Abstract
An epidemiologic survey on the rotavirus strains causing gastroenteritis in young children was conducted in Juiz de Fora, Minas Gerais, in Southern Brazil during two consecutive seasons. Rotavirus was detected in 94 of the 1,056 fecal specimens collected from January 1998 to December 1999. Among the 13 discernible long electrophoretic profiles found, one was highly prevalent (73.4%) and represented the rotavirus strain responsible for the May-August winter epidemic outbreak of 1998, as clearly shown in a three-dimensional graph. This epidemic strain, designated JF98, was characterized as subgroup II and genotype G3P[4] by the original reverse transcription-PCR typing assays. Besides the unusual combination of G and P types, this G3 strain lacked reactivity with anti-G3-specific monoclonal antibodies and presented an uncommon pattern upon digestion of its cDNA-copied VP7 gene with the BstYI restriction enzyme. Strain JF98 affected primarily 6- to 24-month-old children and accounted for 85.5% of the severe rotavirus-associated dehydrating diarrhea cases that required hospitalization. As in our previous studies in neighboring Rio de Janeiro and São Paulo, a remarkably large proportion (44%) of mixed infections was detected, generating a complex set of circulating strains in the community, represented by the many distinct electropherotypes. Other common human types were detected as minor strains in single or in mixed infections, including the JF98 strain. Those were types G1, G4, G8, G9, P[8], and P[6], but not G2 or G5. One specimen contained a mixture of group A and C rotaviruses.
Rotaviruses are the leading cause of nonbacterial childhood diarrhea everywhere in the world. They represent a major public health concern, particularly in the developing world, where they account for high infant mortality rates (3).
As a member of the Reoviridae family, the rotavirus particle is icosahedral and naked and comprises two concentric capsids of characteristic morphology surrounding a core shell with its 11 double-stranded RNA genomic segments. The rotaviruses are antigenically complex and are ubiquitous among mammals and birds. According to the antigenic specificity of the VP6 protein, which constitutes the internal capsid, the rotavirus strains are classified into seven groups (A to G) and at least four subgroups. The outer capsid proteins VP7 and VP4 determine the viral G and P serotypes, respectively (10). Monoclonal antibodies have been very useful in studying the distribution of the four common human serotypes, G1 to G4, but the lack of monoclonal antibodies specific for other G types and for all the P serotypes left a great many isolates untyped in many studies, particularly those conducted in developing countries. Molecular typing assays have largely overcome this problem, and the combined identification of the G and P genotypes of an isolate has revealed the remarkable diversity of rotaviruses and demonstrated the frequent emergence of natural reassortants (13, 19).
Several serotypes infect humans, but the combinations G1P[8], G2P[4], G3P[8], and G4P[8] have been the most frequently identified in children with diarrhea the world over (10, 25). Combinations of either of the above G types with P[6] were found to be endemic in nurseries in several countries, primarily associated with asymptomatic infections in neonates, but occasionally associated with diarrheal disease in young children (1, 6, 7, 32, 36).
In Brazil, despite the limited data available, those conventional combinations of G and P types have also been commonly found, although other serotypes or combinations of serotypes have been reported (16, 26, 27, 33, 36, 37). Among those less common strains, rotavirus type G5 has been outstanding because it was the first non-G1-to-G4 serotype shown to be epidemiologically important in a community (16, 20, 37). This serotype, thought to be exclusively associated with infections of swine and perhaps of equines, caused the majority of diarrheal diseases in children in the state of São Paulo in 1992. Two minor human serotypes, G8 and G9, have been detected with increased frequency in several regions of the world, occasionally showing epidemic potential, such as G8 in Africa (1, 6, 7, 12, 23, 28, 30, 34, 38). Those and other uncommon serotypes have also been described, albeit in small proportions, in Brazilian children with gastroenteritis (27, 33).
Most importantly, studies in São Paulo and Rio de Janeiro have revealed an extraordinarily large proportion of mixed infections and their importance for the generation of new reassortant rotaviruses that may emerge as locally epidemic strains with the potential to spread to other communities (16, 19, 33, 36). The present study describes the rotaviruses causing infant diarrhea in a third, previously unreported state in the southeastern region of Brazil.
MATERIALS AND METHODS
Study area and collection of fecal specimens.
