for using these findings in treating or diagnosing ME/CFS remains under investigation and requires further study to draw definitive conclusions [55–57]. For example, Kashi et al. [58] proposed a “metabolic trap hypothesis” that suggests ME/CFS patients suffer from insufficient kynurenine production due to gene mutations in IDO2 [58]. This leads to elevated tryptophan levels, affecting the central nervous system, gastrointestinal tract, immune system, and energy metabolism. Their study found multiple IDO2 gene mutations in ME/CFS patients, correlating with symptom severity. The resulting lack of IDO2 activity hinders tryptophan conversion to kynurenine. Elevated tryptophan and reduced kynurenine levels were confirmed in these patients, disrupting serotonin and melatonin pathways and contributing to ME/CFS symptoms. Contrarily, while elevated tryptophan levels do not safeguard intestinal integrity from damage induced by LPS, reduced levels of kynurenine appear to offer some protection [58, 59]. The “metabolic trap hypothesis” explores the potential metabolic and immunological mechanisms in ME/CFS, focusing on serotonin and tryptophan metabolism and their effects on patient microbiomes. Crucially, decreased IDO enzyme activity can disrupt tryptophan fermentation, affecting the gut microbiome and potentially leading to an impaired intestinal mucosal barrier, increased endotoxin translocation, and chronic inflammation. These insights are vital for understanding ME/CFS mechanisms and identifying therapeutic targets [60–62]. Recent research into ME/CFS investigates increased kynurenine production rather than decreased levels proposed by the “metabolic trap hypothesis.” The balance between tryptophan depletion and kynurenine generation, marked by the kynurenine and tryptophan (KYN/TRP) ratio, indicates IDO activity and immune responses. High IDO activity, often linked with chronic inflammation, supports theories of increased kynurenine production. This is significant since elevated KYN/TRP ratios and neuroactive metabolites like quinolinic acid are linked with symptoms in disorders, including ME/CFS. Additionally, viral infections such as Epstein-Barr virus activating IDO may convert tryptophan to kynurenine, supporting this hypothesis [63–66]. Although studies suggested that tryptophan and its derivatives could influence the gut microbiome and interact with the gut mucosal immune system, recent clinical investigations proposed that changes in immunity and kynurenine metabolism may trigger fatigue in ME/CFS but do not sustain it over time [61, 67, 68].
Past antibiotic intake hypothesis
There is also a potential link between antibiotic use and ME/CFS development, as altered microbiota can be influenced by antibiotics. While no specific studies have examined antibiotics as a trigger, many patients with ME/CFS have frequent infections treated with antibiotics [47, 69–72]. This may alter the microbiome, impairing anti-inflammatory metabolite production or fostering conditions like D-lactic acidosis by increasing D-lactate-producing bacteria [22, 25, 49]. Women are more often diagnosed with ME/CFS, potentially due to higher antibiotic exposure compared to men [73, 74]. Antibiotics may contribute to oxidative stress by increasing reactive oxygen species (ROS), aligning with observations of elevated ROS levels in ME/CFS patients. Despite this, antibiotics have been explored as a treatment for ME/CFS, targeting the overgrowth of certain bacteria [75–77]. A pilot study by Jackson et al. demonstrated that antibiotics improved sleep quality by reducing lactic acid-producing bacteria and balancing the microbiome with a reduction in Gram-positive bacteria, which lowers proinflammatory cytokines [78]. Similarly, Wallis et al. found that a four-week treatment combining antibiotics and probiotics enhanced neurological symptoms and sleep quality, though it did not alleviate fatigue symptoms linked to ME/CFS [44]. These findings suggest a microbiome-related approach may improve certain aspects of the condition but underline the complexity of addressing fatigue specifically [44, 78].
The stress crash theory
The stress crash theory suggesting that hypothalamic–pituitary–adrenal (HPA) axis hypofunction triggers immune-inflammatory pathways in ME/CFS cannot be substantiated. This theory faces contradictions, such as HPA axis hypofunction being limited to certain patients, no early-stage changes in ME/CFS, and heightened immunosuppressive effects of glucocorticoids in these patients. Alternatively, the HPA axis hypofunction might arise from several mechanisms, including chronic inflammation with elevated TNF-α levels, a regulatory T cells response with increased IL-10 and transforming growth factor (TGF)-β, heightened oxidative and nitrosative stress pathways, notably elevated nitric oxide (NO) production, and infection-related factors like LPS tolerance and viral infections affecting the HPA axis (see Table 2) [79]. These factors may independently or interactively contribute to ME/CFS. For instance, physiological stress is believed to play a role in modifying gut microbiota, reducing beneficial bacteria like Bifidobacterium and Lactobacillus, which could contribute to ME/CFS by enabling harmful bacteria to thrive. This imbalance might cause LPS to be increasingly produced and enter the bloodstream, triggering widespread inflammation through IL-10, disruptions in the HPA axis, and a reduction in cortisol levels, another factor linked with ME/CFS [79, 80]. These intertwined factors potentially maintain a disturbed homeostasis within individuals suffering from ME/CFS. Nevertheless, future research should focus on examining temporal relationships between HPA axis function and immune-inflammatory or nitrosative stress pathways through biomarkers like TNF-α, IL-10, TGF-β, inducible NO synthase, NO production, protein nitrosylation, and bacterial translocation in ME/CFS patients to better understand their roles [79].
Table 2.
Impacts of immune-inflammatory and nitrosative pathways on reducing the functionality of the HPA axis in patients with ME/CFS
(Adapted from Morris et al. [79])
| Biomarkers | Effects |
|---|---|
| ↑ TNF-α |
↓ ACTH production ↓ Cortisol synthesis ↓ ACTH-stimulated cortisol secretion |
| ↑ IL-10 |
↓ Glucocorticoid synthesis ↓ Progesterone levels ↑ Steroid suppression ↑ Dexamethasone binding sites act synergistically with glucocorticoids |
| ↑ TGF-β |
↓ Glucocorticoid secretion ↓ Steroid production ↓ DHEA-S synthesis |
| ↑ NO |
↓ CRH secretion ↓ Steroidogenesis ↓ Transcription of steroidogenesis proteins |
| ↑ LPS |
↑ IL-10 and its effects ↓ HPA axis responses to LPS ↓ Cortisosteron levels |
| ↑ Poly I:C |
↓ Adrenal sensitivity to ACTH ↑ Negative feedback |
TNF-α: tumor necrosis factor-α; IL-10: Interleukin-10; TGF-β: Transforming growth factor-β; NO: Nitric oxide; LPS: Lipopolysaccharides; ACTH: Adrenocorticotropic hormone; DHEA-S: Dehydroepiandrosterone sulfate; CRH: Corticotropin-releasing hormone
The potential therapeutic approaches targeting gut health in ME/CFS
Considering the common links between ME/CFS and dysbiosis, increased gut permeability, and chronic inflammation [12, 21–31, 40, 41], it is reasonable to hypothesize that strategies focused on restoring microbial equilibrium, enhancing mucosal barrier function, and reducing inflammation could have therapeutic potential. In this context, the use of probiotics, prebiotics, specific diets, dietary supplements, fecal microbiota transplantation, some pharmacological interventions, and improved sleep has been suggested.
Probiotics
Probiotics are live microorganisms that can enhance gut health and reduce inflammation [81]. Although this treatment option might benefit ME/CFS treatment [82, 83], only two studies on ClinicalTrials.gov explore this, despite theories linking microbiota to the disease’s progression [12, 84]. An Italian pilot study found that an 8-week course of various probiotics improved well-being and reduced inflammation in patients with ME/CFS, though the study was limited by a small sample size and lack of a control group [12]. Another study assessed Lactobacillus paracasei ssp. paracasei F19, Lactobacillus acidophilus NCFB 1748, and Bifidobacterium lactis Bb12 in 15 ME/CFS patients, observing improvements in neurocognitive function but no significant reduction in fatigue or physical activity levels in only nine patients [84]. The systematic review by Corbitt et al. reveals a lack of evidence for probiotics as a treatment for gastrointestinal symptoms in ME/CFS patients, largely due to poor study quality [85]. This finding aligns with an evaluation in Nature that highlights insufficient impact assessments and therapy recommendations for probiotics, citing conflicting and industry-driven study results [83]. Existing studies typically focus on stool samples, yet recent findings suggest biopsies might better reveal gut interactions, as the small intestine may exhibit distinct patterns. It is also advised to conduct a before-and-after analysis of the gut microbiota due to its highly individual-specific and somewhat resistant nature [86].
The use of Bifidobacterium infantis 35,624 in 48 ME/CFS patients further confirmed probiotics’ capacity to lower systemic inflammatory markers like CRP, TNF-α, and IL-6 [87]. Given the frequent occurrence of anxiety, depression, and other psychiatric conditions in individuals with ME/CFS, exploring alternatives to traditional psychotropic drugs is essential [88]. A 12-week randomized, double-blind, placebo-controlled study found that a combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 effectively reduced inflammation and alleviated psychiatric symptoms in patients with Major Depressive Disorder (MDD) who were on a gluten-free diet [89]. Considering the overlap in psychiatric symptoms between MDD and ME/CFS, it raises the possibility that probiotics could similarly benefit those with chronic fatigue [90]. Early indications suggest that administering Lactobacillus casei strain Shirota daily for two months may significantly lessen anxiety and restore eubiosis in ME/CFS patients [91]. Additionally, notable improvements in neurocognitive functions have been observed in individuals diagnosed with ME/CFS taking L. paracasei spp. paracasei F19, L. acidophilus NCFB 1748, and B. lactis Bb12 [84]. Collectively, these findings indicate that probiotics, whether used alone or in combination, are likely to become an effective component of therapy for ME/CFS.
