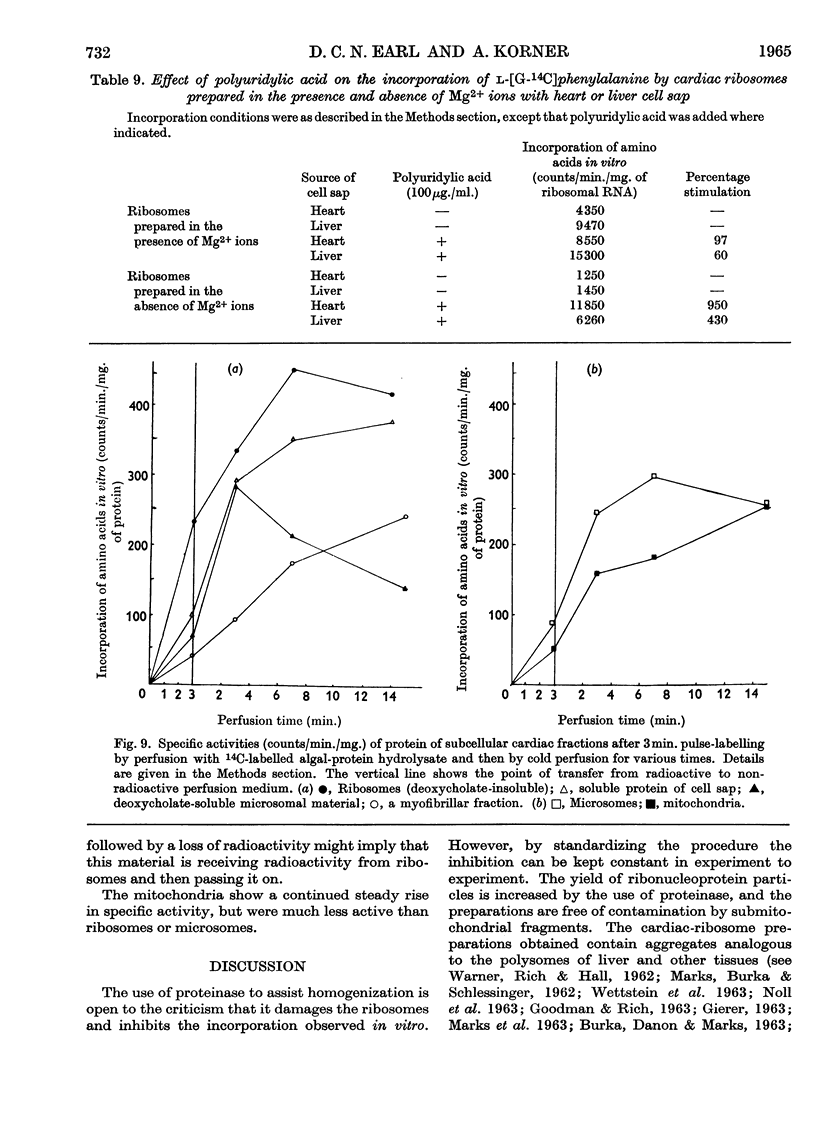
Abstract
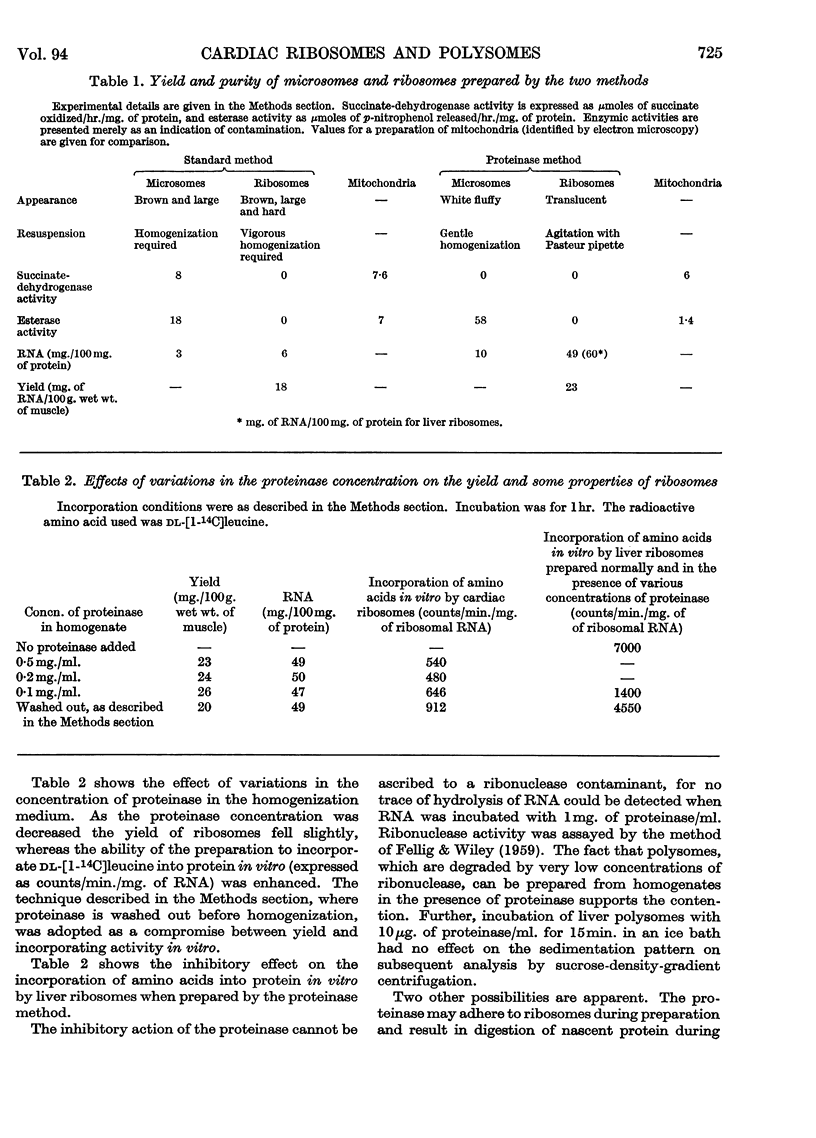
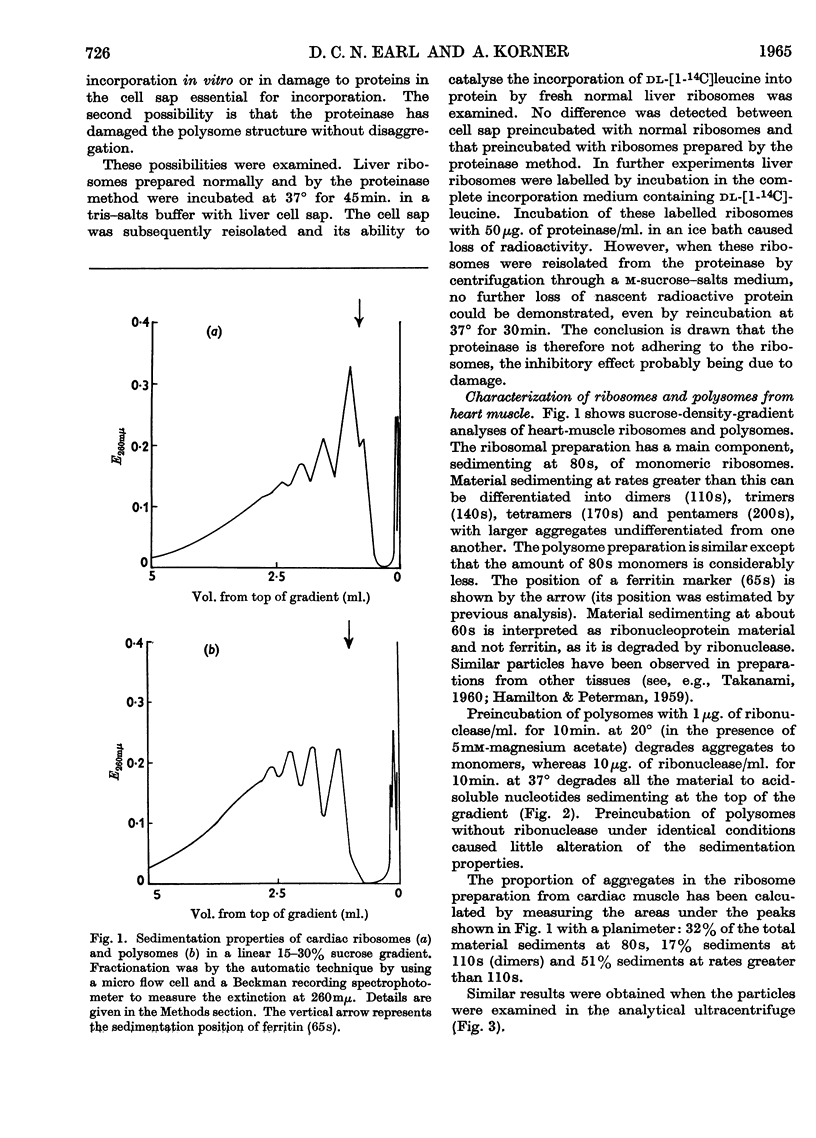
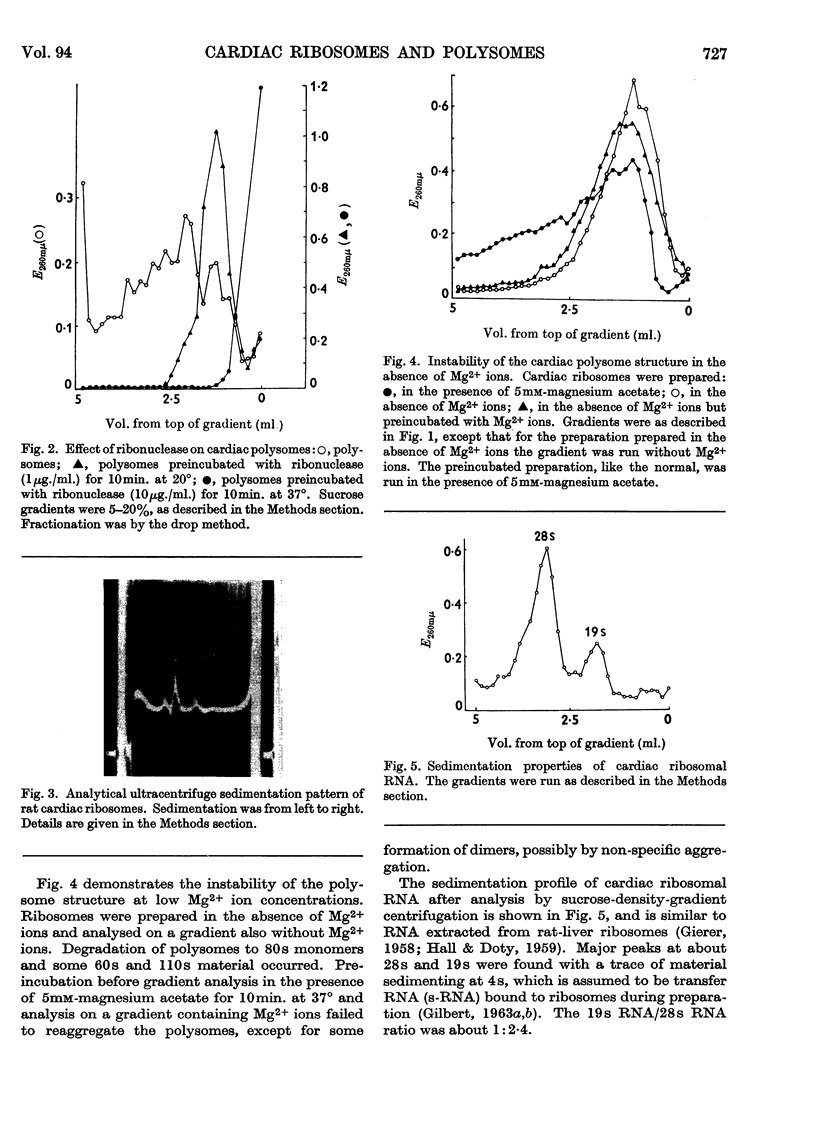
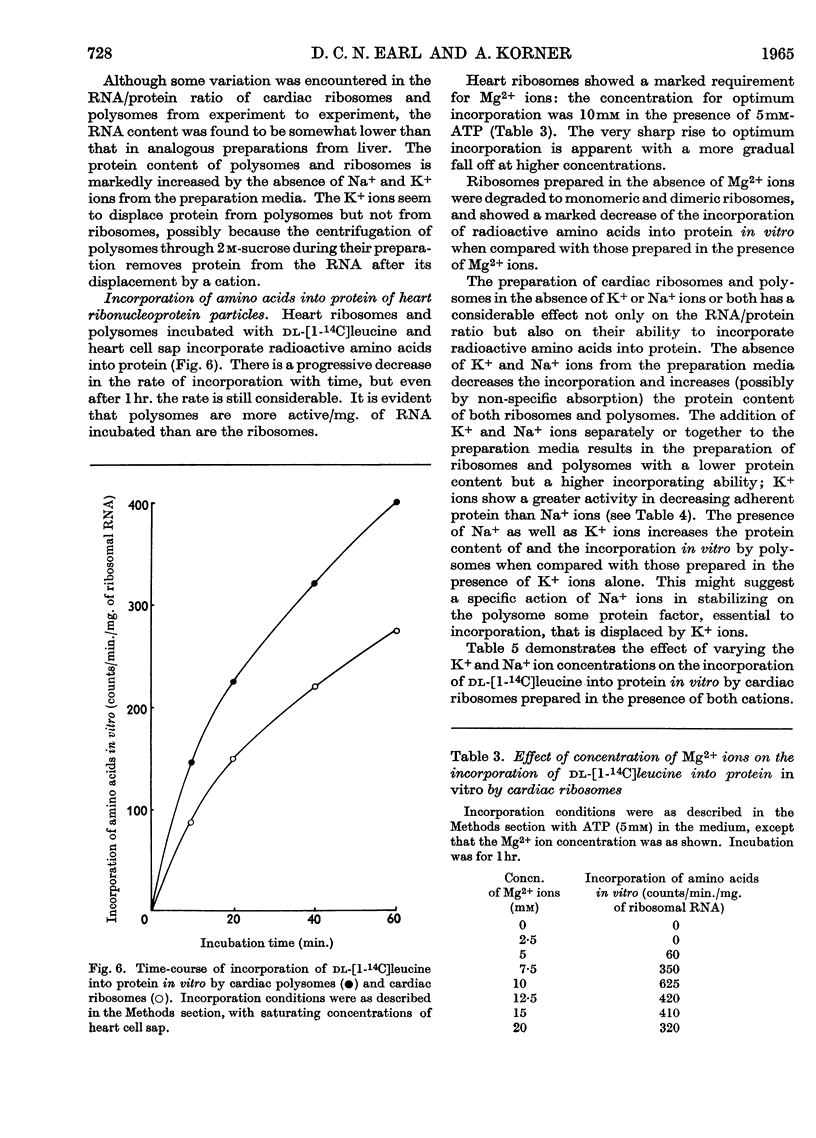
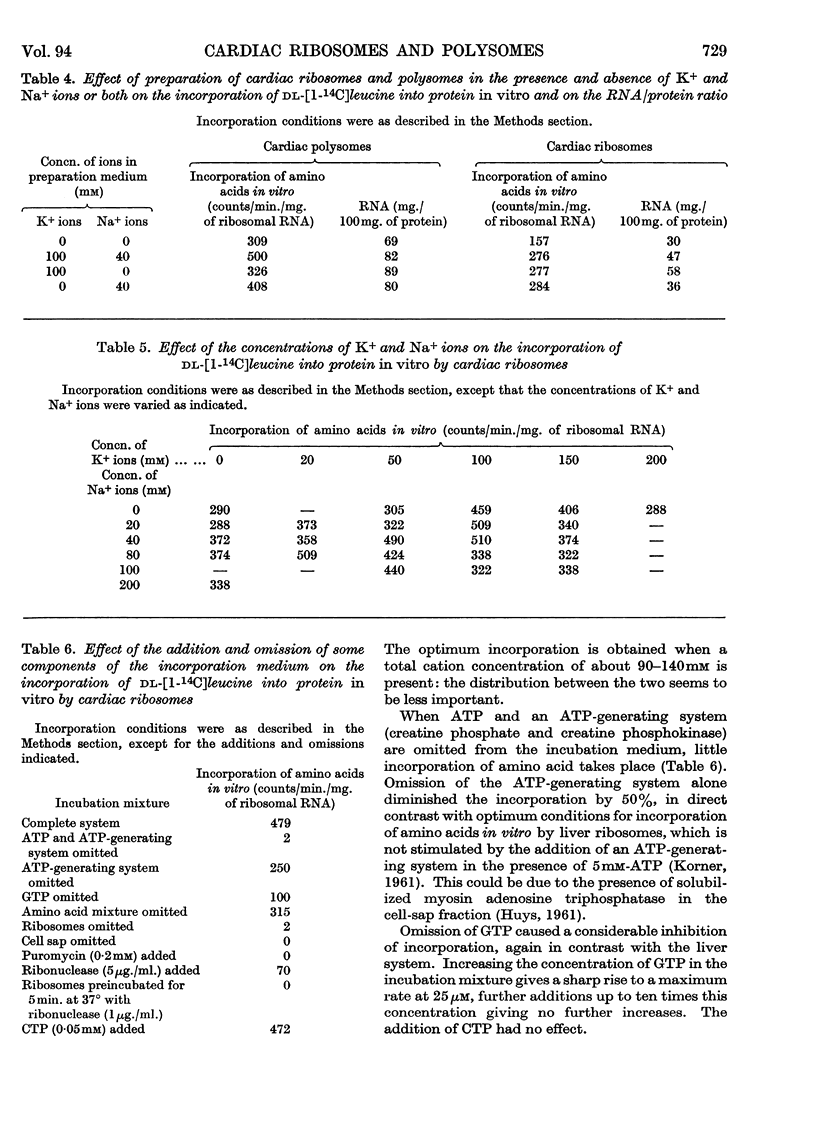
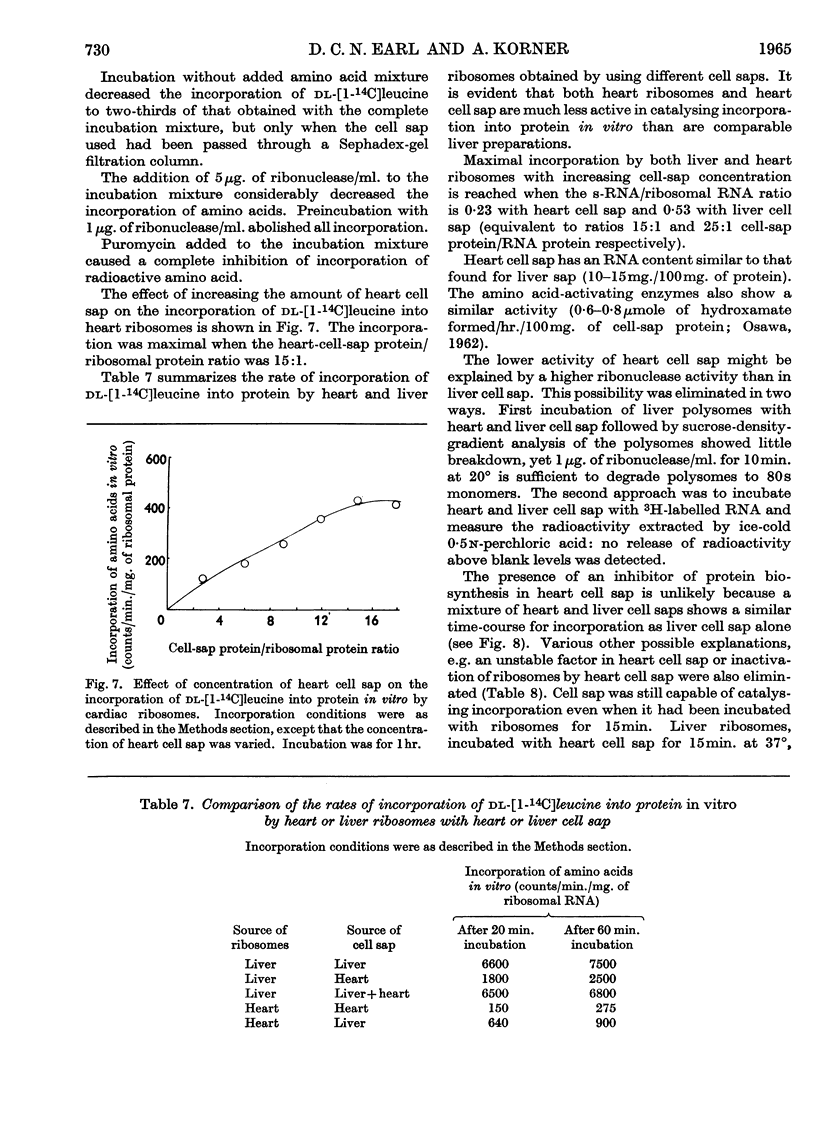
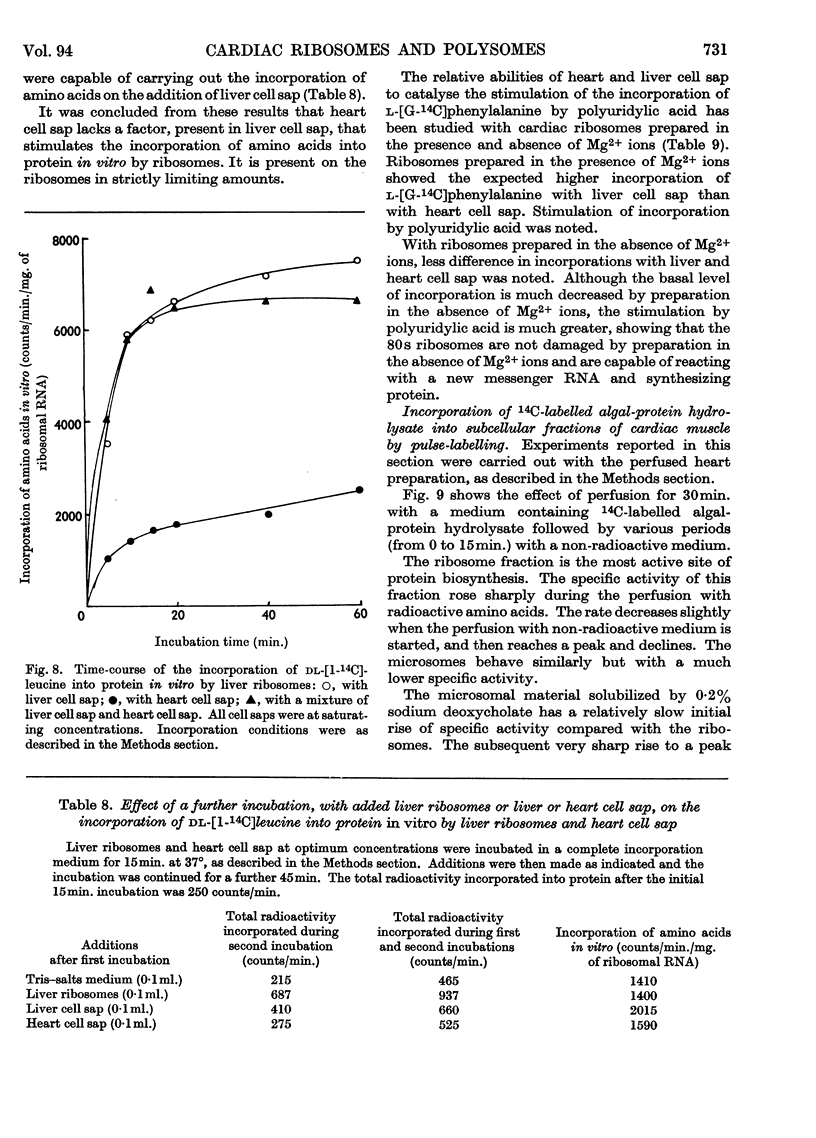
1. A method is described by which good yields of ribosomes and polysomes free of contamination by submitochondrial fragments can be prepared from rat cardiac muscle. These preparations are capable of incorporation of amino acids into protein in vitro. 2. The ribosome preparation consists of 32% of monomeric ribosomes and 68% of ribosomal aggregates or polysomes. The polysome preparation has a decreased monomeric content. Dimers, trimers, tetramers, pentamers and larger components can be differentiated. 