The study area was the city of Juiz de Fora, a middle-sized city of about 424,000 inhabitants, located in the state of Minas Gerais. Drinking water of good quality serves 98.9% of the population, and sewage treatment covers 97.7% of the dwellings. Its climate is that of a tropical highland (altitude, 700 to 1,300 m). Juiz de Fora is 120 km inland from the city of Rio de Janeiro, and there is relatively high traffic between the two cities.
Fecal specimens (one per child) were collected from 1,056 children during a 2-year period (1998 to 1999) from several centers located in different neighborhoods and serving distinct socioeconomic classes. Those included three hospitals: a private hospital, a military hospital, and a public hospital with a large and mostly poor pediatric clientele; two municipal health care posts; and several private clinical laboratories scattered within the community. Fecal specimens were stored at −20°C until processed for rotavirus detection and characterization.
Detection and characterization of rotavirus genomic double-stranded RNA by PAGE.
Screening for rotavirus was performed by polyacrylamide gel electrophoresis (PAGE) (24, 31). This method has the same sensitivity as enzyme immunoassay with the added advantage of giving valuable information on the genome profile of the rotavirus isolate belonging to any serological group. A clarified suspension (40 μl) of approximately 20% fecal material was prepared for each of the 1,056 samples, mixed with sample loading buffer, and applied directly to the gel (9). For samples that presented too-weak bands, viral RNA was purified from 1 ml of fecal suspension by adsorption to hydroxyapatite as previously described (31). The sample, as either fecal suspension or purified RNA, was subjected to electrophoresis at 120 V for 18 h in a standard 10% acrylamide gel, followed by silver staining (24). The rotavirus-positive samples were analyzed and grouped by their electropherotypes.
Genotyping.
Fifty-nine rotavirus-positive specimens were selected, based on their electropherotypes and quantities of fecal material available, to be genotyped by the PCR typing technique (11, 13, 17, 18). RNA was purified from 400 μl of fecal suspension by the hydroxyapatite adsorption method and subjected to reverse transcription (RT)-PCR for the amplification of cDNA copies of the virus VP7 and VP4 genes. Aliquots of the RT-PCR products were then subjected to nested PCR amplifications for identification of the viral G and P types.
Restriction endonuclease analysis.
Further characterization by digestion of the cDNA of the VP7-encoding genes of some isolates with the restriction enzymes HaeIII and BstYI was performed and analyzed as previously described (15).
Subgrouping and G-typing by enzyme immunoassay.
Enzyme immunoassay was performed on 38 selected samples with a pair of monoclonal antibodies (MAbs) for subgrouping and two sets of MAbs for G-typing. MAbs specific for subgroup I (MAb 255-60) and for subgroup II (MAb 631-9) and the set of MAbs specific for serotypes G1 (5E8 and 2C9), G2 (2F1 and 1C10), and G3 (4F8 and 159) were a kind gift from H. B. Greenberg (22, 29). The other set included the MAbs specific for G1 (KU-4), G2 (S2-2G10), G3 (YO-IE2), and G4 (ST-2G7), received as a generous gift from S. Urasawa (35).
RESULTS
A total of 1,056 fecal specimens were collected from January 1998 to December 1999 in Juiz de Fora from children with gastroenteritis. Rotavirus was detected in 94 specimens, representing 29.2% of the pediatric admissions for gastroenteritis, and 28.6% of the nonhospitalized cases that were seen at the public health posts, but only 2.9% of those who sought private practitioners (Table 1).
TABLE 1.
Frequency of rotavirus detection in hospitalized and nonhospitalized children with gastroenteritis in Juiz de Fora, Minas Gerais, from January 1998 to December 1999
| Group | No. of patients | No. (%) rotavirus positive |
|---|---|---|
| Hospitalized | 212 | 62 (29.2) |
| Nonhospitalized | ||
| Public health posts | 28 | 8 (28.6) |
| Private clinical labs | 816 | 24 (2.9) |
| Total | 1,056 | 94 (8.9) |
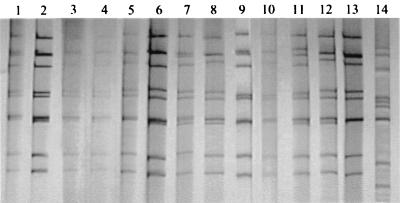
Electrophoretic analysis of rotavirus RNA present in fecal specimens allowed the distinction of at least 13 profiles, designated Y, Q, S, T, U, V, B, G, K, M, N, J, and R (Fig. 1). All were long profiles typical of group A rotaviruses except for profile G, which showed similarities to those of group C rotaviruses. The single specimen with this profile, however, demonstrated the presence of a group A rotavirus strain that was easily typed as G4P[6] by the PCR typing assays. Careful reexamination of this specimen by PAGE revealed faint extra bands at usual positions for segments 6 and 10 of group A strains, but the other bands were too weak or not distinguishable from the stronger bands of profile G. Further characterization of this putative group C strain is under way and will be reported later.