Prebiotics
Prebiotics, which are indigestible carbohydrate nutrients, serve as sustenance for the gut microbiota. The primary categories of prebiotics include fructo-oligosaccharides and galacto-oligosaccharides [92]. These compounds undergo bacterial breakdown, leading to the production of SCFA that enter systemic circulation, thereby affecting both gastrointestinal and systemic health [46]. Due to their selective enhancement of certain beneficial gut bacteria and their ability to modify the composition and function of the gut microbiota, prebiotics are considered a potential supportive treatment for various disorders such as IBS, Crohn’s disease, issues with bowel motility, autism, obesity, and colorectal cancer [92]. Research has shown that various oligosaccharides can counteract imbalances in the microbiota by encouraging the growth of Lactobacilli, reducing Proteobacteria populations, and lowering the Firmicutes/Bacteroidetes ratio in diet-induced obese rodents [93–95]. Moreover, improvements in gut barrier integrity and reductions in systemic inflammation have been observed. Rodents consuming prebiotics like bovine milk oligosaccharides, oligofructose-enriched inulin, spirulina platensis, and a combination of Fructooligosaccharide/Galactooligosaccharide have exhibited decreased plasma levels of lipopolysaccharides, lower serum concentrations of pro-inflammatory cytokines, diminished intestinal inflammation, and enhanced tight-junction integrity [93–97]. Collectively, these findings indicate that prebiotics could be beneficial for managing conditions such as ME/CFS characterized by dysbiosis, increased intestinal permeability, and chronic inflammation. However, further clinical studies are essential to substantiate these potential benefits.
Specific diets
Altering dietary habits provides a swift, consistent, and effective method for altering the composition of the gut microbiota [98]. Recent evidence has shown diets rich in glucose/fructose or prolonged high-protein diets have been linked to negative outcomes such as dysbiosis, increased intestinal permeability, heightened systemic inflammation, and elevated plasma endotoxin levels [99, 100]. Conversely, adopting gluten-free diets or those low in starch and sucrose, as well as calorie-restricted eating plans, can lower levels of C-reactive protein and LPS binding protein, improve gut barrier function, and alleviate both gastrointestinal and systemic symptoms associated with IBS and obesity [89, 101, 102]. The Mediterranean diet and low FODMAP diet may also serve as the models for dietary recommendations. They promote foods that enhance gut microbiota richness and alleviate systemic inflammation and fatigue [103–105]. Moreover, Small partly non-controlled clinical pilot studies have explored the efficacy of ketogenic diets in alleviating fatigue symptoms associated with multiple sclerosis, Parkinson’s disease, and the impact of short-term fasting on cancer-related fatigue [106–108]. Although the exact mechanisms by which ketogenic diets exert their effects are not fully known, it is believed that these effects are partially mediated through interactions with the gut microbiome [109–111]. Consumption of cocoa and dark chocolate has been linked to beneficial health outcomes in relation to chronic diseases. This review highlights a randomized controlled crossover study that explored the therapeutic benefits of cocoa on reducing fatigue and enhancing residual functions in ME/CFS patients, as measured by the London Handicap Scale. The study compared the effects of high-polyphenol cocoa to those of an iso-caloric low polyphenol chocolate. Although the findings were promising, the study’s credibility is tempered by its small sample size (n = 10), short duration of treatment, and the absence of detailed dietary intake data during the trial period [112].
Contrary to the positive findings mentioned above, a systematic review determined that there is no supportive evidence indicating that elimination or modified diets benefit patients with ME/CFS [113]. It is important to acknowledge the potential risk of bias in these dietary studies due to the inability to implement blinding. Additionally, extreme dietary interventions should be approached with caution and implemented under the supervision of qualified healthcare professionals, particularly because it is uncertain if they might exacerbate symptoms in ME/CFS patients, especially during the initial weeks. Overall, further clinical trials in humans are necessary to demonstrate that dietary interventions can simultaneously influence the microbiome, intestinal permeability, inflammation, and neurocognitive symptoms, making them a compelling complementary strategy in the treatment of ME/CFS [10, 11, 13].
Dietary supplements
Enhancing the intake of omega-3 fatty acids and polyphenols has been shown to increase microbiota diversity and reduce metabolic endotoxemia [114]. Specifically, eicosapentaenoic acid found in omega-3 rich fish oil has been effective in reducing symptoms in cases of ME/CFS [115, 116]. In experimental models using diet-induced obese rats and mice, targeted nutrient supplementation has yielded benefits. For instance, treatments with apple polysaccharides, flos lanicera, and Bofutsushosan—an herbal remedy from Japan—have been successful in promoting the growth of beneficial bacteria like Lactobacillus and Bacteroidetes, improving intestinal barrier integrity, and decreasing pro-inflammatory cytokines such as TNF-α and IL-6 [117–119]. Furthermore, incorporating Sarcodon imbricatus or consuming a blend of Angelica gigas, Cnidium officinale, Paeonia lactiflora, moxibustion, cistanche, ginkgo extractsand, and ginseng has shown potential in restoring antioxidant balance and reducing fatigue in ME/CFS models in mice [120–124]. In line with these studies, the findings from a recent systematic review article including 68 patients have highlighted a potential benefit of ginseng therapy in the treatment of ME/CFS [125–127]; results that have also been repeated in an open-label, pilot trial of HRG80™ red ginseng, but this time with a larger sample size (n = 188) [128].
CoQ10–selenium combination, β-nicotinamide adenine dinucleotide (NADH), and NADH-CoQ10 combination were also found to alleviate fatigue in patients with ME/CFS [129–131]. However, the studies did not include assessments of dietary intake either before or after the treatment periods, leaving it uncertain whether changes in diet may have affected the outcomes. Additionally, the reliability of these findings is compromised by the small number of participants and the short duration of the treatments, which lasted only 4 and 8 weeks. Consequently, there is a need for long-term studies with more substantial participant numbers to verify the sustained effectiveness of NADH and CoQ10 in treating ME/CFS [129]. Moreover, the study by Forsyth et al. utilized a questionnaire developed by the researchers to assess symptoms, which, despite undergoing reproducibility tests, raises questions about its ability to accurately capture ME/CFS symptoms [132]. Future research should employ validated measurement tools to ensure reliable results and facilitate comparisons across different studies.
Ubiquinol-10, also known as CoQ10, plays a crucial role in cellular energy production and acts as an antioxidant [7]. Studies have shown that CoQ10 levels are lower in ME/CFS patients than in healthy individuals [133–135]. A single randomized controlled trial examining the effects of 12-week supplementation with Ubiquinol-10 in ME/CFS patients reported no significant improvements in fatigue as measured by Chandler’s Fatigue Scale. However, improvements were noted in other symptoms associated with ME/CFS, such as reduced night-time awakenings, suggesting potential long-term benefits for fatigue management [131]. An animal model study has also shown that the administration of CoQ10 for 21 days significantly altered the gut environment, resulting in an increase in hydrogen concentration and SCFA like butyrate in feces. Additionally, it reduced trimethylamine levels and the relative abundance of Helicobacter while boosting beneficial groups like Ruminococcus and Lachnospiraceae. These changes suggest that CoQ10’s antioxidant effects may involve modifying the gut microbiota’s taxonomic composition and increasing molecular hydrogen production. The rise in butyrate levels could enhance gut barrier protection, contributing to overall gut health [136]. All in all, although the results of a recent meta-analysis have shown that CoQ10 is an effective and safe supplement for reducing fatigue symptoms, more extensive and controlled studies are essential to further substantiate the advantages of Ubiquinol-10 supplementation [137].
Aside from this, recent evidence has found that melatonin and zinc supplementation is safe and possibly effective in managing ME/CFS symptoms. In this respect, a 16-week randomized, placebo-controlled trial was conducted with 50 ME/CFS patients to test the effects of oral melatonin (1 mg) and zinc (10 mg) supplements. The study showed significant reductions in physical fatigue and improvements in quality of life [138].
Thiamine, a crucial co-factor in the TCA cycle and glycolysis, is partially produced by gut bacteria like Bacteroides, which are often reduced in ME/CFS patients [139]. Another bacterium, Faecalibacterium, also found to be diminished in these patients, relies on thiamine for growth [27, 28, 35, 140]. Although gut microbes produce only small amounts of thiamine, its availability significantly influences the competitive microbial ecosystem in the gut. This highlights the potential impact of reduced thiamine levels on gut health in ME/CFS sufferers [141].