3. The polysome aggregate structure is degraded to monomeric ribosomes on incubation with small amounts of ribonuclease or by preparation in the absence of Mg2+ ions. The degradation in the absence of Mg2+ ions was not reversible and drastically decreased the incorporation of amino acids in vitro. 4. The cardiac ribosomes contained two major RNA species sedimenting at 19s and 28s in a 1:2·4 ratio. 5. The RNA/protein ratio of cardiac ribosomes and polysomes was consistently lower than that of similar preparations from liver. The concentrations of Na+ and K+ ions present during preparation had a great effect on the RNA/protein ratio. 6. Optimum conditions for the incorporation of amino acids into protein in vitro are reported. Cardiac ribosomes have a lower rate of incorporation of amino acids in vitro than liver ribosomes. 7. Heart cell sap is less active than liver cell sap: evidence is presented that a factor, present in liver cell sap and concerned with stimulating the synthesis of the peptide chain, is lacking in heart cell sap. 8. Pulse-labelling of perfused hearts followed by examination of the subcellular structures showed that the ribosomal fraction was the most active in the incorporation of amino acids in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOUNCE A. L., WITTER R. F., MONTY K. J., PATE S., COTTONE M. A. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J Biophys Biochem Cytol. 1955 Mar;1(2):139–153. doi: 10.1083/jcb.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS J. G., MATHIAS A. P. APPARATUS FOR THE AUTOMATIC LOCATION OF ABSORBING COMPONENTS SEPARATED BY DENSITY GRADIENT CENTRIFUGATION. Nature. 1963 Aug 10;199:603–604. doi: 10.1038/199603a0. [DOI] [PubMed] [Google Scholar]
- FELLIG J., WILEY C. E. The inhibition of pancreatic ribonuclease by anionic polymers. Arch Biochem Biophys. 1959 Dec;85:313–316. doi: 10.1016/0003-9861(59)90496-5. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R. AMINO ACID INCORPORATION INTO PROTEIN BY CELL-FREE PREPARATIONS FROM RAT SKELETAL MUSCLE. I. PROPERTIES OF THE MUSCLE MICROSOMAL SYSTEM. Biochemistry. 1964 Feb;3:209–215. doi: 10.1021/bi00890a012. [DOI] [PubMed] [Google Scholar]