FIG. 1.
Electropherogram of genomic RNAs of rotaviruses detected in Juiz de Fora in 1998 and 1999. It shows the 13 distinct profiles: Y (lane 1), U (lane 2), Q (lane 3) and Q with an extra fourth segment (lane 4), S (lane 5), J (lane 6), V (lane 7), M (lane 8), T (lane 9), R (lane 10), K (lane 11), B (lane 12), N (lane 13), and G (lane 14).
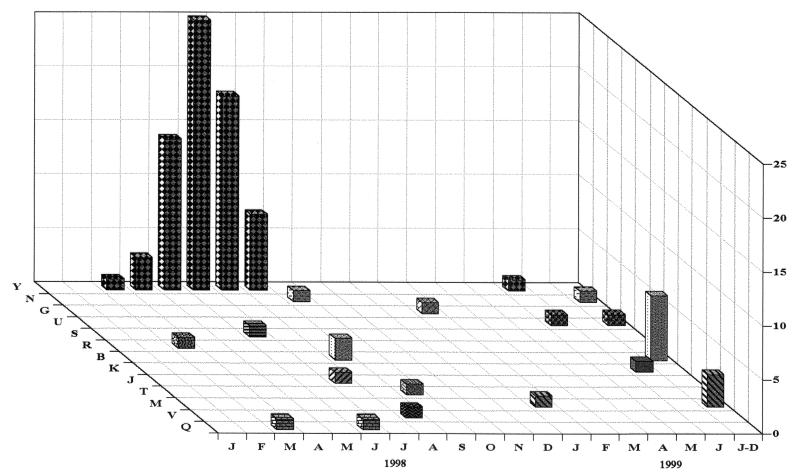
Most of the group A profiles were detected only once (K, S, T, V, N, J, and R), twice (N, Q, and U), or a few times (M and B), whereas profile Y was consistently detected at very high frequencies during the rotavirus epidemic peak that spanned from May to August 1998. There was cocirculation of different electropherotypes during the study period. One of the specimens with profile Q displayed 12 bands, clearly demonstrating mixed infection. The temporal distribution of the distinct electropherotypes is represented in Fig. 2. By plotting of each electropherotype individually, an outstanding epidemic curve for the strain with profile Y is clearly shown in a background of less prevalent, endemic or emerging strains. Most of the specimens collected in the last 6 months of the study were from the clinical laboratories. They were received by early December, and because the precise month of collection was not given, they were plotted as a group under July to December 1999.
FIG. 2.
Temporal distribution of rotavirus electropherotypes associated with gastroenteritis in children in Juiz de Fora, Minas Gerais, from 1998 to 1999. For the last 6-month period, most samples were received without precise dates and were plotted together (J-D, July to December).
Assuming that rotaviruses with identical profiles represent the same virus strain (14), the rotavirus-positive samples were grouped by their electropherotypes, and 59 representative samples were further characterized for their G and P types by PCR typing assays. Results are shown in Table 2. Among the G types, a large prevalence of G3 (60%) was found, both singly and in mixed infections with G8 and/or G9, constituting the samples with profile Y. Mixtures of G3 and G1 presented either profile Y or U, probably depending on the relative amount of the two strains. The second most common type was G1 (27%). Three specimens contained G4 (5%) rotavirus, including the specimen that also contained rotavirus of typical group C profile. The other G types detected, G8 (20%) and G9 (8%), were found in mixed infections with G3 or G1.
TABLE 2.