Overall, although most clinicians commonly recommend that patients with ME/CFS consume supplements such as vitamin A, vitamin C, vitamin D, Vitamin B1, Vitamin B12, vitamin E, folic acid, ferritin, iron, selenium, calcium, magnesium, L-tryptophan, L-carnitine and etc. [129, 142–144], a recent meta-analysis was unable to definitively establish a significant connection between overall vitamin and mineral deficiencies and fatigue in patients with ME/CFS [145], although some uncertainty about the role of vitamin E deficiency persists [146]. Furthermore, this analysis found no clear advantages of nutritional interventions, noting that only a limited number of such trials were assessed [145]. Additionally, another systematic review indicated some potential benefits of nutritional supplements like d-ribose, particularly in symptom management [147]. Despite these findings, the existing limitations of the studies mentioned make it impossible to reach a conclusive verdict at this time [7, 145, 147, 148].
Fecal microbiota transplantation
Fecal microbiota transplantation, also referred to as stool transplantation or bacteriotherapy, involves transferring fecal matter from a healthy donor into the gastrointestinal tract of a patient [149]. This procedure aims to correct imbalances in the gut microbiome by introducing a diverse and healthy microbial community. Typically, this is done through colonoscopy, though other methods such as enemas and oral capsules are also used [150, 151]. Currently, fecal microbiota transplantation is officially sanctioned only for treating persistent or severe infections caused by Clostridium difficile [152]. However, its use is being explored as a potential treatment for a range of conditions including obesity, insulin resistance, metabolic syndrome, non-alcoholic fatty liver disease, fibromyalgia, ulcerative colitis, Crohn’s disease, functional constipation, IBS, and even cancer [153–156]. The technique is also being investigated for its potential benefits in various neuropsychiatric disorders such as autism, Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, although the outcomes of these studies remain uncertain [157–159]. Fecal microbiota transplantation is also being explored in ME/CFS research due to indications that the microbiome may significantly impact the disease, particularly its neurological symptoms [160, 161]. The ability of fecal microbiota transplantation to reduce inflammation, enhance intestinal barrier integrity through the production of SCFA, and rebalance immune function highlights its potential as an emerging treatment modality, particularly for ME/CFS [162]. Interest in fecal microbiota transplantation for treating ME/CFS emerged from a retrospective study involving 60 ME/CFS patients, many of whom also had IBS. This study administered a culture of 13 common fecal bacteria via colon infusion, resulting in 70% of patients responding positively after four weeks. Remarkably, 58% of contactable patients-maintained resolution of ME/CFS symptoms for 15–20 years [160]. Another recent retrospective study showed promising results when comparing fecal microbiota transplantation to an oral treatment consisting of dietary and lifestyle changes, pre- and probiotics, and natural remedies. In this study, 17 out of 21 patients receiving fecal microbiota transplantation from ten different donors achieved at least a 60% improvement [161]. However, in the only randomized, placebo-controlled pilot study to date examining fecal microbiota transplantation in 11 patients with ME/CFS—where 5 patients received transplants from a universal donor and 6 underwent autologous fecal microbiota transplantation through colonoscopy—there were no notable differences in health-related quality of life metrics between the treatment and placebo groups at both 1- and 6-months post-treatment [163]. It seems this contradiction arises from several challenges that remain in the broader application of fecal microbiota transplantation. These include inconsistencies in protocols, a lack of standardized criteria for donor selection and treatment regimens, and unresolved questions regarding long-term safety and efficacy [149, 155, 159, 164–166]. Additionally, the scientific community has yet to reach firm conclusions due to the limited scale of existing studies, underscoring the need for more extensive clinical trials to better understand the effectiveness of fecal microbiota transplantation across various conditions [149, 159, 162, 167, 168]. There is also interest in comparing the effectiveness of using multiple donors versus a single donor in fecal microbiota transplantation procedures, as some preliminary findings suggest potential benefits [169]. Moreover, antibiotic treatment prior to fecal microbiota transplantation significantly enhances engraftment [170, 171]. The success of fecal microbiota transplantation is most strongly linked to the method of administration, especially when combining both upper and lower gastrointestinal applications [172]. Bacteroidetes and Actinobacteria species exhibit better engraftment rates compared to Firmicutes and Proteobacteria, while gram-positive bacteria have lower engraftment compared to gram-negative ones. These findings highlight the importance of characterizing and standardizing donor stool for fecal microbiota transplantation to improve outcomes [172]. While numerous hurdles still exist, the data thus far suggests that leveraging fecal microbiota transplantation for treating a variety of diseases linked to intestinal dysbiosis could soon offer a new therapeutic avenue for cases of ME/CFS [11].
Pharmacological interventions
The exploration of pharmacological interventions for the treatment of ME/CFS reveals both potential benefits and risks, particularly concerning gut health [7]. Numerous studies demonstrate that pharmacological agents can influence gut microbiota, which is increasingly recognized as a factor in ME/CFS pathology [173–176]. For instance, medications that modulate the gut microbiome, such as antibiotics, have been shown to improve some gastrointestinal symptoms associated with ME/CFS, thereby enhancing overall patient well-being [5, 10, 12, 32, 161]. In this context, a study on antibiotics for ME/CFS patients with high stool Streptococcus counts indicated that reducing these counts led to improved sleep [78]. Additionally, neomycin was tested in ME/CFS patients with small intestinal bacterial overgrowth, resulting in reported improvements in pain, depression, and cognitive function [177]. However, the application of these interventions poses a risk of dysbiosis, where medication-induced changes may lead to an imbalance in microbial populations, exacerbating symptoms rather than alleviating them [178–180]. Careful consideration must be given to the individual responses and the existing discrepancies among the microbiomes of ME/CFS patients compared to healthy individuals, emphasizing the delicate interplay between pharmacological treatments and gut health [181–184]. Furthermore, existing literature indicates that drugs aimed at treating specific ME/CFS symptoms may inadvertently affect the gut microbiota, influencing both therapeutic outcomes and overall patient health [10, 11]. For example, non-steroidal anti-inflammatory drugs often prescribed for pain management have been associated with gastrointestinal disturbances, raising concerns about their long-term use in ME/CFS patients who already report high levels of gut-related issues [185, 186].
Research into the effects of various antidepressants on ME/CFS has yielded mixed results [7]. Moclobemide was found to enhance vitality and energy in patients without notably alleviating depression [187]. Conversely, phenelzine showed no significant benefits [186]. A small trial exploring escitalopram for ME/CFS patients with major depressive disorder indicated substantial reductions in both ME/CFS and depression symptoms [189]. However, fluoxetine generally did not show significant improvement in symptoms for either depressed or non-depressed patients, except in one study where it improved depressive symptoms without significantly affecting fatigue levels [190, 191]. Meanwhile, duloxetine, assessed for its analgesic potential, did not reduce general fatigue but resulted in notable improvements in mental fatigue, pain relief, and overall symptom perception compared to a placebo [192]. Nevertheless, antidepressants posed a risk of altering gut flora in several animal models via inhibition of efflux pumps and/or amino acid transporters, which may exacerbate issues related to inflammation and immune responses [193–197]. Additionally, in ME/CFS, the salivary glands undergo pathological changes, including mast cell accumulation and targeting by autoantibodies against muscarinic receptors [198]. These changes affect saliva production, consequently impacting nutrition and oral health. Antidepressants, unlike pyridostigmine or pilocarpine, which are saliva stimulants, may worsen these effects [199]. Recognizing this duality highlights the need for a comprehensive approach that balances symptom relief with potential impacts on gut health, ensuring that interventions contribute positively to the multifactorial nature of ME/CFS [7]. Anti-inflammatory and immunomodulatory agents may also be critical in the context of ME/CFS [7, 10, 200]. Chronic inflammation has been identified as a central feature in many patients, linking immune dysregulation to symptomatology such as fatigue and cognitive impairments. Pharmacological interventions targeting cytokine pathways, particularly those involving TNF-α and ILs, may present viable options for managing these complex immune responses. These agents could foster a more favorable immune profile by reducing inflammation stemming from gut dysbiosis, thereby enhancing the quality of life for patients [201–203].
Ultimately, while pharmacological interventions can offer symptomatic relief for ME/CFS patients, an understanding of the potential benefits and risks associated with such treatments is crucial. The intersections of gut health, microbiome integrity, and medication effects underscore the importance of personalized medicine in this context [6]. By appreciating the potential adverse effects of pharmacological agents on the gut microbiota, researchers and clinicians can develop more effective, tailored therapies that prioritize not only the alleviation of ME/CFS symptoms but also the maintenance of gut health [6, 7, 10, 11]. As the literature expands on the links between gut dysbiosis and the broader symptomatology of ME/CFS, it becomes increasingly necessary to integrate this knowledge into clinical practice, reinforcing perspectives that prioritize holistic treatment methodologies in an often fragmented care landscape.
