Abstract
The susceptibilities of 25 clinical isolates of Aspergillus fumigatus, A. flavus, A. terreus, A. nidulans, and A. ustus to itraconazole and amphotericin B were determined by an agar diffusion-dilution method (the Etest method) and a colorimetric broth microdilution method (the Sensititre method); and the results were compared with those obtained by the NCCLS proposed standard M-38P method for antifungal susceptibility testing of filamentous fungi. Various MIC endpoints for the three methods were determined visually by four different observers in three blinded experiments, and the reproducibilities among the observers (interobserver agreement) and among the replicates (interexperimental agreement) as well as the levels of agreement between the NCCLS, the Etest, and the Sensititre methods were calculated. High levels of reproducibility (within 1 twofold dilution) were found for the NCCLS method (>95%) with the MIC-0 endpoint (complete inhibition of growth) for both drugs and with the MIC-1 endpoint (slight growth) for itraconazole and for the Sensititre method (>90%) with all MIC endpoints, although for the latter the interexperimental agreement for itraconazole was comparatively lower (83 to 93%). The Etest method was less reproducible (67 to 87%) for both drugs. Using the recommended MIC endpoints, high levels of agreement (within one twofold dilution) between the NCCLS and the Sensititre methods for all species were found for amphotericin B (>77%) but not for itraconazole (<66%), for which the MICs by the Sensititre method were up to 3 twofold dilutions lower than the corresponding MICs by the NCCLS method. The use of the first blue well as an endpoint for the Sensititre method and 48 h of incubation improved the levels of agreement with the NCCLS method. Low levels of agreement between the NCCLS and the Etest methods using the recommended MIC endpoints were found for most species, especially after 48 h of incubation (<50%), when the MICs obtained by the Etest method were up to 9 twofold dilutions higher than the corresponding MICs obtained by the NCCLS method. Relatively better agreement was found after 24 h, although it was species dependent, with the highest levels of agreement (>82%) found for A. terreus and A. ustus for amphotericin B and A. fumigatus for both drugs. Overall, better agreement was found when MIC-0 was used as the MIC endpoint for the NCCLS method for both drugs and when the MICs by the Etest method were determined after 48 h of incubation for itraconazole and after 24 h of incubation for amphotericin B.
The numbers of invasive infections caused by Aspergillus species have increased dramatically in recent years, particularly in immunocompromised patients. The rate of mortality due to these infections remains high, despite the use of antifungal therapy (7). Amphotericin B in its various formulations and itraconazole are at present the only drugs licensed in Europe for the treatment of these infections. The development of resistance to itraconazole during antifungal therapy (3, 8) and the inability of in vitro susceptibility testing methods to detect amphotericin B resistance have been reported (16). Despite the considerable progress made by use of the NCCLS proposed standard M-38P method for the susceptibility testing of filamentous fungi in vitro (23), problems still arise when determining the MICs (19). Consequently, several modifications of the broth microdilution format of the M-38P method (5, 19, 21, 22) as well as other alternative methodologies based on agar methods (31, 38) have been developed for testing of the in vitro susceptibilities of filamentous fungi to antifungal drugs.
The Sensititre YeastOne method (the Sensititre method; Trek Diagnostic Systems Ltd., East Grinsted, Sussex, England) is a commercial colorimetric microdilution method that uses the oxidation-reduction indicator Alamar blue. This indicator has been used to test the susceptibilities of various yeasts to different antifungal drugs, resulting in high levels of agreement with the reference methods (6, 11, 12, 17, 25, 30, 36, 37). The use of Alamar blue to determine MIC endpoints for filamentous fungi has also been shown to achieve high levels of inter- and intralaboratory agreement (10, 15).
The Etest method (AB Biodisk, Solna, Sweden) is a commercial agar diffusion-dilution method which has been used to test the susceptibilities of various yeasts to different antifungal drugs, resulting in high levels of agreement with the NCCLS reference method, depending upon the species tested and the medium used (1, 13, 26-28, 34, 40, 42). However, the levels of agreement detected when the susceptibilities of different species of filamentous fungi to itraconazole and amphotericin B were tested ranged from nil to 100% (29, 35).
Given the variable agreement between the commercially available methods and the NCCLS reference methods and the absence of a comparative study of all these methods done under the same conditions, a study was undertaken in which the susceptibilities to itraconazole and amphotericin B of 25 clinical isolates of the genus Aspergillus belonging to five different species were determined by the Sensititre, the Etest, and the NCCLS proposed standard M-38P methods. Various MIC endpoints were determined visually for each method by four different observers in three blinded experiments conducted on different days, and the reproducibilities of these methods among the observers and the replicates as well as the levels of agreement between the two commercially available methods (the Sensititre and the Etest methods) and the NCCLS reference (M-38P) method were calculated.
MATERIALS AND METHODS
Test isolates.
A total of 25 isolates of Aspergillus species from the private collection of the Department of Medical Microbiology, University Medical Center Nijmegen, were tested. These included five clinical isolates of each of the following species: Aspergillus fumigatus (isolates AZN5161, AZN5241, AZNV09-20, AZN8248, and AZN8244), Aspergillus flavus (isolates AZN137, AZN510, AZN2865, AZN4094, and AZN4132), Aspergillus nidulans (isolates AZN2867, AZN8033, AZN8236, AZN8933, and AZN4606), Aspergillus terreus (isolates AZN286, AZN515, AZN2868, AZN7320, and AZN9152), and Aspergillus ustus (isolates AZN677, AZN2725, AZN6989, AZN9420, and AZN7843). Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used for quality control since they are recommended for use for quality control for all three methodologies. Paecilomyces variotii (ATCC 22319) was also included in tests by the Etest method since it is recommended by the manufacturer for the testing of filamentous fungi. For each replicate the quality control strains were tested twice.
Inoculum preparation and medium.
The isolates were passaged twice at an interval of 5 to 7 days at 30°C by first subculturing them onto Sabouraud glucose agar and then onto Takashio agar in order to obtain adequate sporulation. Conidia were collected with a cotton swab and suspended in sterile saline with 0.05% Tween 20. After the heavy particles were allowed to settle, the turbidities of the supernatants were measured spectrophotometrically (Spectronic 20D; Milton Roy, Rochester, N.Y.) at 530 nm, and transmittance was adjusted to 80 to 82% so that the inoculum corresponded to 0.5 × 106 to 4.5 × 106 CFU/ml. The inoculum size was confirmed by plating serial dilutions onto Sabouraud glucose agar plates. All isolates were tested three times on 3 different days. The conidia of the isolates were obtained from fresh cultures each time.
The inoculum for the yeast quality controls was prepared from 1- to 2-day-old colonies of C. krusei and C. parapsilosis, which were suspended in saline, and the transmittance was adjusted to 75 to 77% at 530 nm. The blastoconidial suspensions were then diluted 1:1,000 to obtain double the final inoculum, which ranged from 0.5 × 103 to 2.5 × 103 CFU/ml. For P. variotii, fresh cultures were prepared as described above and conidial suspensions were adjusted to 75 to 77% transmittance at 530 nm. The suspensions were diluted 1:50 in order to obtain double the final inoculum, which ranged from 0.5 × 104 to 2 × 104 CFU/ml.