Genotypes and electropherotypes of rotaviruses recovered from children with diarrhea in Juiz de Fora, Minas Gerais, during 1998 and 1999
| Genotype | No. of isolates with electropherotypea:
|
Total no. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | U | N | Gb | R | J | V | S | B | T | K | M | Q | ||
| G3P[4] | 18 | 18 | ||||||||||||
| G3P[4] + G8 | 6 | 6 | ||||||||||||
| G3P[4] + G8 + G9 | 4 | 4 | ||||||||||||
| G3P[4] + G9 | 1 | 1 | ||||||||||||
| G3P[4] + G1 | 3 | 1 | 4 | |||||||||||
| G3P[4] + P[8] | 1 | 1 | ||||||||||||
| G3P[?]c | 1 | 1 | ||||||||||||
| G?P[4] | 8 | 8 | ||||||||||||
| G4P[?] | 2 | 2 | ||||||||||||
| G4P[6] | 1 | 1 | ||||||||||||
| G?P[6] | 1 | 1 | ||||||||||||
| G1P[4] | 1 | 1 | ||||||||||||
| G1P[8] | 1 | 1 | ||||||||||||
| G1P[8] + P[4] | 1 | 3 | 1 | 1 | 6 | |||||||||
| G1P[8] + P[6] | 2 | 2 | ||||||||||||
| G1P[8] + G8P[4] | 2 | 2 | ||||||||||||
| Not done | 27 | 1 | 5 | 2 | 35 | |||||||||
| Total | 69 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 8 | 1 | 1 | 4 | 2 | 94 |
All are long profiles.
Profile for group A rotavirus too weak to be determined. Profile G refers to group C strain.
[?], genotype not determined.
Among the P types, only the three types commonly described in human infections, P[8], P[4], and P[6], were found. Surprisingly, however, P[4] was associated with all G3 rotaviruses (71%), P[8] accounted for 20% of the infections, and P[6] accounted for 7%. Most of the specimens containing G1 rotavirus presented either a mixture of P[8] and P[4] (15%) or of P[8] and P[6] (4%) and a variety of electropherotypes (Table 2).
Digestion of some of the amplicons produced by RT-PCR amplification with endonucleases HaeIII and BstYI of samples with profile S, K, T, and Q generated fragments with pattern h1b7, compatible with human G1 strains, but digestion of amplicons obtained from four samples with profile Y showed pattern h1b6, not commonly seen in human or animal G3 strains (15).
Some specimens were selected for subgrouping and serotyping by enzyme immunoassay with MAbs. Almost all (20 of 23, or 87%) were found to be subgroup II, and the remainder (3 of 23, or 13%) were nonreactive with either subgroup I- or II-specific MAbs. Serotyping with MAbs was inconsistent and mostly unsuccessful. None of 11 specimens of profile Y reacted with the monoclonal antibodies used. Nine specimens, two each with rotavirus profiles Q, B, and M and one each of profiles J, V, and U, reacted with MAb 5E8 or KU-4, confirming their G1 specificity. Some cross-reacted with MAb 159, specific for G3, as has been seen for other G1 strains in previous studies (21; V. Gouvea, unpublished results). The two specimens with profile N reacted exclusively with the anti-G4 MAb ST-2G7. However, the one with profile G was not reactive, probably due to the presence of small numbers or degraded particles of the group A rotavirus, which were greatly outnumbered by those of group C rotavirus in the fecal specimen.
Rotavirus G3P[4] with profile Y (or profile U in mixed infections with G1 virus) accounted for the majority (76.5%) of the diarrheal cases in Juiz de Fora in 1998, clearly representing an epidemic strain, then designated JF98. To better characterize the epidemiology of rotavirus diarrhea in this community, we analyzed this strain isolated from the other strains, examining its contribution to rotavirus-associated diarrhea cases by the child's age and the center of attendance. All children studied were less than 5 years old, although information on the child's precise age could not be obtained for the specimens collected at the clinical laboratories and for a few of the children who were hospitalized or were attended at the health care posts. The importance of the JF98 strain as a causal agent of diarrhea, particularly in the 6- to 24-month-old children in the community, is depicted in Table 3.
TABLE 3.
Age distribution by epidemic (JF98) and endemic rotavirus strains recovered in Juiz de Fora, Minas Gerais, in the 1998 to 1999 period
| Strain | No. of children in age group (mo):
|
Total (%) | |||||
|---|---|---|---|---|---|---|---|
| <6 | 6-12 | 13-24 | 25-36 | 37-60 | Unknowna | ||
| JF98 | 5 | 17 | 16 | 3 | 4 | 24 | 69 (73.4) |
| Others | 2 | 1 | 0 | 3 | 1 | 18 | 25 (26.5) |
Exact age unknown, but <5 years old. Data include all 24 samples received from the clinical laboratories.