- GIERER A. Function of aggregated reticulocyte ribosomes in protein synthesis. J Mol Biol. 1963 Feb;6:148–157. doi: 10.1016/s0022-2836(63)80131-x. [DOI] [PubMed] [Google Scholar]
- GIERER A. Vergleichende Untersuchungen an hochmolekularer Ribosenucleinsäure. Z Naturforsch B. 1958 Dec;13B(12):788–792. [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. I. Ribosomes and the active complex. J Mol Biol. 1963 May;6:374–388. doi: 10.1016/s0022-2836(63)80050-9. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- GOODMAN H. M., RICH A. MECHANISM OF POLYRIBOSOME ACTION DURING PROTEIN SYNTHESIS. Nature. 1963 Jul 27;199:318–322. doi: 10.1038/199318a0. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., MII S., KOHOUT P. M. Studies on the terminal electron transport system. I. Succinic dehydrogenase. J Biol Chem. 1955 Dec;217(2):551–567. [PubMed] [Google Scholar]
- HAMILTON M. G., PETERMANN M. L. Ultracentrifugal studies on ribonucleoprotein from rat liver microsomes. J Biol Chem. 1959 Jun;234(6):1441–1446. [PubMed] [Google Scholar]
- HULSMANS H. A. "Microsomes" in heart-muscle homogenates. Biochim Biophys Acta. 1961 Nov 25;54:1–14. doi: 10.1016/0006-3002(61)90932-5. [DOI] [PubMed] [Google Scholar]
- KORNER A. Studies on incorporation of amino acids into protein in isolated rat-liver ribosomes. Biochem J. 1961 Oct;81:168–178. doi: 10.1042/bj0810168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROON A. M. Amino acid incorporation into the protein of mitochondria and mitochondrial fragments from beef heart. Biochim Biophys Acta. 1963 Jan 1;69:184–185. doi: 10.1016/0006-3002(63)91245-9. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., KELLER E. B., GROSS J., ZAMECNIK P. C. Studies on cytoplasmic ribonucleoprotein particles from the liver of the rat. J Biol Chem. 1955 Nov;217(1):111–123. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKHAM R. A graphical method for the rapid determination of sedimentation coefficients. Biochem J. 1960 Dec;77:516–519. doi: 10.1042/bj0770516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKS P. A., RIFKIND R. A. DANON D: POLYRIBOSOMES AND PROTEIN SYNTHESIS DURING RETICULOCYTE MATURATION IN VITRO. Proc Natl Acad Sci U S A. 1963 Aug;50:336–342. doi: 10.1073/pnas.50.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Schlessinger D. PROTEIN SYNTHESIS IN ERYTHROID CELLS, I. RETICULOCYTE RIBOSOMES ACTIVE IN STIMULATING AMINO ACID INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2163–2171. doi: 10.1073/pnas.48.12.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLEAN J. R., COHN G. L., BRANDT I. K., SIMPSON M. V. Incorporation of labeled amino acids into the protein of muscle and liver mitochondria. J Biol Chem. 1958 Sep;233(3):657–663. [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Mar 25;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACHS H. A stabilized enzyme system for amino acid incorporation. J Biol Chem. 1957 Sep;228(1):23–39. [PubMed] [Google Scholar]
- SCOTT J. F., FRACCASTORO A. P., TAFT E. B. Studies in histochemistry. I. Determination of nucleic acids in microgram amounts of tissue. J Histochem Cytochem. 1956 Jan;4(1):1–10. doi: 10.1177/4.1.1. [DOI] [PubMed] [Google Scholar]
- SIMKIN J. L., WORK T. S. Protein synthesis in guinea-pig liver; incorporation of radioactive amino acids into proteins of the microsome fraction in vivo. Biochem J. 1957 Feb;65(2):307–315. doi: 10.1042/bj0650307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON M. V., MCLEAN J. R. The incorporation of labeled amino acids into the cytoplasmic particles of rat muscle. Biochim Biophys Acta. 1955 Dec;18(4):573–575. doi: 10.1016/0006-3002(55)90156-6. [DOI] [PubMed] [Google Scholar]
- TAKANAMI M. On the molecular weigt of a ribonucleic acid preparation from a ribonucleoprotein complex. Biochim Biophys Acta. 1960 Mar 25;39:152–154. doi: 10.1016/0006-3002(60)90133-5. [DOI] [PubMed] [Google Scholar]
- TRUMAN D. E., KORNER A. Incorporation of amino acids into the protein of isolated mitochondria. A search for optimum conditions and a relationship to oxidative phosphorylation. Biochem J. 1962 Jun;83:588–596. doi: 10.1042/bj0830588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- WINNICK R. E., WINNICK T. Protein synthesis in skeletal muscle, with emphasis on myofibrils. J Biol Chem. 1960 Sep;235:2657–2661. [PubMed] [Google Scholar]
- WOOL I. G. Effect of insulin on distribution of radioactivity in protein of cell fractions from isolated rat diaphragm. Biochim Biophys Acta. 1961 Sep 30;52:574–576. doi: 10.1016/0006-3002(61)90417-6. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Rich A., Hall C. E. Electron Microscope Studies of Ribosomal Clusters Synthesizing Hemoglobin. Science. 1962 Dec 28;138(3548):1399–1403. doi: 10.1126/science.138.3548.1399. [DOI] [PubMed] [Google Scholar]