RPMI 1640 medium (with l-glutamine but without bicarbonate; GIBCO BRL, Life Technologies, Woerden, The Netherlands) at a concentration of 35.4 g/liter buffered to pH 7.0 with 0.165 M 3-(N-morpholino)propanesulfonic acid (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) (RPMI) was used throughout the study.
Antifungal susceptibility testing methods. (i) The NCCLS method.
A broth microdilution method (the M-38P method) was performed according to the guidelines of the NCCLS (16). The degree of growth was assessed visually by four different observers and graded to four scales according to the NCCLS guidelines. Four MIC endpoints (MIC-0, MIC-1, MIC-2, and MIC-3) were determined and were the lowest drug concentrations with growth scaled to the corresponding score (0, absence of visible growth; 1, slight growth; 2, prominent reduction in growth compared with the growth in the drug-free well; 3, slight reduction in growth compared with the growth in the drug-free well). MIC-4 was the highest drug concentration with a growth score of 4 (no reduction in growth compared with the growth in the drug-free well).
(ii) Etest method.
The Etest method was performed in accordance with the instructions of the manufacturer. The MIC was determined as the drug concentration at which the elliptical inhibition zone intersected the Etest strip (MIC-I) (9, 35).
(iii) Sensititre method.
The colorimetric Sensititre method was performed in accordance with the instructions of the manufacturer. Three MIC endpoints were determined visually: the lowest concentration of drug with a blue color (MIC-B) and a purple color (MIC-P) and the highest concentration of drug with a red color (MIC-R) (6, 12).
For all methods, MIC endpoints were determined after 24 and 48 h of incubation at 37°C under ambient conditions.
Analysis of results.
The levels of agreement between the four observers and the three replicates as well as between the NCCLS, the Etest, and the Sensititre methods were calculated for each strain-drug-MIC endpoint-incubation time combination.
(i) Reproducibilities of the methods.
The relative and absolute reproducibilities of the NCCLS, the Etest, and the Sensititre methods among the four observers (interobserver agreement) and among the three replicates (intraexperimental agreement) were calculated. The relative and absolute interobserver agreements were defined as the proportion of the MICs among the four observers which fell within 1 dilution of the median MIC for each strain and the proportion of the MICs among the four observers that were the same for each strain, respectively. The results for all strains were pooled for each replicate; and the average percentage for the three replicates was calculated, together with the 95% confidence interval for interobserver agreement. The relative and absolute interexperimental agreements were defined as the proportion of the results among the replicate MIC endpoints which fell within 1 dilution of the median MIC for each strain and the proportion of the results among the replicate MIC endpoints that were the same for each strain, respectively. The results for all strains were pooled for each observer, and the average percentage for all four observers was calculated, together with the 95% confidence interval of the interexperimental agreement. The average agreement was reported for each drug, MIC endpoint, and method after 24 and 48 h of incubation.
(ii) Agreement between the methods.
The results of the Etest and the Sensititre methods were compared with those of the NCCLS method, and the overall level of agreement was estimated. For this purpose, MICs determined by the Etest method that fell between the twofold dilutions of the MICs of the NCCLS method were elevated to the next drug concentration so that they matched the twofold dilution scheme; i.e., MICs of 0.38, 1.5, and 12 mg/liter by the Etest method were elevated to MICs of 0.5, 2, and 16 mg/liter of the twofold dilution scheme, respectively. For each replicate, the percent agreement between the methods was calculated for all 25 Aspergillus strains as the proportion of the MIC endpoints determined by each observer for each strain by the Etest or the Sensititre method which fell within 1 or 2 twofold dilutions of the corresponding MIC endpoint determined by the NCCLS method. The average percent agreement among three replicates between the Etest method and the NCCLS method as well as between the Sensititre method and the NCCLS method was calculated. The agreement between the NCCLS method and the Sensititre and the Etest method was calculated by using the following MIC endpoints recommended for each method: for the NCCLS method, MIC-0 and MIC-2; for the Sensititre method, the first blue well and the first purple well for amphotericin B and itraconazole, respectively. For the Etest method, the unique MIC endpoint was used.
(iii) Statistical analysis.
In order to approximate a normal distribution, the drug concentrations were transformed to the log2 dilution and the percent agreement was transformed by angular transformation to arcsine √P values (41). The differences in the average log2 MICs for the four observers for each replicate between the MICs determined by the Etest method and the Sensititre method compared with those determined by the NCCLS method were analyzed by repeated-measures one-way analysis of variance with the Dunnett posttest, which was applied to each species-drug-MIC endpoint-incubation period combination. P values less than 0.05 were considered statistically significant. Statistical analysis was carried out with Graphpad software (Prism Software, San Diego, Calif.). The transformed percent agreement was used in order to estimate the average agreement and the variations between the replicates and the species. The high and low off-scale MICs were included in the analysis by converting each one to the next higher or the next lower drug concentration, respectively. All tests were performed with the investigators blinded.
RESULTS
MICs.
The MICs for the reference strains obtained by the three methods were within the reference ranges. The susceptibilities of the 25 Aspergillus strains to itraconazole and amphotericin B after 24 and 48 h of incubation determined by the NCCLS, the Etest, and the Sensititre methods are shown in Table 1. The geometric mean MICs were obtained after all the MICs determined by the four observers and in the three replicates were pooled. All strains produced visible growth within 24 h, but the growth was more obvious after 48 h for the NCCLS method. The MICs determined by the NCCLS method were in agreement with those obtained in previous studies (9, 34, 38).
TABLE 1.