All hospitalized children studied presented symptoms of gastroenteritis at the time of admission, indicating that they had acquired their infections in the community rather than nosocomially. The severity of the illness varied, but it was severe in at least the 62 rotavirus-positive children who were hospitalized for oral or parenteral rehydration, most of them at the public hospital, during mid-1998. Strain JF98 was responsible for the majority (53 of 62, or 85.5%) of the severe cases requiring hospitalization (Table 4). Among the nonhospitalized children, the eight who were attended at the health care posts had mild to moderate JF98-associated diarrhea. The severity of symptoms for the remaining 24 rotavirus-positive cases is not known but is assumed to be mild, as the specimens were obtained from the clinical laboratories that had received them for parasitological examination.
TABLE 4.
Distribution of epidemic strain JF98 (profile Y) and other rotaviruses in both inpatient and outpatient groups by collection centera
| Yr | No. of patients
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inpatients (n = 62)
|
Outpatients (n = 32)
|
|||||||||
| SUS (n = 53)
|
HM (n = 5)
|
HAS (n = 4)
|
PS (n = 8)
|
LAC (n = 24)
|
||||||
| JF98 | Others | JF98 | Others | JF98 | Others | JF98 | Others | JF98 | Others | |
| 1998 | 46 | 5 | 3 | 2 | 4 | 0 | 7 | 0 | 8 | 3 |
| 1999 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 13 |
Includes single and mixed infections of profile Y. Centers: a private (HAS), a military (HM), and a public (SUS) hospital, two public health care posts (PS), and several private clinical laboratories (LAC).
The possible influence of the climate conditions on the rotavirus epidemic curve was also investigated for the year 1998. Rotavirus gastroenteritis was detected from March to September 1998 (Fig. 2 and Table 5). The number of cases gradually increased by the end of the rainy season, intensified when the temperature dropped in May, peaked at the coolest and driest months of June and July, and decreased in the following month. By October, coincidental with the return of the rainy season, the epidemic was resolved, and only sporadic cases caused by endemic strains were detected in the summer. The next rotavirus season was marked by a small number of gastroenteritis cases associated mainly with endemic strains. The epidemic strain JF98 (G3P[4] profile Y) was recovered singly only once in 1999 but, interestingly, was often recovered in double infections (as indicated by the P[4] specificity), probably with endemic G1P[8] strains, presenting a variety of new profiles such as U, S, T, K, and B.
TABLE 5.
Average temperature and rainfall in Juiz de Fora, Minas Gerais, and number of rotavirus-associated diarrhea cases during 1998a
| Mo | Temp (°C) | Precipitation (mm) | No. of cases |
|---|---|---|---|
| January | 22.8 | 194 | 0 |
| February | 23.1 | 143 | 0 |
| March | 22.0 | 110 | 3 |
| April | 21.0 | 87 | 3 |
| May | 17.4 | 88 | 14 |
| June | 15.5 | 4 | 27 |
| July | 16.3 | 7 | 19 |
| August | 19.4 | 54 | 10 |
| September | 18.9 | 21 | 2 |
| October | 18.4 | 192 | 0 |
| November | 18.3 | 205 | 0 |
| December | 21.4 | 193 | 0 |
Source: Laboratório de Climatologia, Department of Geology, Universidade Federal de Juiz de Fora.
DISCUSSION
The present work is a continuation of our epidemiological survey in Brazil. It was conducted in the city of Juiz de Fora, Minas Gerais. Previous surveys in the neighboring states of Rio de Janeiro and São Paulo had revealed an enormous diversity of rotavirus strains, displaying uncommon and emerging strains circulating in many cities, prompting our interest to investigate communities in Minas Gerais (16, 33, 36, 37).
A new, epidemiologically important JF98 strain characterized as G3P[4], subgroup II, and long profile Y was found in the southeast region of Brazil in 1998. Type G3 has been consistently present in relatively high proportions in most surveys conducted in Brazil (8, 26, 33, 36). This type has been universally found in combination with P[8] and occasionally with P[6], subgroup II and a long electropherotype, whereas P[4] has classically been associated with G2 specificity, subgroup I and a short electropherotype (3, 10, 25). Nevertheless, rotaviruses with unusual combinations of G and P types are often found, usually in very small numbers, in most epidemiologic studies (4, 6, 7, 12, 23, 26, 27, 28, 30, 33, 34, 36, 39). They represent new reassortant strains that emerge upon coinfections with rotavirus strains of distinct G and/or P types and may spread in the community, occasionally achieving epidemic proportions.