Improved sleep
Research on sleep disorders, both in animal models and humans, has been aimed at understanding how sleep deprivation or disturbance can impact the gut microbiome [204–207]. A study has found that short-term sleep deprivation can indirectly affect human microbiota by altering the balance between Firmicutes and Bacteroidetes, with the ratio of these bacteria doubling after just two days of partial sleep deprivation compared to normal sleep conditions [208]. Similar findings have been observed in mice; those subjected to experimentally induced sleep fragmentation and fed a low-fat diet exhibited increased food consumption and shifts in gut microbiota, including changes in the Firmicutes/Bacteroidetes ratio and a decrease in Actinobacteria species. These shifts disrupt metabolic balance, potentially leading to inflammation in systemic and adipose tissues, possibly due to the translocation of microbial metabolites and insulin resistance. However, these effects were found to be reversible when sleep fragmentation occurred intermittently [209, 210]. Further studies in mice have revealed that a five-day sleep disruption affects both the microbiome and metabolome, with consequences lasting at least four days post-interruption. This disruption results in decreased levels of beneficial bacteria, altered metabolic functions within the microbiome, and changes in fecal bacterial metabolite levels [211]. A recent study examined the connection between the gut microbiome and sleep patterns in young males, utilizing tools such as actigraphy, cognitive assessments, and gut microbiome sequencing. The research found a positive correlation between the diversity and richness of gut microbes and sleep quality, as well as a negative correlation with sleep fragmentation. Notably, increased richness in the Bacteroidetes and Firmicutes phyla was linked to improved sleep efficiency. Conversely, Bacteroidetes alone was negatively related to post-sleep onset wakefulness. Actinobacteria diversity showed a negative correlation with awakening frequency. The study also investigated the role of IL-6, an immune system marker regulating sleep, finding its levels positively associated with microbiota diversity and various sleep metrics, including time in bed and total sleep duration. Certain Proteobacteria were associated with increased IL-6 levels. Despite these findings, the precise mechanisms connecting gut microbes, sleep, and immune functions remain unclear [212]. Liu et al. discovered that insomnia is associated with significant changes in the structure and function of gut microbiota, showing reduced diversity (both α-diversity and β-diversity) and altered microbial interactions in individuals with insomnia compared to healthy controls [213]. The study observed a reduction in the Firmicutes/Bacteroidetes ratio in the gut microbiota of individuals with insomnia, alongside an increase in gram-negative and potentially pathogenic bacteria compared to a control group. This finding contrasts with prior research on sleep deprivation, which reported increased ratios. While insomnia and sleep deprivation both lead to reduced sleep, they differently affect gut microbiota and metabolic processes. Liu and colleagues also noted increased vitamin B6 catabolism and folate biosynthesis but reduced arachidonic acid biosynthesis in the insomnia group [213]. Although further research is necessary, numerous studies have demonstrated that there is a bidirectional relationship between gut microbiota and sleep disorders. Disruptions in circadian rhythms can adversely affect sleep quality and lead to imbalances in gut microbiota, resulting in alterations to the community structure, ecological parameters of the microbial ecosystem, and the inflammatory state of the body [45, 214–221]. This overview highlights the complex relationship between sleep patterns and gut microbiota, suggesting a possible bidirectional influence where disturbances in one can lead to changes in the other. However, specific investigations into intestinal dysbiosis following improvements in sleep quality among individuals with ME/CFS have not been conducted, indicating a need for further research in this area.
Moderate exercise training
Physical activity, particularly moderate exercise, is a common approach for self-rehabilitation among ME/CFS patients due to its potential to reduce fatigue symptoms [222]. Research indicates that structured regimens of moderate physical activity over 12 to 26 weeks can significantly help in reducing fatigue compared to conventional or passive therapies [223]. A survey in Norway revealed that about 80% of ME/CFS patients experienced a decline in health after participating in graded exercise therapy [224]. This observation contrasts with research suggesting the combination of graded exercise therapy and cognitive behavioral therapy can alleviate fatigue, indicating ongoing debate about these therapies’ effectiveness [225]. Nevertheless, although moderate exercise training reduces gut transit time, prolonged strenuous exercise can increase gut permeability, leading to issues like diarrhea, bacterial translocation, gastrointestinal bleeding, and disorders [9]. In a study by Shukla et al., the relationship between bacterial translocation and post-exertional malaise was investigated. The researchers observed phenotypic characteristics, post-exertional malaise, and microbiome changes shortly after a maximal exercise test at intervals of 15 min, 48 h, and 72 h. They noted significant changes in pain, fatigue, and confusion levels post-exercise. Furthermore, they discovered interesting shifts in the relative abundance of intestinal microbiota compared to a control group. Specifically, the genus Clostridium appeared in the blood 15 min following exercise, while Bacilli levels significantly increased after 48 h. These findings suggest that increased bacterial translocation may occur after exertion. The authors emphasized that future research should focus more on changes in intestinal composition following exercise rather than just examining dysbiosis itself [24].
Conclusions
In summary, research has identified a link between the gut microbiome and ME/CFS, although causation has not been established. Changes in the gut microbiome may significantly impact ME/CFS, potentially due to increased gut permeability that allows bacteria to enter the body, or through fermentation products affecting cells and immune responses, exacerbating cognitive and physical impairment in ME/CFS patients. In this respect, future research should explore diagnostic methods like fecal and plasma analyses and consider treatments such as fecal microbiota transplantation. However, the widespread use of prebiotics and probiotics needs careful reconsideration because it is unclear whether gut dysbiosis contributes to the disease or results from it, or if it is related to factors like inactivity or antibiotic use. Additionally, to advance the understanding of ME/CFS pathophysiology, longitudinal studies should account for variables like physical activity and antibiotic use before disease onset. The role of other life events, including pregnancy, should also be examined as potential risk factors. Moreover, analyzing the microbiome in ME/CFS patients presents significant challenges, as studies often produce conflicting findings, particularly concerning gut imbalances. These discrepancies may arise from the varied diagnostic criteria employed across different research efforts, leading to diverse participant groups that exhibit distinct neurological, immune, infectious, muscular, and hormonal issues. Additionally, it is crucial to recognize the existence of ME/CFS subtypes, such as those arising post-infection versus other causes, as well as the fluctuating nature and intensity of symptoms. There is a general agreement in the scientific community on the need for improved research methodologies. This includes the consistent application of case definitions, enhancing study quality, and conducting more longitudinal research to gain clearer insights into ME/CFS. Future studies should take these considerations into account to advance understanding in this field. Furthermore, as highlighted in the existing literature, integrating microbiota assessments into clinical practice can serve as a significant step toward implementing individualized treatment strategies, thereby enhancing patient care and recovery prospects. Finally, it is imperative to acknowledge the complexity and heterogeneity of ME/CFS, which challenges researchers and clinicians in identifying effective therapeutic approaches. The intersection of genetic, environmental, and microbial factors emphasizes the need for a multidisciplinary approach in addressing this condition; systematic reviews point out inconsistencies in methodologies and outcomes in current research, indicating a critical gap in our understanding of how gut health can be effectively targeted for therapeutic gain in ME/CFS patients. By fostering collaborative research efforts that prioritize standardized diagnostic criteria and enhanced longitudinal studies, the potential for innovative treatments targeting gut health can be realized. Ultimately, the development of targeted therapeutic interventions related to gut health for ME/CFS can lead to improved quality of life and symptom management for those affected by this often-misunderstood disorder.
Acknowledgements
The authors are thankful to the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/152/46.
Author contributions
Chou-Yi Hsu, Irfan Ahmad, Rana Warid Maya, Mohsen Khosravi: Conceptualization, Methodology, Investigation, Data curation, Visualization, Supervision, Writing-Original draft preparation, Writing-Reviewing and Editing. Mayada Ahmed Abass, Jitendra Gupta, Abhayveer Singh, Kamal Kant Joshig, Premkumar J, Samir Sahoo: Writing- Original draft preparation, Writing-Reviewing and Editing.
Funding
No funding.