Susceptibilities of five strains of five Aspergillus spp. to amphotericin B and itraconazole determined by four observers in three experiments on different days by the NCCLS, Etest, and Sensititre methods after 24 and 48 h of incubationa
| Drug | Incubation time (h) | Species | Geometric mean range MIC (mg/liter)
|
||
|---|---|---|---|---|---|
| NCCLS | Etest | Sensititre | |||
| Itraconazole | 24 | A. fumigatus | 2.39 (0.015->32) | 2.84 (0.125->32) | 0.09 (0.025->16) |
| A. flavus | 0.14 (0.062-0.25) | 0.16 (0.015->32) | 0.04 (0.008-0.25) | ||
| A. nidulans | 0.13 (0.031-0.5) | 0.16 (0.016-1) | 0.02 (0.008-0.25) | ||
| A. terreus | 0.09 (0.031-0.25) | 0.05 (0.006-0.75) | 0.02 (0.008-0.06) | ||
| A. ustus | 0.40 (0.062-1) | 15.17 (0.125->32) | NGb | ||
| 48 | A. fumigatus | 2.89 (0.125->32) | 8.98 (0.75->32) | 1.11 (0.03->16) | |
| A. flavus | 0.20 (0.125-0.5) | 1.04 (0.25->32) | 0.08 (0.03-0.5) | ||
| A. nidulans | 0.16 (0.062-0.25) | 0.56 (0.095->32) | 0.05 (0.03-0.5) | ||
| A. terreus | 0.15 (0.062-0.5) | 0.27 (0.02-4) | 0.06 (0.016-0.25) | ||
| A. ustus | 1.48 (0.125->32) | 28.04 (1->32) | 8.37 (0.03->16) | ||
| Amphotericin B | 24 | A. fumigatus | 0.68 (0.125-4) | 0.59 (0.125-24) | 0.35 (0.25-1) |
| A. flavus | 1.19 (0.5-4) | 6.81 (0.064->32) | 0.75 (0.03->16) | ||
| A. nidulans | 0.74 (0.25-4) | 1.26 (0.064-32) | 0.45 (0.125-1) | ||
| A. terreus | 1.11 (0.5-4) | 1.07 (0.38-3) | 0.78 (0.5-1) | ||
| A. ustus | 1.11 (0.5-2) | 1.01 (0.064-6) | NG | ||
| 48 | A. fumigatus | 1.50 (1-4) | 7.26 (1->32) | 1.27 (0.5-2) | |
| A. flavus | 1.64 (1-4) | 45.34 (2->32) | 1.82 (0.5-8) | ||
| A. nidulans | 1.10 (0.25-4) | 9.84 (0.25->32) | 0.86 (0.5-2) | ||
| A. terreus | 2.89 (2-4) | 29.29 (1->32) | 2.17 (2-4) | ||
| A. ustus | 1.91 (1-4) | 1.74 (0.38->32) | 2.12 (1->16) | ||
The MICs of amphotericin B and intraconzole were the lowest drug concentrations that resulted in the complete or prominent inhibition of growth for the NCCLS method and the first purple or blue well for the Sensititre method.
NG, insufficient growth.
Although visible growth was also obtained by the Etest method after 24 h, determination of the MICs after this period of incubation was difficult since the edge of the elliptical inhibition zone was barely visible. A broad range of MICs was obtained by the Etest method, with the geometric mean MICs generally being higher than those obtained by the NCCLS method, especially after 48 h of incubation, when up to 10-fold differences in geometric mean MICs were obtained in many cases (Table 1). This difference was especially noted with A. terreus and A. flavus strains tested with amphotericin B and A. ustus strains tested with itraconazole, for which this difference was apparent even after 24 h of incubation. The geometric mean MICs after 48 h of incubation were significantly elevated compared with those after 24 h of incubation, with the increase being 30-fold in some cases (A. terreus with amphotericin B) (Table 1).
In the case of the Sensititre method, 24 h of incubation was not sufficient for the complete conversion of Alamar blue to its pink derivative for any A. ustus strain, two A. terreus strains, and one A. fumigatus strain; but a bright pink color was attained for the growth controls of all strains after 48 h of incubation. The geometric mean MICs obtained by the Sensititre method were lower than those obtained by the NCCLS method, especially after 24 h of incubation. After further incubation, no clear endpoints were obtained in some cases since all the intermediate colors between pink and blue were found. This phenomenon occurred less often after 48 h of incubation, although visible growth without a color change was often observed.
Reproducibilities of the methods.
The relative agreement (agreement within 1 dilution) and the absolute agreement (no divergence in agreement) among the four observers (interobserver agreement) and among the three replicates (interexperimental agreement) for the various endpoints of each method are shown in Table 2. The NCCLS method showed high levels of relative interobserver and interexperimental agreement for the MIC-0 endpoint after 24 and 48 h of incubation for amphotericin B (>99%) and for the MIC-0 and MIC-1 endpoints after 24 h of incubation and the MIC-0, MIC-1, and MIC-2 endpoints after 48 h of incubation for itraconazole (>92%). Lower levels of agreement (<82%) were detected for the MIC-3 and MIC-4 endpoints for itraconazole. The Etest method showed the poorest reproducibility, since the levels of relative interobserver and interexperimental agreement seldom exceeded 86% for both drugs. The Sensititre method showed high levels of relative interobserver agreement (>91%) but comparatively lower levels of interexperimental agreement (83 to 93%) for all MIC endpoints after 24 and 48 h of incubation for itraconazole, while high levels of reproducibility were obtained for amphotericin B (>90%) in all cases. Among the three MIC endpoints, the MIC-B endpoint showed the highest levels of absolute interobserver and interexperimental agreement for both drugs (Table 2). In most of the cases the interexperimental agreement was lower than the interobserver agreement, and incubation for up to 48 h increased the levels of both interobserver and interexperimental agreement compared to those at 24 h.
TABLE 2.
Relative and absolute reproducibilities of various endpoints of the NCCLS, Sensititre, and E-test methods among the four observers (interobserver agreement) and among the three replicates (interexperimental agreement) for all Aspergillus spp.
| Drug | Method | Endpointa | % Interobserver agreementb
|
% Interexperimental agreementc
|
||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |||
| Itraconazole | NCCLS | MIC-0 | 96.6 (80.9) | 97.6 (88.1) | 95.2 (77.4) | 97.1 (83.7) |
| MIC-1 | 97.0 (82.6) | 97.5 (75.2) | 95.6 (69.2) | 95.0 (77.4) | ||
| MIC-2 | 86.6 (68.9) | 92.1 (69.2) | 85.6 (65.8) | 92.6 (71.3) | ||
| MIC-3 | 79.6 (62.3) | 77.2 (65.1) | 82.0 (62.6) | 82.1 (67.6) | ||
| MIC-4 | 71.3 (60.6) | 76.5 (63.8) | 73.8 (61.7) | 78.7 (65.8) | ||
| E-test | MIC-I | 76.7 (54.9) | 86.9 (68.1) | 73.2 (55.6) | 80.4 (61.4) | |
| Sensititre | MIC-R | 98.1 (83.8) | 95.5 (83.7) | 83.4 (65.7) | 92.7 (79.9) | |
| MIC-P | 98.9 (84.9) | 91.1 (84.0) | 85.8 (67.8) | 89.0 (80.3) | ||
| MIC-B | 97.7 (88.2) | 93.7 (91.4) | 84.9 (69.2) | 87.9 (83.1) | ||
| Amphotericin B | NCCLS | MIC-0 | 99.3 (88.3) | 100.0 (88.1) | 99.2 (81.6) | 99.7 (78.0) |
| Etest | MIC-I | 81.5 (55.5) | 85.5 (69.1) | 67.1 (51.2) | 76.8 (67.4) | |
| Sensititre | MIC-R | 94.5 (82.6) | 99.2 (86.8) | 90.0 (72.6) | 99.4 (75.3) | |
| MIC-P | 99.9 (84.3) | 99.2 (85.8) | 94.1 (73.9) | 99.4 (75.0) | ||
| MIC-B | 98.6 (87.3) | 99.2 (93.7) | 93.3 (76.9) | 99.7 (82.1) | ||
MIC-0, the lowest drug concentration with an absence of growth; MIC-1, the lowest drug concentration that resulted in slight growth; MIC-2, the lowest drug concentration that resulted in a prominent reduction in growth; MIC-3, the lowest drug concentration with that resulted in a slight reduction in growth; MIC-4: the highest drug concentration that resulted in no reduction of growth compared always with the control growth; MIC-R, the red well with the highest drug concentration; MIC-P, the first purple well with the lowest drug concentration; MIC-B, the blue well with the lowest drug concentration; MIC-I, the intersect of the elliptical inhibition zone with the Etest strip.