Strain JF98 caused diarrhea in children of all age groups, but the highest incidence was in the 6- to 24-month-old group. This age group is known to have the highest risk for severe rotavirus-associated dehydrating diarrhea (3). In the present study, 85.5% of the cases severe enough to require hospitalization and fluid replacement were caused by JF98. This shows the high susceptibility of young children to this strain, suggesting that this might indeed be a new strain in the community. Strain JF98 demonstrated an epidemic curve with marked winter seasonality similar to that described for rotavirus infections in regions of temperate climate (3, 5). Most of the JF98-associated cases occurred in the coolest (May to July) period, peaking during the driest month of June. It was the prevalent (87%) rotavirus strain in 1998, but only a minor (6%) strain in the following year.
This is the second epidemiologically relevant nonconventional rotavirus strain described in Brazil. The first was an IAL28-like G5P[8] strain that was prevalent in São Paulo in 1992 (20, 37). Rotavirus G5P[8] strains had been present, albeit in minor proportions, all over Brazil before and after that epidemic season in São Paulo (16, 26, 33). In the present study, rotavirus types G5 and G2 were not detected, and types G9 and G8 were found only in mixed infections with the epidemic G3P[4] strain (18.6%). An incredibly high proportion (44%) of mixed infections was detected in this study, confirming our previous studies in Rio de Janeiro and São Paulo and predictions for the tropical areas of the world (19).
Further analysis of strain JF98 indicated that, besides the unusual combination with the P[4] type, the G3 type demonstrated some uncommon characteristics: it was not recognized by any of the G3-specific MAbs used, and the BstYI digestion profile of its amplified gene 9 cDNA fragment was not the typical b1 or b2 seen with most human G3 strains, but rather presented the b6 profile common to some G1 and G8 human strains (15). Those findings show that the G3 specificity of JF98 is somewhat distinct from the G3 specificity of other strains previously found in this region.
A few G3P[4] strains were found sporadically, often in mixed infections, in Hubei Province, China, in 1994 (39), in three Mexican states (1.5%) in 1996 to 1997 (4), and in Blantyre, Malawi, in 1997 to 1998 (7). The Chinese strains had subgroup II and short electropherotypes, and the single Malawi strain presented a long profile. At the time that this work was being revised, two reports from studies carried out in the Kasena Nankana district of the Upper East region of Ghana came out describing the prevalence of G and P types in two consecutive 3- to 4-month periods (1, 2). Unusual G3P[4] strains were prevalent (62%) from August to December of 1998 (2) but disappeared from January to April 1999, when rotavirus strains G2P[6], which were present before (12%), became the dominant (50%) rotavirus in this rural region of Ghana (1).
Unlike the Brazilian JF98 strain characteristics of subgroup II and a long electropherotype, the G3P[4] strains from Ghana displayed a short profile and subgroup I specificity, similar to the cocirculating G2P[6] and G8P[6] strains (1, 2). Together, those findings clearly demonstrate that the Brazilian and African strains are distinct G3P[4] rotaviruses derived from local strains. Most likely they are reassortants that emerged during mixed infections (19). The continual surveillance of rotavirus genotypes in communities scattered among distinct regions of the country is essential to monitor the appearance of new types and changes in existing ones.
Acknowledgments
We thank André Luiz S. Domingues and Felipe G. Naveca for assistance with the figures.
This work is part of the doctoral thesis of M.L.R.S., who received a fellowship from CAPES, Brazil. We acknowledge partial support from FUJB and FAPERJ, Rio de Janeiro, and CNPq, Brasília, Brazil.