Availability of data and materials
The data are available from the corresponding author on a reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they had no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deumer US, Varesi A, Floris V, et al. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): an overview. J Clin Med. 2021;10(20):4786. 10.3390/jcm10204786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eun-Jin L, Ahn YC, Eun-Su J, Si-Woo L, Su-Hwa L, Chang-Gue S. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100. 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96(11):2861–78. 10.1016/j.mayocp.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Noor N, Urits I, Degueure A, et al. A comprehensive update of the current understanding of chronic fatigue syndrome. Anesth Pain Med. 2021;11(3): e113629. 10.5812/aapm.113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JH, Choi Y, Lee JS, Hwang SJ, Gu J, Son CG. Clinical evidence of the link between gut microbiome and myalgic encephalomyelitis/chronic fatigue syndrome: a retrospective review. Eur J Med Res. 2024;29(1):148. 10.1186/s40001-024-01747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurek JM, Castro-Marrero J. A narrative review on gut microbiome disturbances and microbial preparations in myalgic encephalomyelitis/chronic fatigue syndrome: implications for long COVID. Nutrients. 2024;16(11):1545. 10.3390/nu16111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seton KA, Espejo-Oltra JA, Giménez-Orenga K, Haagmans R, Ramadan DJ, Mehlsen J. Advancing research and treatment: an overview of clinical trials in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and future perspectives. J Clin Med. 2024;13(2):325. 10.3390/jcm13020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves BS, Patel M, Newgent H, et al. Chronic fatigue syndrome: diagnosis, treatment, and future direction. Cureus. 2024;16(10): e70616. 10.7759/cureus.70616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Sun W, Guo S, et al. Research progress on pathogenesis of chronic fatigue syndrome and treatment of traditional Chinese and Western medicine. Auton Neurosci. 2024;255: 103198. 10.1016/j.autneu.2024.103198. [DOI] [PubMed] [Google Scholar]
- 10.König RS, Albrich WC, Kahlert CR, et al. The gut microbiome in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Front Immunol. 2022;12: 628741. 10.3389/fimmu.2021.628741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varesi A, Deumer US, Ananth S, Ricevuti G. The emerging role of gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): current evidence and potential therapeutic applications. J Clin Med. 2021;10(21):5077. 10.3390/jcm10215077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupo GF, Rocchetti G, Lucini L, et al. Potential role of microbiome in chronic fatigue syndrome/myalgic encephalomyelits (CFS/ME). Sci Rep. 2021;11(1):7043. 10.1038/s41598-021-86425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stallmach A, Quickert S, Puta C, Reuken PA. The gastrointestinal microbiota in the development of ME/CFS: a critical view and potential perspectives. Front Immunol. 2024;2024(15):1352744. 10.3389/fimmu.2024.1352744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martín F, Blanco-Suárez M, Zambrano P, et al. Increased gut permeability and bacterial translocation are associated with fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: Implications for disease-related biomarker discovery. Front Immunol. 2023;14:1253121. 10.3389/fimmu.2023.1253121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nacul L, O’Boyle S, Palla L, et al. How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol. 2020;11:826. 10.3389/fneur.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arron HE, Marsh BD, Kell DB, Khan MA, Jaeger BR, Pretorius E. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: the biology of a neglected disease. Front Immunol. 2024;15:1386607. 10.3389/fimmu.2024.1386607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweetman E, Noble A, Edgar C, et al. Current research provides insight into the biological basis and diagnostic potential for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Diagnostics. 2019;9(3):73. 10.3390/diagnostics9030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. 2019;9(3):80. 10.3390/diagnostics9030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang K, Lidbury BA, Thomas N, Gooley PR, Armstrong CW. Machine learning and multi-omics in precision medicine for ME/CFS. J Transl Med. 2025;23(1):68. 10.1186/s12967-024-05915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLure M. ‘Clarity bordering on stupidity’: where’s the quality in systematic review? J Educ Policy. 2005;20(4):393–416. 10.1080/02680930500131801. [Google Scholar]
- 21.Maes M, Mihaylova I, Leunis JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut–intestinal permeability. J Affect Disord. 2007;99(1–3):237–40. 10.1016/j.jad.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Sheedy JR, Wettenhall RE, Scanlon D, et al. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo. 2009;23(4):621–8. [PubMed] [Google Scholar]
- 23.Frémont M, Coomans D, Massart S, De Meirleir K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe. 2013;22:50–6. 10.1016/j.anaerobe.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Shukla SK, Cook D, Meyer J, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE. 2015;10(12): e0145453. 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. The association of fecal microbiota and fecal, blood serum and urine metabolites in myalgic encephalomyelitis/chronic fatigue syndrome. Metabolomics. 2017;13:8. 10.1007/s11306-016-1145-z. [Google Scholar]
- 26.Giloteaux L, Hanson MR, Keller BA. A pair of identical twins discordant for myalgic encephalomyelitis/chronic fatigue syndrome differ in physiological parameters and gut microbiome composition. Am J Case Rep. 2016;2016(17):720–9. 10.12659/AJCR.900314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):30. 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy-Szakal D, Williams BL, Mishra N, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5(1):44. 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandarano AH, Giloteaux L, Keller BA, Levine SM, Hanson MR. Eukaryotes in the gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome. PeerJ. 2018;2018(6): e4282. 10.7717/peerj.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitami T, Fukuda S, Kato T, et al. Deep phenotyping of myalgic encephalomyelitis/chronic fatigue syndrome in Japanese population. Sci Rep. 2020;10(1):19933. 10.1038/s41598-020-77105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He G, Cao Y, Ma H, et al. Causal effects between gut microbiome and myalgic encephalomyelitis/chronic fatigue syndrome: a two-sample Mendelian randomization study. Front Microbiol. 2023;14:1190894. 10.3389/fmicb.2023.1190894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Preez S, Corbitt M, Cabanas H, Eaton N, Staines D, Marshall-Gradisnik S. A systematic review of enteric dysbiosis in chronic fatigue syndrome/myalgic encephalomyelitis. Syst Rev. 2018;7(1):241. 10.1186/s13643-018-0909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navaneetharaja N, Griffiths V, Wileman T, Carding SR. A role for the intestinal microbiota and virome in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)? J Clin Med. 2016;5(6):55. 10.3390/jcm5060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proal A, Marshall T. Myalgic encephalomyelitis/chronic fatigue syndrome in the era of the human microbiome: persistent pathogens drive chronic symptoms by interfering with host metabolism, gene expression, and immunity. Front Pediatr. 2018;6:373. 10.3389/fped.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newberry F, Hsieh SY, Wileman T, Carding SR. Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome? Clin Sci. 2018;132(5):523–42. 10.1042/CS20171330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin JD, Atkinson G, Atkinson JM, Batterham AM. Peak oxygen uptake in chronic fatigue syndrome/myalgic encephalomyelitis: a meta-analysis. Int J Sports Med. 2019;40(2):77–87. 10.1055/a-0802-9175. [DOI] [PubMed] [Google Scholar]
- 38.Montagnani M, Bottalico L, Potenza MA, et al. The crosstalk between gut microbiota and nervous system: a bidirectional interaction between microorganisms and metabolome. Int J Mol Sci. 2023;24(12):10322. 10.3390/ijms241210322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–25. 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson MR, Giloteaux L. The gut microbiome in Myalgic Encephalomyelitis. Biochem. 2017;39(2):10–3. 10.1042/BIO03902010. [Google Scholar]
- 41.Tsai SY, Chen HJ, Chen C, et al. Increased risk of chronic fatigue syndrome following psoriasis: a nationwide population-based cohort study. J Transl Med. 2019;17(1):55. 10.1186/s12967-019-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes Rivera M, Mastronardi C, Silva-Aldana CT, Arcos-Burgos M, Lidbury BA. Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics. 2019;9(3):91. 10.3390/diagnostics9030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25. 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallis A, Ball M, Butt H, et al. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: neuropsychological symptoms and sex comparisons. J Transl Med. 2018;16(1):24. 10.1186/s12967-018-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–72. 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu L, Valencia IJ, Garvert DW, Montoya JG. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. 2019;7:12. 10.3389/fped.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–38. 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathew SJ, Mao X, Keegan KA, et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T 1H MRS imaging study. NMR Biomed. 2009;22(3):251–8. 10.1002/nbm.1315. [DOI] [PubMed] [Google Scholar]