The 95% confidence intervals of the average percent interobserver agreement (relative agreement and absolute agreement [in parentheses]) among the three experiments (for each experiment, 125 comparisons [five species × five strains × four observers]) were calculated after angular transformation and ranged from ±0 to ±9.8% (median, 2.7%).
The 95% confidence intervals of the average percent interexperimental agreement (relative agreement and absolute agreement [in parentheses]) among the four observers (for each of observer, 75 comparisons [five species × five strains × three replicates]) were calculated after angular transformation and ranged from ±0.9 to ±8.6% (median, 3.6%).
Overall, for itraconazole the highest levels of absolute interobserver and interexperimental agreement were found after 48 h of incubation with the MIC-B endpoint of the Sensititre method (91 and 83%, respectively) followed by the MIC-0 endpoint of the NCCLS method (88 and 83%, respectively). The same was found for amphotericin B, with the NCCLS method showing high levels of reproducibility even after 24 h of incubation.
Agreement of the Etest and the Sensititre methods with the NCCLS method.
Use of the MIC-I endpoint of the Etest method for both drugs, the MIC-B endpoint of the Sensititre method for amphotericin B, and the MIC-P endpoint of the Sensititre method for itraconazole was compared with use of the MIC-0 endpoint of the NCCLS method for amphotericin B and the MIC-2 endpoint of the NCCLS method for itraconazole, respectively; and the levels of agreement within 1 and 2 log2 dilutions are shown in Table 3.
TABLE 3.
Agreement between the NCCLS method and the Sensititre or the Etest method for each of five Aspergillus species for amphotericin B and itraconazole after 24 and 48 h of incubation
| Drug | Incubation time (h) | Species | % Agreement for Etest method
|
P value for Etest method | % Agreement for Sensititre method
|
P value for Sensititre method | ||
|---|---|---|---|---|---|---|---|---|
| ± 1 log2 dilution | ± 2 log2 dilution | ± 1 log2 dilution | ± 2 log2 dilution | |||||
| Itraconazolea | 24 | A. fumigatus | 82.0 ± 18.5c | 93.3 ± 14.8 | 2.2 ± 8.1 | 12.0 ± 7.1 | <0.05 | |
| A. flavus | 43.9 ± 15.6 | 82.7 ± 14.8 | 16.7 ± 8.1 | 72.5 ± 7.7 | <0.01 | |||
| A. nidulans | 58.9 ± 22.3 | 92.9 ± 13.5 | 4.8 ± 8.9 | 48.2 ± 12.0 | <0.01 | |||
| A. terreus | 53.5 ± 8.7 | 83.0 ± 10.0 | 21.1 ± 15.0 | 50.0 ± 27.9 | <0.01 | |||
| A. ustus | 2.6 ± 4.9 | 3.8 ± 7.3 | <0.001 | NCe | NC | |||
| Overall | 45.7 ± 36.1d | 72.5 ± 38.9 | <0.05 | 9.7 ± 10.3 | 44.6 ± 29.1 | <0.01 | ||
| 48 | A. fumigatus | 53.4 ± 13.0 | 62.8 ± 25.5 | <0.01 | 63.3 ± 3.3 | 96.6 ± 6.7 | <0.01 | |
| A. flavus | 17.2 ± 14.4 | 51.5 ± 8.9 | <0.01 | 50.2 ± 10.0 | 90.4 ± 5.7 | <0.01 | ||
| A. nidulans | 33.2 ± 8.7 | 73.0 ± 19.1 | 34.9 ± 5.7 | 86.8 ± 3.4 | <0.01 | |||
| A. terreus | 45.0 ± 5.7 | 81.6 ± 16.2 | 66.7 ± 6.4 | 96.7 ± 6.5 | <0.05 | |||
| A. ustus | 9.0 ± 8.8 | 12.6 ± 9.1 | <0.01 | 23.9 ± 18.2 | 32.8 ± 16.9 | <0.05 | ||
| Overall | 30.0 ± 21.2 | 56.0 ± 30.9 | 47.6 ± 20.8 | 84.6 ± 24.1 | ||||
| Amphotericin Bb | 24 | A. fumigatus | 91.8 ± 3.4 | 97.8 ± 4.3 | 77.3 ± 7.9 | 93.9 ± 13.4 | <0.05 | |
| A. flavus | 16.6 ± 3.2 | 40.0 ± 5.7 | <0.01 | 93.9 ± 13.4 | 97.2 ± 5.4 | |||
| A. nidulans | 37.9 ± 20.7 | 78.3 ± 19.7 | 93.0 ± 15.7 | 100.0 ± 0.0 | ||||
| A. terreus | 88.9 ± 8.2 | 99.4 ± 2.2 | 97.0 ± 5.8 | 100.0 ± 0.0 | ||||
| A. ustus | 95.0 ± 16.7 | 99.4 ± 2.4 | NC | 0.0 ± 0.0 | ||||
| Overall | 69.7 ± 37.3 | 89.2 ± 22.2 | <0.05 | 91.4 ± 8.7 | 98.9 ± 2.9 | |||
| 48 | A. fumigatus | 33.3 ± 6.4 | 45.0 ± 9.7 | <0.01 | 100.0 ± 0.0 | 100.0 ± 0.0 | ||
| A. flavus | 2.2 ± 4.3 | 5.0 ± 0.0 | <0.01 | 96.7 ± 6.5 | 99.4 ± 2.2 | |||
| A. nidulans | 34.8 ± 10.0 | 40.0 ± 0.0 | <0.05 | 92.0 ± 14.6 | 100.0 ± 0.0 | |||
| A. terreus | 16.4 ± 6.8 | 26.4 ± 8.5 | <0.01 | 100.0 ± 0.0 | 100.0 ± 0.0 | |||
| A. ustus | 75.1 ± 5.7 | 97.8 ± 4.3 | 99.4 ± 2.2 | 99.4 ± 2.2 | ||||
| Overall | 29.3 ± 30.9 | 43.6 ± 41.5 | 98.8 ± 3.0 | 99.9 ± 0.3 | ||||
MIC-2 was used as the endpoint for the NCCLS method, and MIC-P was used as the endpoint for the Sensititre method.
MIC-0 was used as the endpoint for the NCCLS method, and MIC-B was used as the endpoint for the Sensititre method.
For each of the five Aspergillus species, the average percent agreement and the 95% confidence intervals among the three replicates were calculated after angular transformation.
For the overall result, the average percent agreement and the 95% confidence intervals among the five Aspergillus species were calculated after angular transformation.
NC, not calculated.
(i) Etest method.