REFERENCES
- 1.Armah, G. E., C. T. Pager, R. H. Asmah, F. R. Anto, A. R. Oduro, F. Binka, and D. Steele. 2001. Prevalence of unusual human rotavirus strains in Ghanaian children. J. Med. Virol. 63:67-71. [PubMed] [Google Scholar]
- 2.Asmah, R. H., J. Green, G. E. Armah, C. I. Gallimore, J. J. Gray, M. Iturriza-Gomara, F. Anto, A. Oduro, F. N. Binka, D. W. G. Brown, and F. Cutts. 2001. Rotavirus G and P genotypes in rural Ghana. J. Clin. Microbiol. 39:1981-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, R. F. 1994. Natural history of rotavirus infections, p. 131-167. In A. Z. Kapikian (ed.). Viral infections of the gastrointestinal tract, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 4.Castillo, A. R., A. V. Villa, J. E. R. González, E. M. Pimentel, M. M. Munguía, B. D. De Jésus, H. O. Díaz, and H. G. Lozano. 2000. VP4 and VP7 genotyping by reverse transcription-PCR of human rotavirus in Mexican children with acute diarrhea. J. Clin. Microbiol. 38:3876-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook, S. M., R. I. Glass, C. W. LeBaron, and M.-S. Ho. 1990. Global seasonality of rotavirus infections. Bull. W.H.O. 66:171-177. [PMC free article] [PubMed] [Google Scholar]
- 6.Cubitt, W. D., A. D. Steele, and M. Iturriza. 2000. Characterization of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6]. J. Med. Virol. 61:150-154. [DOI] [PubMed] [Google Scholar]
- 7.Cunliffe, N. A., J. S. Gondwe, R. L. Broadhead, M. E. Molyneux, P. A. Woods, J. S. Bresee, R. I. Glass, J. R. Gentsch, and C. A. Hart. 1999. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J. Med. Virol. 57:308-312. [PubMed] [Google Scholar]
- 8.deCastro, L. 1994. Electropherotypes, subgroups and serotypes of rotaviruses in Brazil. M.Sc. thesis. FIOCRUZ, Rio de Janeiro, Brazil.
- 9.Dolan, K. T., E. M. Twist, P. Horton-Slight, C. Forrer, L. M. Bell, Jr., S. A. Plotkin, and H. F. Clark. 1985. Epidemiology of rotavirus electropherotypes determined by a simplified diagnostic technique with RNA analysis. J. Clin. Microbiol. 21:753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes, M. K. 1996. Rotaviruses and their replication, p. 1625-1655. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 11.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bahn. 1992. Identification of group A rotavirus gene types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerna, G., A. Sarasini, L. Zentilin, A. DiMatteo, P. Miranda, M. Parea, M. Batttaglia, and G. Milanesi. 1990. Isolation in Europe of 69M-like (serotype G8) human rotavirus strains with either subgroup I or II specificity and a long RNA electropherotype. Arch. Virol. 112:27-40. [DOI] [PubMed] [Google Scholar]
- 13.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z.-Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouvea, V., M.-S. Ho, R. I. Glass, P. Woods, B. Forrester, C. Robinson, R. Ashley, M. Riepenhoff-Talty, H. F. Clark, K. Taniguchi, E. Meddix, B. McKellar, and L. Pickering. 1990. Serotypes and electropherotypes of human rotavirus in the USA: 1987-1989. J. Infect. Dis. 162:362-367. [DOI] [PubMed] [Google Scholar]
- 15.Gouvea, V., C. Ramirez, B. Li, N. Santos, L. Saif, H. F. Clark, and Y. Hoshino. 1993. Restriction endonuclease analysis of the vp7 genes of human and animal rotaviruses. J. Clin. Microbiol. 31:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouvea, V., L. de Castro, M. C. Timenetsky, H. Greenberg, and N. Santos. 1994. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J. Clin. Microbiol. 32:1408-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouvea, V., N. Santos, and M. C. Timenetsky. 1994. VP4 typing of bovine and porcine group A rotaviruses by PCR. J. Clin. Microbiol. 32:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouvea, V., N. Santos, and M. C. Timenetsky. 1994. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 32:1338-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouvea, V., and M. Brantly. 1995. Is rotavirus a population of reassortants? Trends Microbiol. 3:159-162. [DOI] [PubMed] [Google Scholar]
- 20.Gouvea, V., and N. Santos. 1999. Rotavirus serotype G5: an emerging cause of epidemic childhood diarrhea. Vaccine 17:1291-1292. [DOI] [PubMed] [Google Scholar]