- 50.Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–90. 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos. 2015;43(10):1522–35. 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blankfield A. A brief historic overview of clinical disorders associated with tryptophan: the relevance to chronic fatigue syndrome (CFS) and fibromyalgia (FM). Int J Tryptophan Res. 2012;5:27–32. 10.4137/IJTR.S10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Korecka A, Dona A, Lahiri S, et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016;2:16014. 10.1038/npjbiofilms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8(20):1–27. 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- 56.Eleftheriadis T, Pissas G, Sounidaki M, et al. Indoleamine 2, 3-dioxygenase, by degrading L-tryptophan, enhances carnitine palmitoyltransferase I activity and fatty acid oxidation, and exerts fatty acid-dependent effects in human alloreactive CD4+ T-cells. Int J Mol Med. 2016;38(5):605–1613. 10.3892/ijmm.2016.2750. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt SV, Schultze JL. New insights into IDO biology in bacterial and viral infections. Front Immunol. 2014;2014(5):384. 10.3389/fimmu.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashi AA, Davis RW, Phair RD. The IDO metabolic trap hypothesis for the etiology of ME/CFS. Diagnostics. 2019;9(3):82. 10.3390/diagnostics9030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen M, Liu Y, Xiong S, et al. Dietary l-tryptophan alleviated LPS-induced intestinal barrier injury by regulating tight junctions in a Caco-2 cell monolayer model. Food Funct. 2019;10(5):2390–8. 10.1039/c9fo00123a. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;2018(8):13. 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2, 3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24(8):1113–20. 10.1038/s41591-018-0060-4. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki Y, Suda T, Furuhashi K, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67(3):361–5. 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Badawy AA, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2, 3-dioxygenase: time for appraisal. Int J Tryptophan Res. 2019;12:1–10. 10.1177/1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu WL, Lin YH, Xiao H, et al. Epstein-Barr virus infection induces indoleamine 2, 3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-κB pathways: impairment in T cell functions. J Virol. 2014;88(12):6660–71. 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18(7):1214–20. 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blomberg J, Gottfries CG, Elfaitouri A, Rizwan M, Rosén A. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol. 2018;9:229. 10.3389/fimmu.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maes M, Ringel K, Kubera M, et al. In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J Affect Disord. 2013;150(2):223–30. 10.1016/j.jad.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Nicolson GL, Gan R, Haier J. Multiple co-infections (mycoplasma, chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: association with signs and symptoms. APMIS. 2003;111(5):557–66. 10.1034/j.1600-0463.2003.1110504.x. [DOI] [PubMed] [Google Scholar]
- 73.Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163(13):1530–6. 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- 74.Schröder W, Sommer H, Gladstone BP, et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1800–6. 10.1093/jac/dkw054. [DOI] [PubMed] [Google Scholar]
- 75.Thambirajah AA, Sleigh K, Grant Stiver H, Chow AW. Differential heat shock protein responses to strenuous standardized exercise in chronic fatigue syndrome patients and matched healthy controls. Clin Invest Med. 2008;31(6):E319–27. 10.25011/cim.v31i6.4917. [DOI] [PubMed] [Google Scholar]
- 76.Shungu DC, Weiduschat N, Murrough JW, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25(9):1073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. 2015;11:1626–39. 10.1007/s11306-015-0816-5. [Google Scholar]
- 78.Jackson ML, Butt H, Ball M, Lewis DP, Bruck D. Sleep quality and the treatment of intestinal microbiota imbalance in chronic fatigue syndrome: a pilot study. Sleep Sci. 2015;8(3):124–33. 10.1016/j.slsci.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris G, Anderson G, Maes M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. 2017;54(9):6806–19. 10.1007/s12035-016-0170-2. [DOI] [PubMed] [Google Scholar]
- 80.Herane-Vives A, Papadopoulos A, De Angel V, et al. Cortisol levels in chronic fatigue syndrome and atypical depression measured using hair and saliva specimens. J Affect Disord. 2020;267:307–14. 10.1016/j.jad.2020.01.146. [DOI] [PubMed] [Google Scholar]
- 81.Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 82.Colletti A, Pellizzato M, Cicero AF. The possible role of probiotic supplementation in inflammation: a narrative review. Microorganisms. 2023;11(9):2160. 10.3390/microorganisms11092160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–29. 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan A, Nord CE, Evengård B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. 10.1186/1475-2891-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corbitt M, Campagnolo N, Staines D, Marshall-Gradisnik S. A systematic review of probiotic interventions for gastrointestinal symptoms and irritable bowel syndrome in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Probiotics Antimicrob Proteins. 2018;10(3):466–77. 10.1007/s12602-018-9397-8. [DOI] [PubMed] [Google Scholar]
- 86.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–23. 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 87.Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4(4):325–39. 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daniels J, Brigden A, Kacorova A. Anxiety and depression in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): examining the incidence of health anxiety in CFS/ME. Psychol Psychother. 2017;90(3):502–9. 10.1111/papt.12118. [DOI] [PubMed] [Google Scholar]
- 89.Karakula-Juchnowicz H, Rog J, Juchnowicz D, et al. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. 2019;18(1):50. 10.1186/s12937-019-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffith JP, Zarrouf FA. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Prim Care Companion J Clin Psychiatry. 2018;10(2):120–8. 10.4088/pcc.v10n0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boudry G, Hamilton MK, Chichlowski M, et al. Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J Dairy Sci. 2017;100(4):2471–81. 10.3168/jds.2016-11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu T, Wang Y, Chen X, Xiong W, Tang Y, Lin L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J Cell Mol Med. 2020;24(15):8603–13. 10.1111/jcmm.15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, Lin T, Meng Y, et al. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism. 2021;119: 154767. 10.1016/j.metabol.2021.154767. [DOI] [PubMed] [Google Scholar]
- 96.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103. 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nettleton JE, Klancic T, Schick A, et al. Low-dose stevia (rebaudioside A) consumption perturbs gut microbiota and the mesolimbic dopamine reward system. Nutrients. 2019;11(6):1248. 10.3390/nu11061248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Snelson M, Clarke RE, Nguyen TV, et al. Long term high protein diet feeding alters the microbiome and increases intestinal permeability, systemic inflammation and kidney injury in mice. Mol Nutr Food Res. 2021;65(8): e2000851. 10.1002/mnfr.202000851. [DOI] [PubMed] [Google Scholar]
- 100.Do MH, Lee E, Oh MJ, Kim Y, Park HY. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 2018;10(6):761. 10.3390/nu10060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nilholm C, Roth B, Ohlsson B. A dietary intervention with reduction of starch and sucrose leads to reduced gastrointestinal and extra-intestinal symptoms in IBS patients. Nutrients. 2019;11(7):1662. 10.3390/nu11071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ott B, Skurk T, Hastreiter L, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7(1):11955. 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrasco-Querol N, Cabricano-Canga L, Bueno Hernández N, et al. Effectiveness of the SYNCHRONIZE+ brief intervention in improving Mediterranean diet adherence, nutritional quality and intake pattern in persons with fibromyalgia and chronic fatigue syndrome. Nutrients. 2024;17(1):11. 10.3390/nu17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merra G, Noce A, Marrone G, et al. Influence of mediterranean diet on human gut microbiota. Nutrients. 2020;13(1):7. 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kortlever TL, Ten Bokkel HS, Offereins M, et al. Low-FODMAP diet is associated with improved quality of life in IBS patients—a prospective observational study. Nutr Clin Pract. 2019;34(4):623–30. 10.1002/ncp.10233. [DOI] [PubMed] [Google Scholar]
- 106.Brenton JN, Banwell B, Bergqvist AC, et al. Goldman, Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(4): e565. 10.1212/NXI.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phillips MC, Murtagh DK, Gilbertson LJ, Asztely FJ, Lynch CD. Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov Disord. 2018;33(8):1306–14. 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bauersfeld SP, Kessler CS, Wischnewsky M, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263-1275.e16. 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Craig C. Mitoprotective dietary approaches for myalgic encephalomyelitis/chronic fatigue syndrome: caloric restriction, fasting, and ketogenic diets. Med Hypotheses. 2015;85(5):690–3. 10.1016/j.mehy.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 111.Cossington J, Coe DS, Liu Y, Dawes H. Potential benefits of a ketogenic diet to improve response and recovery from physical exertion in people with Myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a feasibility study. Int J Sport Exerc Health Res. 2019;3(2):33–9. 10.31254/sportmed.3202. [Google Scholar]
- 112.Sathyapalan T, Beckett S, Rigby AS, Mellor DD, Atkin SL. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55. 10.1186/1475-2891-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campagnolo N, Johnston S, Collatz A, Staines D, Marshall-Gradisnik S. Dietary and nutrition interventions for the therapeutic treatment of chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. J Hum Nutr Diet. 2017;30(3):247–59. 10.1111/jhn.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaliannan K, Wang B, Li XY, Kim KJ, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5:11276. 10.1038/srep11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puri BK. The use of eicosapentaenoic acid in the treatment of chronic fatigue syndrome. Prostaglandins Leukot Essent Fatty Acids. 2004;70(4):399–401. 10.1016/j.plefa.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 116.Puri BK. Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome). J Clin Pathol. 2007;60(2):122–4. 10.1136/jcp.2006.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang JH, Bose S, Kim GC, et al. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE. 2014;9(1): e86117. 10.1371/journal.pone.0086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang S, Li Q, Zang Y, et al. Apple Polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int J Biol Macromol. 2017;99:282–92. 10.1016/j.ijbiomac.2017.02.074. [DOI] [PubMed] [Google Scholar]
- 119.Fujisaka S, Usui I, Nawaz A, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep. 2020;10(1):5544. 10.1038/s41598-020-62506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X, Qu Y, Zhang Y, et al. Antifatigue potential activity of Sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress. Oxid Med Cell Longev. 2018;2018:9140896. 10.1155/2018/9140896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwon DA, Kim YS, Kim SK, Baek SH, Kim HK, Lee HS. Antioxidant and antifatigue effect of a standardized fraction (HemoHIM) from Angelica gigas, Cnidium officinale, and Paeonia lactiflora. Pharm Biol. 2021;59(1):391–400. 10.1080/13880209.2021.1900878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J, Sun C, Zheng Y, Pan H, Zhou Y, Fan Y. The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Arch Pharm Res. 2014;37(4):530–8. 10.1007/s12272-013-0235-y. [DOI] [PubMed] [Google Scholar]