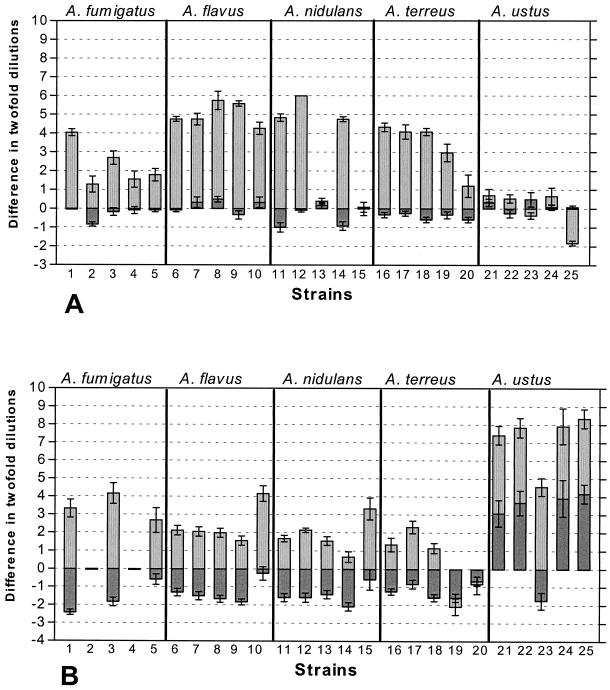
The level of agreement between the Etest method and the NCCLS method depended on the drug-species combination and the incubation period used. Overall, higher levels of agreement were found after 24 h of incubation than after 48 h of incubation, with most of the differences after 48 h being statistically significant for both drugs. Overall, higher levels of agreement were found for amphotericin B for all species compared to those for itraconazole, particularly after 24 h of incubation (70 and 46%, respectively). The majority of the MICs of itraconazole (>82%) and amphotericin B (>78%) obtained by the Etest method after 24 h of incubation were within 2 log2 dilutions of the corresponding MICs obtained by the NCCLS method for all species except A. ustus (4%) and A. flavus (40%), respectively (Table 3). Extremely poor agreement was found for A. ustus when it was tested with itraconazole, although the level of agreement increased up to 20% when the MIC-0 endpoint was used (data not shown). Relatively high levels of agreement (>82%) were found after 24 h of incubation for both drugs for A. fumigatus and for A. terreus and A. ustus when they were tested with amphotericin B. The levels of agreement after 48 h of incubation were very low since the majority (20 to 95%) of the MICs obtained by the Etest method were more than 2 log2 dilutions different from the corresponding MICs obtained by the NCCLS method for both drugs and all species with the exception of A. ustus with amphotericin B. A comparison of the results of the two methods for each strain is shown in Fig. 1. For most of the strains, the results obtained by the four observers and the three replicates were up to ninefold higher by the Etest method than by the NCCLS method, especially for itraconazole. The only exceptions in which the MICs determined by the Etest method were lower than the corresponding MICs determined by the NCCLS method were for two strains of A. terreus (strains 19 and 20 in Fig. 1B) tested with itraconazole and one strain of A. ustus (strain 25 in Fig. 1A) tested with amphotericin B.
FIG. 1.
Schematic representation of the results of testing of the susceptibilities of 25 Aspergillus strains to amphotericin B (A) and itraconazole (B) on the basis of the results of the Sensititre and the Etest methods compared with those of the NCCLS method after 48 h of incubation. The MIC endpoints defined in footnotes a and b of Table 3 were used. The MICs obtained by the NCCLS method are represented by the line with 0 difference in twofold dilutions. Bars represent differences in twofold dilutions between the MICs obtained by the NCCLS method and those obtained by the Sensititre method (bars with heavy shading) and the Etest method (bars with light shading) by four different observers in triplicate for each strain of the five Aspergillus species tested (indicated above each panel). Error bars represent the standard errors of the means for the 12 differences (four observers × three replicates) for each strain. The geometric means of the 12 MICs (four observers × three replicates) obtained by the NCCLS method, represented by the line with 0 difference in twofold dilutions, were as follows for amphotericin B (A): for A. fumigatus strains 1 to 5, 1, 3.56, 1.41, 1.19, and 1.26 mg/liter, respectively; for A. flavus strains 6 to 10, 2.12, 1.41, 1.26, 1.26, and 2.52 mg/liter, respectively; for A. nidulans strains 11 to 15, 2.24, 1, 0.5, 2.38, and 0.59 mg/liter, respectively; for A. terreus strains 16 to 20, 2.52, 2.38, 3, 3.78, and 3.21 mg/liter, respectively; and for A. ustus strains 21 to 25, 2.67, 1.89, 1.5, 1.78, and 1.89 mg/liter, respectively. The geometric means of the 12 MICs (four observers × three replicates) obtained by the NCCLS method, represented by the line with 0 difference in twofold dilutions, were as follows for itraconazole (B): for A. fumigatus strains 1 to 5, 0.4, >32, 0.31, >32, and 0.4 mg/liter, respectively; for A. flavus strains 6 to 10, 0.22, 0.19, 0.21, 0.17, and 0.22 mg/liter, respectively; for A. nidulans strains 11 to 15, 0.18, 0.14, 0.16, 0.14, and 0.17 mg/liter, respectively; for A. terreus strains 16 to 20, 0.12, 0.15, 0.18, 0.19, and 0.13 mg/liter, respectively; and for A. ustus strains 21 to 25, 1.59, 1.78, 0.94, 1.5, and 1.78 mg/liter, respectively.
Since the levels of agreement between the Etest method and the NCCLS method were very poor, with the MIC endpoints of the Etest method being systematically higher than those of the NCCLS method, various other MIC endpoints were chosen for comparison of the NCCLS method with the Etest method. In addition, the levels of agreement of the results of the NCCLS method after 48 h of incubation were compared with the results of the Etest method after 24 h of incubation. The levels of agreement are shown in Table 4. Although a linear correlation was found between the MICs obtained by the NCCLS method and the MICs obtained by the Etest method (data not shown), the levels of agreement were dependent on the drug, the incubation period, and the species tested. For itraconazole, the levels of agreement (within 1 log2 dilution) were less than 50% for most of the MIC endpoint-incubation period combinations. Higher levels of agreement were found when the MIC-I endpoint of the Etest method after 24 h of incubation was compared with the MIC-0 endpoint (54%) and the MIC-1 endpoint (51%) of the NCCLS method after 48 h of incubation. However, the highest levels of agreement (70%) were found when the MIC-I endpoint of the Etest method was compared with the MIC-0 endpoint of the NCCLS method after 48 h of incubation. For amphotericin B the MIC-0 endpoint after 24 h of incubation showed the highest level of agreement (64%) compared to that for the other incubation period.
TABLE 4.