- 21.Green, K. Y., H. D. James, and A. Z. Kapikian. 1990. Evaluation of three pannels of monoclonal antibodies for the identification of human rotavirus VP7 serotypes by ELISA. Bull. W.H.O. 68:601-610. [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg, H., V. McAuliffe, J. Valdesuso, R. Wyatt, J. Flores, A. Kalica, Y. Hoshino, and N. Singh. 1983. Serological analysis of the subgroup protein of rotavirus with monoclonal antibodies. Infect. Immun. 39:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin, D. D., C. D. Kirkwood, U. D. Parashar, P. A. Woods, J. S. Bresee, R. I. Glass, J. R. Gentsch, and The National Rotavirus Strain Surveillance System Collaborating Laboratories. 2000. Surveillance of rotavirus strains in the United States: identification of unusual strains. J. Clin. Microbiol. 38:2784-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring, A. J., N. F. Inglis, C. K. Ojeh, D. R. Snodgrass, and J. D. Menzies. 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 16:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshino, Y., and A. Z. Kapikian. 1996. Classification of rotavirus VP4 and VP7 serotypes. Arch. Virol. Suppl. 12:99-111. [DOI] [PubMed] [Google Scholar]
- 26.Leite, J. P. G., A. A. Alfieri, P. A. Woods, R. I. Glass, and J. R. Gentsch. 1996. Rotavirus G and P types circulating in Brazil: characterization by RT-PCR, probe hybridization, and sequence analysis. Arch. Virol. 141:2365-2374. [DOI] [PubMed] [Google Scholar]
- 27.Linhares, A. C., Y. B. Gabbay, J. A. P. Mascarenhas, R. B. Freitas, T. H. Flewett, and G. M. Beards. 1988. Epidemiology of rotavirus subgroup and serotypes in Belém, Brazil: a three-year study. Ann. Inst. Pasteur Virol. 139:89-99. [DOI] [PubMed] [Google Scholar]
- 28.Nakata, S., Z. Gatheru, S. Ukae, N. Adachi, N. Kobayashi, S. Honma, J. Muli, P. Ogaja, J. Nyangao, E. Kiplagat, P. M. Tukei, and S. Chiba. 1999. Epidemiological study of the G serotype distribution of group A rotaviruses in Kenya from 1991 to 1994. J. Med. Virol. 58:296-303. [DOI] [PubMed] [Google Scholar]
- 29.Padilla-Noriega, L., C. F. Arias, S. López, F. Puerto, D. R. Snodgrass, K. Taniguchi, and H. B. Greenberg. 1990. Diversity of rotavirus serotypes in Mexican infants with gastroenteritis. J. Clin. Microbiol. 28:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palombo, E. A., R. Clark, and R. F. Bishop. 2000. Characterisation of a “European-like” serotype G8 human rotavirus isolated in Australia. J. Med. Virol. 60:56-62. [PubMed] [Google Scholar]
- 31.Santos, N., and V. Gouvea. 1994. Improved method for purification of RNA from fecal specimens for rotavirus detection. J. Virol. Methods 46:11-21. [DOI] [PubMed] [Google Scholar]
- 32.Santos, N., V. Gouvea, M. C. Timenetsky, H. F. Clark, M. Riepenhoff-Talty, and A. Garbarg-Chenon. 1994. Comparative analysis of VP8∗ sequences from rotaviruses possessing M37-like VP4 recovered from children with and without diarrhoea. J. Gen. Virol. 75:1775-1780.8021606 [Google Scholar]
- 33.Santos, N., R. C. C. Lima, C. F. A. Pereira, and V. Gouvea. 1998. Detection of rotavirus types G8 and G10 among Brazilian children with diarrhea. J. Clin. Microbiol. 36:2727-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele, A. D., S. P. Parker, I. Peenze, C. T. Pager, M. B. Taylor, and W. D. Cubitt. 1999. Comparative studies of human rotavirus serotype G8 strains recovered in South Africa and the United Kingdom. J. Gen. Virol. 80:3029-3034. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi, K., T. Urasawa, Y. Morita, H. B. Greenberg, and S. Urasawa. 1987. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay with serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J. Infect. Dis. 155:11159-111166. [DOI] [PubMed] [Google Scholar]
- 36.Timenetsky, M. C. S. T., N. Santos, and V. Gouvea. 1994. Survey of rotavirus G and P types associated with human gastroenteritis in São Paulo, Brazil, from 1986 to 1992. J. Clin. Microbiol. 32:2622-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timenetsky, M. C. S. T., V. Gouvea, N. Santos, R. C. C. Carmona, and Y. Hoshino. 1997. A novel human rotavirus serotype with dual G5-G11 specificity. J. Gen. Virol. 78:1373-1378. [DOI] [PubMed] [Google Scholar]
- 38.Unicomb, L. E., G. Podder, J. R. Gentsch, P. A. Woods, K. Z. Hasan, A. S. G. Faruque, M. J. Albert, and R. I. Glass. 1999. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J. Clin. Microbiol. 37:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, H., K. Taniguchi, T. Urasawa, and S. Urasawa. 1998. Serological and genomic characterization of human rotaviruses detected in China. J. Med. Virol. 55:168-176. [DOI] [PubMed] [Google Scholar]