- 123.Kan J, Cheng J, Hu C, et al. A botanical product containing Cistanche and Ginkgo extracts potentially improves chronic fatigue syndrome symptoms in adults: a randomized, double-blind, and placebo-controlled study. Front Nutr. 2021;8: 658630. 10.3389/fnut.2021.658630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo H, Gong R, Zheng R, et al. Dose–effect of long-snake-like moxibustion for chronic fatigue syndrome: a randomized controlled trial. J Transl Med. 2023;21(1):430. 10.1186/s12967-023-04250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J, Shin KM, Abu Dabrh AM, et al. Ginseng for the treatment of chronic fatigue syndrome: a systematic review of clinical studies. Glob Adv Health Med. 2022. 10.1177/2164957X221079790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bentler SE, Hartz AJ, Kuhn EM. Prospective observational study of treatments for unexplained chronic fatigue. J Clin Psychiatry. 2005;66(5):625–32. 10.4088/jcp.v66n0513. [DOI] [PubMed] [Google Scholar]
- 127.Sung WS, Kang HR, Jung CY, Park SS, Lee SH, Kim EJ. Efficacy of Korean red ginseng (Panax ginseng) for middle-aged and moderate level of chronic fatigue patients: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2020;48: 102246. 10.1016/j.ctim.2019.102246. [DOI] [PubMed] [Google Scholar]
- 128.Teitelbaum J, Goudie S. An open-label, pilot trial of HRG80™ red ginseng in chronic fatigue syndrome, fibromyalgia, and post-viral fatigue. Pharmaceuticals. 2021;15(1):43. 10.3390/ph15010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dorczok MC, Mittmann G, Mossaheb N, et al. Dietary supplementation for fatigue symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review. Nutrients. 2025;17(3):475. 10.3390/nu17030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Castro-Marrero J, Domingo JC, Cordobilla B, et al. Does coenzyme Q10 plus selenium supplementation ameliorate clinical outcomes by modulating oxidative stress and inflammation in individuals with myalgic encephalomyelitis/chronic fatigue syndrome? Antioxid Redox Signal. 2022;36(10–12):729–39. 10.1089/ars.2022.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Castro-Marrero J, Segundo MJ, Lacasa M, Martinez-Martinez A, Sentañes RS, Alegre-Martin J. Effect of dietary coenzyme Q10 plus NADH supplementation on fatigue perception and health-related quality of life in individuals with myalgic encephalomyelitis/chronic fatigue syndrome: a prospective, randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13(8):2658. 10.3390/nu13082658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Forsyth LM, Preuss HG, MacDowell AL, Chiazze L Jr, Birkmayer GD, Bellanti JA. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol. 1999;82(2):185–91. 10.1016/S1081-1206(10)62595-1. [DOI] [PubMed] [Google Scholar]
- 133.Kurup RK, Kurup PA. Isoprenoid pathway dysfunction in chronic fatigue syndrome. Acta Neuropsychiatr. 2003;15(5):266–73. 10.1034/j.1601-5215.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 134.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuroendocrinol Lett. 2009;30(4):470–6. [PubMed] [Google Scholar]
- 135.Castro-Marrero J, Cordero MD, Sáez-Francas N, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013;19(15):1855–60. 10.1089/ars.2013.5346. [DOI] [PubMed] [Google Scholar]
- 136.Ivanova AY, Shirokov IV, Toshchakov SV, et al. Effects of coenzyme Q10 on the biomarkers (Hydrogen, Methane, SCFA and TMA) and composition of the gut microbiome in rats. Pharmaceuticals. 2023;16(5):686. 10.3390/ph16050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsai I, Hsu CW, Chang CH, Tseng PT, Chang KV. Effectiveness of coenzyme Q10 supplementation for reducing fatigue: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13: 883251. 10.3389/fphar.2022.883251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castro-Marrero J, Zaragozá MC, López-Vílchez I, et al. Effect of melatonin plus zinc supplementation on fatigue perception in myalgic encephalomyelitis/chronic fatigue syndrome: a randomized, double-blind, placebo-controlled trial. Antioxidants. 2021;10(7):1010. 10.3390/antiox10071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Putnam EE, Goodman AL. B vitamin acquisition by gut commensal bacteria. PLoS Pathog. 2020;16(1): e1008208. 10.1371/journal.ppat.1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soto-Martin EC, Warnke I, Farquharson FM, et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. MBio. 2020;11(4):e00886-e920. 10.1128/mBio.00886-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Costliow ZA, Degnan PH. Thiamine acquisition strategies impact metabolism and competition in the gut microbe Bacteroides thetaiotaomicron. mSystems. 2017;2(5):e00116-17. 10.1128/mSystems.00116-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kreijkamp-Kaspers S, Brenu EW, Marshall S, Staines D, Van Driel ML. Treating chronic fatigue syndrome: a study into the scientific evidence for pharmacological treatments. Aust Fam Physician. 2011;40(11):907–12. [PubMed] [Google Scholar]
- 143.Bansal AS. Investigating unexplained fatigue in general practice with a particular focus on CFS/ME. BMC Fam Pract. 2016;17:81. 10.1186/s12875-016-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Raij T, Raij K. Association between fatigue, peripheral serotonin, and L-carnitine in hypothyroidism and in chronic fatigue syndrome. Front Endocrinol. 2024;15:1358404. 10.3389/fendo.2024.1358404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joustra ML, Minovic I, Janssens KA, Bakker SJ, Rosmalen JG. Vitamin and mineral status in chronic fatigue syndrome and fibromyalgia syndrome: a systematic review and meta-analysis. PLoS ONE. 2017;12(4): e0176631. 10.1371/journal.pone.0176631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vecchiet J, Cipollone F, Falasca K, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335(3):151–4. 10.1016/s0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 147.Jones K, Probst Y. Role of dietary modification in alleviating chronic fatigue syndrome symptoms: a systematic review. Aust N Z J Public Health. 2017;41(4):338–44. 10.1111/1753-6405.12670. [DOI] [PubMed] [Google Scholar]
- 148.Toogood PL, Clauw DJ, Phadke S, Hoffman D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): where will the drugs come from? Pharmacol Res. 2021;165: 105465. 10.1016/j.phrs.2021.105465. [DOI] [PubMed] [Google Scholar]
- 149.Tan P, Li X, Shen J, Feng Q. Fecal microbiota transplantation for the treatment of inflammatory bowel disease: an update. Front Pharmacol. 2020;11: 574533. 10.3389/fphar.2020.574533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gupta A, Khanna S. Fecal microbiota transplantation. JAMA. 2017;318(1):102. 10.1001/jama.2017.6466. [DOI] [PubMed] [Google Scholar]
- 151.Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. A review of microbiota and irritable bowel syndrome: future in therapiesm. Adv Ther. 2018;35(3):289–310. 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Juul FE, Garborg K, Bretthauer M, et al. Fecal microbiota transplantation for primary Clostridium difficile infection. N Engl J Med. 2018;378(26):2535–6. 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 153.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention ofclostridium difficileInfections. Am J Gastroenterol. 2014;108(4):478–98. 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 154.Malnick SD, Fisher D, Somin M, Neuman MG. Treating the metabolic syndrome by fecal transplantation—current status. Biology. 2021;10(5):447. 10.3390/biology10050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Choi HH, Cho YS. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. 2016;49(3):257–65. 10.5946/ce.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer. 2019;145(8):2021–31. 10.1002/ijc.32003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim MS, Kim Y, Choi H, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69(2):283–94. 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 158.Xu MQ, Cao HL, Wang WQ, et al. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol. 2015;21(1):102–11. 10.3748/wjg.v21.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Evrensel A, Ceylan ME. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci. 2016;14(3):231–7. 10.9758/cpn.2016.14.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borody TJ, Nowak A, Finlayson S. The GI microbiome and its role in chronic fatigue syndrome: a summary of bacteriotherapy. J Australas Coll Nutr Environ Med. 2012;31(3):3–8. 10.3316/informit.119626231492520. [Google Scholar]
- 161.Kenyon JN, Coe S, Izadi H. A retrospective outcome study of 42 patients with Chronic Fatigue Syndrome, 30 of whom had Irritable Bowel Syndrome. Half were treated with oral approaches, and half were treated with Faecal Microbiome Transplantation. Hum Microbiome J. 2019;13:100061. 10.1016/j.humic.2019.100061. [Google Scholar]
- 162.Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24(1):5–14. 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salonen T, Jokinen E, Satokari R, Lahtinen P. Randomized, double-blinded, placebo-controlled pilot study: efficacy of faecal microbiota transplantation on chronic fatigue syndrome. J Transl Med. 2023;21(1):513. 10.1186/s12967-023-04227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schmulson M, Bashashati M. Fecal microbiota transfer for bowel disorders: efficacy or hype? Curr Opin Pharmacol. 2018;43:72–80. 10.1016/j.coph.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 165.Lopetuso LR, Ianiro G, Allegretti JR, et al. Fecal transplantation for ulcerative colitis: current evidence and future applications. Expert Opin Biol Ther. 2020;20(4):343–51. 10.1080/14712598.2020.1733964. [DOI] [PubMed] [Google Scholar]
- 166.Shanahan F, Quigley EM. Manipulation of the microbiota for treatment of IBS and IBD—challenges and controversies. Gastroenterology. 2014;146(6):1554–63. 10.1053/j.gastro.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 167.Imdad A, Pandit NG, Zaman M, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11(11):CD012774. 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29(1):79–84. 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 169.Levy AN, Allegretti JR. Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Therap Adv Gastroenterol. 2019;12:1756284819836893. 10.1177/1756284819836893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Keshteli AH, Millan B, Madsen KL. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: a meta-analysis. Mucosal Immunol. 2017;10(2):565–6. 10.1038/mi.2016.123. [DOI] [PubMed] [Google Scholar]