Overall agreement for all 25 Aspergillus strains between the different MIC endpoints of the NCCLS, Sensititre, and Etest methods
| Incubation time (h) | Druga | NCCLS endpointb | % Agreement with 1 twofold dilution (2 twofold dilutions)
|
|||
|---|---|---|---|---|---|---|
| Etest method | Sensititre method
|
|||||
| MIC-I | MIC-R | MIC-P | MIC-B | |||
| 24 | ICZ | MIC-0 | 46.7 (64.5)c | 3.3 (4.4) | 3.5 (7.7) | 7.1 (22.6) |
| MIC-1 | 49.5 (70.2) | 4.4 (7.5) | 6.0 (25.3) | 18.0 (61.0) | ||
| MIC-2 | 48.1 (70.2) | 7.5 (14.1) | 11.9 (46.2) | 39.1 (74.8) | ||
| MIC-3 | 43.2 (59.9) | 9.8 (35.7) | 34.3 (71.5) | 66.7 (84.3) | ||
| MIC-4 | 27.8 (47.4) | 38.0 (70.4) | 69.6 (85.9) | 73.9 (86.8) | ||
| AMB | MIC-0 | 64.2 (81.9) | 11.2 (62.0) | 60.8 (91.7) | 87.9 (96.9) | |
| 48 | ICZ | MIC-0 | 70.4 (83.1) | 23.6 (26.0) | 25.7 (41.3) | 29.1 (60.9) |
| MIC-1 | 42.9 (66.8) | 16.6 (27.8) | 27.4 (67.6) | 42.6 (82.3) | ||
| MIC-2 | 32.2 (55.7) | 21.4 (50.3) | 48.0 (80.1) | 63.5 (84.6) | ||
| MIC-3 | 21.5 (37.9) | 41.3 (72.2) | 68.2 (82.8) | 75.4 (82.4) | ||
| MIC-4 | 15.0 (21.5) | 66.9 (81.5) | 68.7 (80.8) | 62.2 (76.1) | ||
| AMB | MIC-0 | 32.6 (42.7) | 45.6 (90.3) | 89.6 (99.3) | 96.4 (99.6) | |
| 24 and 48d | ICZ | MIC-0 | 54.1 (60.0) | 17.6 (23.6) | 22.8 (55.1) | 31.7 (74.7) |
| MIC-1 | 51.4 (64.5) | 19.0 (46.9) | 44.2 (75.7) | 59.4 (81.2) | ||
| MIC-2 | 48.3 (68.8) | 32.5 (61.6) | 56.6 (79.5) | 66.6 (80.2) | ||
| MIC-3 | 46.9 (64.0) | 51.1 (72.6) | 66.2 (78.9) | 66.5 (76.8) | ||
| MIC-4 | 37.6 (54.6) | 65.8 (79.2) | 52.1 (73.4) | 41.0 (63.9) | ||
| AMB | MIC-0 | 54.4 (67.9) | 86.6 (98.4) | 92.2 (99.2) | 84.3 (99.3) | |
ICZ, itraconazole; AMB, amphotericin B.
See footnote a of Table 2 for the definitions of the endpoints.
The 95% confidence intervals of the average percent agreement among the three replicates (for each replicate, 100 comparisons [five strains × five species × four observers] were calculated after angular transformation and ranged from ±0.9 to ±11.9% (median, 5%).
For the Etest method, the values are for the Etest method at 24 h and the NCCLS method at 48 h; and for the sensititre method, the values are for the NCCLS method at 24 h and the sensititre method at 48 h.
(ii) Sensititre method.
Overall, the levels of agreement (within 1 log2 dilution) between the Sensititre method and the NCCLS method were high for amphotericin B (91% after 24 h and 99% after 48 h of incubation) but not for itraconazole (9.7 and 48%, respectively) (Table 3) on the basis of the MIC endpoints chosen for the two methods (see above). The levels of agreement increased after 48 h of incubation compared to those after 24 h of incubation for both drugs. Extremely poor agreement was found after 24 h for itraconazole when it was tested against A. fumigatus and A. nidulans (<5%), and the differences for all of the species were statistically significant after both 24 and 48 h of incubation. However, the majority of the MICs obtained by the Sensititre method (>86%) after 48 h of incubation for both drugs were within 2 log2 dilutions of the corresponding MICs obtained by the NCCLS method for all species except A. ustus tested with itraconazole (33%). An analytical schematic representation of the differences between these two methods after 48 h of incubation is shown in Fig. 1. Overall, the MICs generated by the Sensititre method, in particular with itraconazole, were up to threefold lower than those generated by the NCCLS method (Fig. 1). The only exceptions were the MICs for four A. ustus strains, for which the itraconazole MICs were higher by the Sensititre method.
Since the MICs determined by the Sensititre method were lower than those determined by the NCCLS method, the results obtained after 24 h of incubation by the NCCLS method were compared with the results obtained by the Sensititre method after 48 h of incubation. The results, together with the levels of agreement between any combination of various MIC endpoints between the Sensititre method and the NCCLS method, are presented in Table 4. Although the MICs determined by the NCCLS method were linearly correlated with the MICs determined by the Sensititre method (data not shown), the color development was associated with large amounts of growth. For itraconazole, lower levels of agreement were found with the MIC-P and MIC-R endpoints of the Sensititre method and the MIC-0 and MIC-1 endpoints of the NCCLS method. The highest levels of agreement were found with the MIC-B endpoint of the Sensititre method and the MIC-4 endpoint of the NCCLS method after 24 h of incubation (74%) or the MIC-3 endpoint of the NCCLS method after 48 h of incubation (75%). Among the MIC endpoints of the NCCLS method, the MIC-2 and the MIC-3 endpoints showed the highest levels of agreement (66%) when they were compared after 24 h of incubation with the MIC-B endpoint of the Sensititre method after 48 h of incubation. With this MIC endpoint-incubation period combination, high levels of agreement (71 to 96%) were obtained for all species except A. ustus (2%), for which the MIC-0 endpoint showed the highest level of agreement for any combination (data not shown). For amphotericin B the highest level of agreement was found when the MIC-B endpoint of the Sensititre method was compared with the MIC-0 endpoint of the NCCLS method after both 24 and 48 h of incubation (88 and 96%, respectively).
DISCUSSION
Overall, broader ranges of MICs and higher MICs were obtained by the Etest method than by the other methods, while the Sensititre method yielded the lowest MICs. High levels of reproducibility among the observers and the experiments were achieved with the NCCLS and the Sensititre methods but not the Etest method. By using the recommended MIC endpoints, the level of agreement (within 1 twofold dilution) between the Etest and the NCCLS method was low after 24 h of incubation and was even lower after 48 h of incubation (although there was a large variation among the species), while between the Sensititre method and the NCCLS methods, low levels of agreement were found for itraconazole, especially after 24 h of incubation, but not for amphotericin B. By comparison of the various combinations of MIC endpoints and incubation periods for the three methods, the highest overall levels of agreement between the Etest method and the NCCLS method were found when the MIC-0 endpoint after 24 h of incubation was used for amphotericin B and the MIC-0 endpoint after 48 h of incubation was used for itraconazole. Overall, the highest levels of agreement between the Sensititre method and the NCCLS method were found after 48 h when the MIC-B endpoint of the Sensititre method was compared with the MIC-0 endpoint of the NCCLS method for amphotericin B and the MIC-3 endpoint of the NCCLS method for itraconazole.