- 171.Podlesny D, Durdevic M, Paramsothy S, et al. Identification of clinical and ecological determinants of strain engraftment after fecal microbiota transplantation using metagenomics. Cell Rep Med. 2022;3(8): 100711. 10.1016/j.xcrm.2022.100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ianiro G, Punčochář M, Karcher N, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022;28(9):1913–23. 10.1038/s41591-022-01964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vich Vila A, Collij V, Sanna S, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11(1):362. 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510–9. 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–8. 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Verdegaal AA, Goodman AL. Integrating the gut microbiome and pharmacology. Sci Transl Med. 2024;16(732):eadg8357. 10.1126/scitranslmed.adg8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pimentel M, Hallegua D, Chow EJ, Wallace D, Bonorris G, Lin HC. Eradication of small intestinal bacterial overgrowth decreases symptoms in chronic fatigue syndrome: a double blind, randomized study. Gastroenterology. 2000;118(4):A414. 10.1016/S0016-5085(00)83765-8. [Google Scholar]
- 178.Wei S, Bahl MI, Baunwall SM, Hvas CL, Licht TR. Determining gut microbial dysbiosis: a review of applied indexes for assessment of intestinal microbiota imbalances. Appl Environ Microbiol. 2021;87(11):e00395-e421. 10.1128/AEM.00395-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat Rev Microbiol. 2011;9(4):233–43. 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 180.Abdulsalam M, Hamisu AA, Ahmad AM, Wakili FB, Annu FS, Garba MM. Microbiome dynamics and strategic management strategies: exploring synergies between integrative medicine modalities and microbial balance. Biol Sci. 2024;4(3):725–35. 10.55006/biolsciences.2024.4307. [Google Scholar]
- 181.Wang S, Ju D, Zeng X. Mechanisms and clinical implications of human gut microbiota-drug interactions in the precision medicine era. Biomedicines. 2024;12(1):194. 10.3390/biomedicines12010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mousa S, Sarfraz M, Mousa WK. The interplay between gut microbiota and oral medications and its impact on advancing precision medicine. Metabolites. 2023;13(5):674. 10.3390/metabo13050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao Q, Chen Y, Huang W, Zhou H, Zhang W. Drug-microbiota interactions: an emerging priority for precision medicine. Signal Transduct Target Ther. 2023;8(1):386. 10.1038/s41392-023-01619-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.WanY ZT. Interplays between drugs and the gut microbiome. Gastroenterol Rep. 2022;10:goac009. 10.1093/gastro/goac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maseda D, Ricciotti E. NSAID–gut microbiota interactions. Front Pharmacol. 2020;11:1153. 10.3389/fphar.2020.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Theoharides TC, Asadi S, Weng Z, Zhang B. Serotonin-selective reuptake inhibitors and nonsteroidal anti-inflammatory drugs-important considerations of adverse interactions especially for the treatment of myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Psychopharmacol. 2011;31(4):403–5. 10.1097/JCP.0b013e318225848c. [DOI] [PubMed] [Google Scholar]
- 187.Hickie IB, Wilson AJ, Wright JM, Bennett BK, Wakefield D, Lloyd AR. A randomized, double-blind, placebo-controlled trial of moclobemide in patients with chronic fatigue syndrome. J Clin Psychiatry. 2000;61(9):643–8. 10.4088/jcp.v61n0909. [DOI] [PubMed] [Google Scholar]
- 188.Natelson BH, Pareja J, Policastro T, Cheu J, Ellis SP, Findley TW. Randomized, double blind, controlled placebo-phase in trial of low dose phenelzine in the chronic fatigue syndrome. Psychopharmacology. 1996;124(3):226–30. 10.1007/BF02246661. [DOI] [PubMed] [Google Scholar]
- 189.Amsterdam JD, Shults J, Rutherford N. Open-label study of s-citalopram therapy of chronic fatigue syndrome and co-morbid major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):100–6. 10.1016/j.pnpbp.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 190.Vercoulen JH, Hoofs MP, Bleijenberg G, et al. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet. 1996;347(9005):858–61. 10.1016/s0140-6736(96)91345-8. [DOI] [PubMed] [Google Scholar]
- 191.Wearden AJ, Morriss RK, Mullis R, et al. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry. 1998;172:485–90. 10.1192/bjp.172.6.485. [DOI] [PubMed] [Google Scholar]
- 192.Arnold LM, Blom TJ, Welge JA, Mariutto E, Heller A. A randomized, placebo-controlled, double-blinded trial of duloxetine in the treatment of general fatigue in patients with chronic fatigue syndrome. Psychosomatics. 2015;56(3):242–53. 10.1016/j.psym.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 193.McGovern AS, Hamlin AS, Winter G. A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust N Z J Psychiatry. 2019;53(12):1151–66. 10.1177/0004867419877954. [DOI] [PubMed] [Google Scholar]
- 194.Zhang W, Qu W, Wang H, Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2021;11(1):131. 10.1038/s41398-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li J, Qu W, Hu C, Liu Z, Yan H. Antidepressants amitriptyline, fluoxetine, and traditional Chinese medicine Xiaoyaosan caused alterations in gut DNA virome composition and function in rats exposed chronic unpredictable mild stress. Front Microbiol. 2023;14:1132403. 10.3389/fmicb.2023.1132403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shi Y, Chen C, Wu X, et al. Exposure to amitriptyline induces persistent gut damages and dysbiosis of the gut microbiota in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol. 2022;260: 109417. 10.1016/j.cbpc.2022.109417. [DOI] [PubMed] [Google Scholar]
- 197.Ninomiya K, Paudel D, Uehara O, et al. Effect of systemic administration of amitriptyline on oral microbes in rats. In Vivo. 2022;36(5):2134–42. 10.21873/invivo.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Woo SB, Schacterle RS, Komaroff AL, Gallagher GT. Salivary gland changes in chronic fatigue syndrome: a case-controlled preliminary histologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(1):82–7. 10.1067/moe.2000.107363. [DOI] [PubMed] [Google Scholar]
- 199.Freitag H, Szklarski M, Lorenz S, et al. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med. 2021;10(16):3675. 10.3390/jcm10163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gil A, Hoag GE, Salerno JP, Hornig M, Klimas N, Selin LK. Identification of CD8 T-cell dysfunction associated with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID and treatment with a nebulized antioxidant/anti-pathogen agent in a retrospective case series. Brain Behav Immun Health. 2023;36: 100720. 10.1016/j.bbih.2023.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12: 578386. 10.3389/fimmu.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay-Kumar M, Saha P. Crosstalk between gut microbiota and host immunity: impact on inflammation and immunotherapy. Biomedicines. 2023;11(2):294. 10.3390/biomedicines11020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou P, Chen C, Patil S, Dong S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front Nutr. 2024;11:1355542. 10.3389/fnut.2024.1355542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Z, Wang Z, Lu T, et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022;65: 101691. 10.1016/j.smrv.2022.101691. [DOI] [PubMed] [Google Scholar]
- 205.Sun J, Fang D, Wang Z, Liu Y. Sleep deprivation and gut microbiota dysbiosis: current understandings and implications. Int J Mol Sci. 2023;24(11):9603. 10.3390/ijms24119603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neroni B, Evangelisti M, Radocchia G, et al. Relationship between sleep disorders and gut dysbiosis: what affects what? Sleep Med. 2021;87:1–7. 10.1016/j.sleep.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 207.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53: 101340. 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 208.Benedict C, Vogel H, Jonas W, et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. 2016;5(12):1175–86. 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu P, Wang J, Hong F, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4): e12399. 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- 210.Poroyko VA, Carreras A, Khalyfa A, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bowers SJ, Vargas F, González A, et al. Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS ONE. 2020;15(2): e0229001. 10.1371/journal.pone.0229001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Smith RP, Easson C, Lyle SM, et al. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE. 2019;14(10): e0222394. 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu B, Lin W, Chen S, et al. Gut microbiota as an objective measurement for auxiliary diagnosis of insomnia disorder. Front Microbiol. 2019;10:1770. 10.3389/fmicb.2019.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–29. 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 215.Louis P, O’byrne CP. Life in the gut: microbial responses to stress in the gastrointestinal tract. Sci Prog. 2010;93(1):7–36. 10.3184/003685009X12605525292307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res. 2014;76(1):2–10. 10.1038/pr.2014.49. [DOI] [PubMed] [Google Scholar]
- 217.Salzman NH. The role of the microbiome in immune cell development. Ann Allergy Asthma Immunol. 2014;113(6):593–8. 10.1016/j.anai.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 218.Lobionda S, Sittipo P, Kwon HY, Lee YK. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019;7(8):271. 10.3390/microorganisms7080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vitetta L, Briskey D, Alford H, Hall S, Coulson S. Probiotics, prebiotics and the gastrointestinal tract in health and disease. Inflammopharmacology. 2014;22(3):135–54. 10.1007/s10787-014-0201-4. [DOI] [PubMed] [Google Scholar]
- 220.Mohammed RN, Khosravi M, Rahman HS, et al. Anastasis: cell recovery mechanisms and potential role in cancer. Cell Commun Signal. 2022;20(1):81. 10.1186/s12964-022-00880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.El-Sehrawy AA, Ayoub II, Uthirapathy S, et al. The microbiota-gut-brain axis in myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review of an emerging field. Eur J Transl Myol. 2025;35(1):13690. 10.4081/ejtm.2025.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Clark LV, Pesola F, Thomas JM, Vergara-Williamson M, Beynon M, White PD. Guided graded exercise self-help plus specialist medical care versus specialist medical care alone for chronic fatigue syndrome (GETSET): a pragmatic randomised controlled trial. Lancet. 2017;390(10092):363–73. 10.1016/S0140-6736(16)32589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2019;10(10):CD003200. 10.1002/14651858.CD003200.pub8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bjørkum T, Wang CE. Patients’ experience with treatment of chronic fatigue syndrome. Tidsskr Nor Laegeforen. 2009;129(12):1214–6. 10.4045/tidsskr.09.35791. [DOI] [PubMed] [Google Scholar]
- 225.White PD, Goldsmithb KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomized trial. Lancet. 2011;377(9768):823–36. 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on a reasonable request.