The Etest method was easier and less time-consuming, but poor reproducibility was found, in particular after 24 h of incubation. At that time, the intersect of the elliptical inhibition zone with the Etest strip was ambiguous, and therefore, MICs might be determined erroneously. The same levels of reproducibility found after 48 h of incubation in the present study were found in a previous study (75%; 9 of 12 strains) (35). In another study, Johnson et al. (16) found that the inoculum size is a critical factor for the reproducibility of the Etest method since the use of inocula lower than 106 CFU/ml resulted in poor reproducibilities. Furthermore, the medium might also be important for obtaining reproducible results since in that study reproducibility increased when yeast nitrogen base medium was used instead of RPMI.
Depending on the species-drug-MIC endpoint-incubation period combination used, poor agreement of results with those of the NCCLS M-38P reference method was found. This is in agreement with previous findings, in which levels of agreement were higher only for some species-drug combinations tested (35) and when the results of the Etest method after 24 h were compared with the results of the NCCLS method after 48 h (29). However, lower levels of agreement of the results of the present study, particularly for amphotericin B after 48 h of incubation, compared with those of previous studies were found, although evaluations of interobserver and interexperimental variations were not included in the previous studies (9, 35). The discrepancies caused by the higher MICs obtained by the Etest method confirms the results of previous studies (29, 35; I. Gergopoulos, A. Skiada, P. Giakkoupi, and G. Petrikkos, Abstr. 6th Cong. Eur. Confederation Med. Mycol., abstr. P9-013, 2000). The higher levels of agreement found between the complete growth inhibition endpoint of the NCCLS method and the results of the Etest method for both drugs was expected since by the latter method the inhibition zone differentiates growth from no growth. The overall poor agreement between the Etest method and the NCCLS method might be due to the medium used, which is an important factor for the growth of filamentous fungi (20) and for the Etest method with yeasts (28), as well as to the patterns of the inhibition zones, whose borders are determined subjectively in many cases. Furthermore, the results of agar-based susceptibility testing methods might be influenced by problems with the diffusion of the drugs (32) and factors such as limited contact of the fungi with the drugs (43), the different nature of growth on a surface (i.e., due to differences in oxygen tension), and the different growth rates of fungi on agar medium compared with those in broth solutions. These factors together with the nature of the antifungal actions might be responsible for the species-dependent differential agreement between the NCCLS method and the Etest method for itraconazole and amphotericin B, for which high levels of agreement were found after 24 h but not after 48 h, in contrast to the results for itraconazole, in which the opposite was observed.
In vitro resistant strains might be better distinguished from the susceptible strains by the Etest method than the NCCLS method since differences of 6 twofold dilutions were observed after 48 h of incubation, confirming the findings of Szekely et al. (35). Higher itraconazole MICs were obtained for all A. ustus strains, even after 24 h of incubation, by the Etest method, although the MICs obtained by the NCCLS method were much lower. Since aspergillosis caused by this species has previously been reported to be refractory to itraconazole treatment (14, 33, 39), the Etest method might detect itraconazole-resistant A. ustus strains better than the NCCLS method does. In the case of A. terreus, even though the MICs for all five strains were similar by the NCCLS method (0.5 to 1 mg/liter), two strains were differentiated by the Etest method, with the MICs for the two strains being threefold lower those for the other three strains. Furthermore, the MICs of amphotericin B for A. flavus, A. terreus, and three strains of A. nidulans determined by the Etest were very high (>16 mg/liter) compared with those determined by the NCCLS method (1 to 4 mg/liter). In vivo resistance to amphotericin B has been reported for A. flavus (24) and A. terreus (4, 16), even though the MICs determined by the NCCLS method in vitro are low. Again, this indicates that the Etest method might be superior to the NCCLS method in detecting amphotericin B resistance (9), as has previously been found for yeasts (2, 18, 40).
The results of the Sensititre method had high levels of interobserver agreement, comparable to those of the results of the NCCLS method, confirming the results of previous studies (10). However, relatively lower levels of interexperimental agreement were obtained, particularly with itraconazole. In comparison with the NCCLS method, high levels of agreement were found with the Sensititre method for amphotericin B but not for itraconazole. The discrepancies were caused by the lower MICs determined by the Sensititre method compared to those determined by the NCCLS method, particularly the MICs of itraconazole. The presence of more than 50% of the visible growth compared to the growth of the growth control was required for the color to change from blue to purple. For some species-drug-incubation period combinations, previous studies (6, 11, 25, 36) also found higher and lower MICs by the Sensititre method than by the NCCLS method. While no clear explanation has been offered for the trailing phenomenon, such a phenomenon might be the reason for the discrepancies in the MICs. On the basis of our observations, the blue color did not always correspond to the absence of growth since small amounts of visible growth did not cause a color change. Because this method is based on the conversion of an oxidation-reduction indicator by living fungi, any metabolic inhibition caused by the antifungal agents may result in a lack of a color change, despite the presence of visible growth, resulting in lower MICs by the Sensititre method than by the NCCLS method.
In addition, To et al. (37) suggested that the inoculum size may be an important factor when azoles are tested in the presence of Alamar blue. Jahn et al. (15) found that A. fumigatus strains had poor reactivities in the Alamar blue test. The poor reactivities might possibly be explained by the low surface oxidase activities exhibited by these strains compared with those exhibited by Candida albicans strains. The different rates of conversion of Alamar blue by some Aspergillus species (A. ustus, A. terreus) found in the present study after 24 h of incubation might be related to differential compositions of the cell walls, which may affect the rates of penetration of Alamar blue. Moreover, although 48 h of incubation is sufficient for the growth control to convert Alamar blue, it might not be long enough for organisms in the drug-containing wells to do so because of metabolic inhibition and different stages of growth. The dye Alamar blue was used in a multicenter study to test the susceptibilities of different filamentous fungi to itraconazole and amphotericin B, and high levels of agreement with the NCCLS method were found after 48 h of incubation, with even higher levels of agreement found after 72 h of incubation (10). Since the Sensititre method was initially developed for the testing of yeasts, different adjustments might be required for filamentous fungi.
In conclusion, the Sensititre method was very reproducible, but it showed low levels of agreement with the NCCLS method for itraconazole. Prolonged incubation up to 72 h and the use of the concentration in the first blue well as an MIC endpoint might decrease the levels of discrepancy, resulting in higher levels of agreement with the NCCLS method, particularly for itraconazole, for which an endpoint at 50% growth inhibition was correlated better with the color change of the Sensititre method. The optimal concentration of Alamar blue, the optimal inoculum size, and the optimal incubation conditions should be explored. The importance of the difference between the color change and the presence of visible growth should be investigated in detail, and the appropriateness of various steps of the protocol like the sealing of the microtiter plates should be studied. Although the Etest method is applicable for filamentous fungi, resulting in a broad range of MICs, it showed poor reproducibility and its results agreed poorly with those of the NCCLS method, particularly for itraconazole, for which the use of a complete growth inhibition endpoint after 48 h of incubation could increase the levels of agreement. The use of richer media, the use of 24 h of incubation, particularly for amphotericin B, the determination of another MIC endpoint (i.e., the most outside border of the elliptical inhibition zone when more than one zone is obvious [29]), and the use of inocula higher than 106 CFU/ml might improve the results. The effect of the inoculum as well as of the incubation conditions on the results of the Etest method should be evaluated in depth with large collections of isolates.
Both the Etest and the Sensititre methods are promising but require further investigation to identify the optimum conditions for their use in the testing of the susceptibilities of filamentous fungi to antifungal agents including new azoles and the new class of candins. Optimization of these tests might require adjustments depending on the species tested, particularly for A. ustus and A. flavus. Head-to-head comparisons in combination with studies with animals and correlations with clinical outcomes are necessary in order to test the validities and reliabilities of these tests.
Acknowledgments
This work was supported by European Commission Training and Mobility of Researchers grant FMRX-CT970145 to Joseph Meletiadis and by the Mycology Research Center Nijmegen.
We thank J. Peter Donnelly for critical comments.
REFERENCES
- 1.Aller, A. I., E. Martin-Mazuelos, M. J. Gutierrez, S. Bernal, M. Chavez, and F. J. Recio. 2000. Comparison of the Etest and microdilution method for antifungal susceptibility testing of Cryptococcus neoformans to four antifungal agents. J. Antimicrob. Chemother. 46:997-1000. [DOI] [PubMed] [Google Scholar]
- 2.Clancy, C. J., and M. H. Nguyen. 1999. Correlation between in vitro susceptibility determined by E test and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob. Agents Chemother. 43:1289-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannaoui, E., E. Borel, M. F. Monier, M. A. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 4.Dannaoui, E., E. Borel, F. Persat, M. A. Piens, and S. Picot. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601-606. [DOI] [PubMed] [Google Scholar]
- 5.Dannaoui, E., F. Persat, M. F. Monier, E. Borel, M. A. Piens, and S. Picot. 1999. Use of spectrophotometric reading for in vitro antifungal susceptibility testing of Aspergillus spp. Can. J. Microbiol. 45:871-874. [PubMed] [Google Scholar]
- 6.Davey, K. G., A. Szekely, E. M. Johnson, and D. W. Warnock. 1998. Comparison of a new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J. Antimicrob. Chemother. 42:439-444. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 8.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 2001. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J. Clin. Microbiol. 39:1360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, S. Killian, H. A. Norris, and M. A. Ghannoum. 1999. Multicenter comparison of the Sensititre YeastOne Colorimetric Antifungal Panel with the National Committee for Clinical Laboratory standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeasts and yeast-like organisms. J. Clin. Microbiol. 37:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., J. L. Rodriguez-Tudela, and J. V. Martinez-Suarez. 1995. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J. Clin. Microbiol. 33:3154-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favel, A., C. Chastin, A. L. Thomet, P. Regli, A. Michel-Nguyen, and A. Penaud. 2000. Evaluation of the E test for antifungal susceptibility testing of Candida glabrata. Eur. J. Clin. Microbiol. Infect. Dis. 19:146-148. [DOI] [PubMed] [Google Scholar]
- 14.Iwen, P. C., M. E. Rupp, M. R. Bishop, M. G. Rinaldi, D. A. Sutton, S. Tarantolo, and S. H. Hinrichs. 1998. Disseminated aspergillosis caused by Aspergillus ustus in a patient following allogeneic peripheral stem cell transplantation. J. Clin. Microbiol. 36:3713-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahn, B., A. Stuben, and S. Bhakdi. 1996. Colorimetric susceptibility testing for Aspergillus fumigatus: comparison of menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide and Alamar blue tests. J. Clin. Microbiol. 34:2039-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E. M., K. L. Oakley, S. A. Radford, C. B. Moore, P. Warn, D. W. Warnock, and D. W. Denning. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85-93. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman, C. A., and L. T. Zarins. 1999. Colorimetric method for susceptibility testing of voriconazole and other triazoles against Candida species. Mycoses 42:539-542. [DOI] [PubMed] [Google Scholar]
- 18.Lozano-Chiu, M., V. L. Paetznick, M. A. Ghannoum, and J. H. Rex. 1998. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J. Clin. Microbiol. 36:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meletiadis, J., J. F. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, and P. E. Verweij. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. Document M-38P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Odds, F. C., F. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., and A. L. Barry. 1994. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 32:1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., S. A. Messer, and A. Bolmstrom. 1998. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn. Microbiol. Infect. Dis. 32:223-227. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., S. A. Messer, A. Bolmstrom, F. C. Odds, and J. H. Rex. 1996. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 34:1691-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., S. A. Messer, A. Karlsson, and A. Bolmstrom. 1998. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., S. A. Messer, K. Mills, and A. Bolmstrom. 2000. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J. Clin. Microbiol. 38:3359-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., Q. Vu, M. Lancaster, A. Espinel-Ingroff, A. Fothergill, C. Grant, M. R. McGinnis, L. Pasarell, M. G. Rinaldi, and L. Steele-Moore. 1994. Multisite reproducibility of colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 32:1625-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provine, H., and S. Hadley. 2000. Preliminary evaluation of a semisolid agar antifungal susceptibility test for yeasts and molds. J. Clin. Microbiol. 38:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricci, R. M., J. S. Evans, J. J. Meffert, L. Kaufman, and L. C. Sadkowski. 1998. Primary cutaneous Aspergillus ustus infection: second reported case. J. Am. Acad. Dermatol. 38:797-798. [DOI] [PubMed] [Google Scholar]
- 34.Sewell, D. L., M. A. Pfaller, and A. L. Barry. 1994. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J. Clin. Microbiol. 32:2099-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szekely, A., E. M. Johnson, and D. W. Warnock. 1999. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 37:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiballi, R. N., X. He, L. T. Zarins, S. G. Revankar, and C. A. Kauffman. 1995. Use of a colorimetric system for yeast susceptibility testing. J. Clin. Microbiol. 33:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To, W. K., A. W. Fothergill, and M. G. Rinaldi. 1995. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 33:2660-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tortorano, A. M., E. Dannaoui, M. Cogliati, M. A. Piens, A. L. Rigoni, R. Grillot, M. A. Viviani, and EGBA Network. 2000. Evaluation of different in vitro procedures for testing amphotericin B and itraconazole susceptibility of Aspergillus fumigatus. J. Mycol. Med. 10:123-127. [Google Scholar]
- 39.Verweij, P. E., M. F. van den Bergh, P. M. Rath, B. E. de Pauw, A. Voss, and J. F. Meis. 1999. Invasive aspergillosis caused by Aspergillus ustus: case report and review. J. Clin. Microbiol. 37:1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardlaw, A. C. 1989. Practical statistics for experimental biologists. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 42.Warnock, D. W., E. M. Johnson, T. R. Rogers, et al. 1998. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. J. Antimicrob. Chemother. 42:321-331. [DOI] [PubMed] [Google Scholar]
- 43.Yamada, H., S. Kohno, S. Maesaki, H. Koga, M. Kaku, K. Hara, and H. Tanaka. 1993. Rapid and highly reproducible method for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 31:1009-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]