Abstract

Degraders with dual activity against BRD4 and CBP/EP300 were designed. A structure-guided design approach was taken to assess and test potential exit vectors on the dual BRD4 and CBP/EP300 inhibitor, ISOX-DUAL. Candidate degrader panels revealed that VHL-recruiting moieties could mediate dose-responsive ubiquitination of BRD4. A panel of CRBN-recruiting thalidomide-based degraders was unable to induce ubiquitination or degradation of target proteins. High-resolution protein cocrystal structures revealed an unexpected interaction between the thalidomide moiety and Trp81 on the first bromodomain of BRD4. The inability to form a ternary complex provides a potential rationale for the lack of degrader activity with these compounds, some of which have remarkable affinities close to those of (+)-JQ1, as low as 65 nM in a biochemical assay, vs 1.5 μM for their POI ligand, ISOX-DUAL. Such a “degrader collapse” may represent an under-reported mechanism by which some putative degrader molecules are inactive with respect to target protein degradation.
Introduction
The MYC proto-oncogene is a master regulator of transcription with a central role in cancer cell pathophysiology. The expression of MYC is tightly controlled in healthy cells but, in up to 70% of human cancers, MYC expression is elevated or dysregulated.1,2 Pharmacological inactivation of the c-Myc oncoprotein is therefore considered an attractive approach as a therapeutic strategy for cancer, with multiple lines of evidence suggesting that MYC inactivation leads to tumor regression.3−5 Directly targeting the c-Myc oncoprotein as a therapeutic strategy is challenging, however, due to a lack of a binding pocket amenable to small-molecule inhibitor development.6
Alternative strategies to target c-Myc indirectly have therefore been pursued, through inhibition or degradation of upstream and downstream proteins including BET bromodomains7,8 and the immune cell-specific transcription factor, interferon regulatory factor 4 (IRF4).9 Knockdown of IRF4 is toxic in multiple myeloma (MM) cell lines, while pharmacological inhibition of the bromodomain histone acetyltransferases CBP/EP300 is reported to lead to direct transcriptional suppression of IRF4 and concomitant reduction in MYC expression.10,11 More recent work suggests that MM cell death following treatment with CBP/EP300 inhibitors is not mediated by reduced IRF4 expression, but indirectly through MYC itself. CBP/EP300 bromodomain inhibition was sufficient to reduce IRF4 mRNA levels but not IRF4 protein levels, which might partly be explained by the long half-life of IRF4 in MM cell lines (33–61 h), compared to the short half-life of c-Myc (30 min).12
The strategy of targeted protein degradation through proteolysis targeting chimeras (PROTACs, commonly referred to as degraders) provides an alternative approach for small-molecule modulation of target proteins. The advantages of degraders as a modality have been extensively reviewed elsewhere and include an altered pharmacokinetic/pharmacodynamic (PK/PD) regime that can elicit beneficial outcomes compared to small-molecule inhibition.13−22 For example, degraders can induce durable PD responses extending beyond the detectable presence of the degrader itself, resulting in a long-lasting reduction in protein levels, particularly those with long half-lives.23 Further, efficacious degraders can be designed using poorly active small-molecule inhibitors.24,25
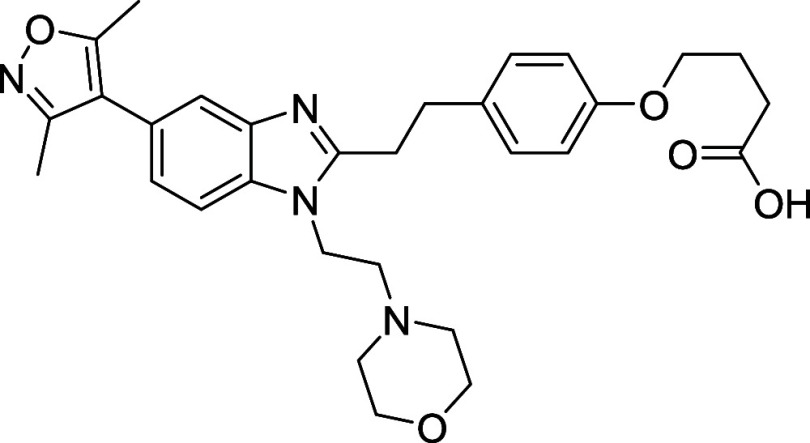
Reduction of c-Myc levels has been demonstrated following treatment with degraders independently targeting both CBP/EP30026−31 and BRD4.32−42 Here, we pursued a strategy of targeting c-Myc through dual degradation of both CBP/EP300 and BRD4, via the small-molecule inhibitor ISOX-DUAL 1(43) (Figure 1). The latter inhibits BRD4 and CBP/EP300 with micromolar potency and was deemed to be a suitable candidate to assess whether a dual degradation approach would offer a synergistic benefit in the reduction of c-Myc, compared to inhibition or degradation of the proteins individually. We recently successfully optimized the synthesis of 1,44 providing ready access to larger quantities of precursors suitable for onward chemistry to enable synthesis of panels of candidate degraders. Here, we report the design, synthesis, and evaluation of libraries of molecules based on 1, as the protein of interest binder (POI), with suitable exit vectors, various linkers, and E3 ligase ligands, to explore the potential for rational design of a degrader with a balanced dual degradation profile toward CBP/EP300 and BRD4.
Figure 1.
Choice of the starting scaffold and exit vectors. (A) Crystal structure of the CBP bromodomain with bound inhibitor 2 (5CGP). (B) Structure of the first bromodomain of BRD4 (5CFW) with the same inhibitor, showing ether (OMe) and morpholine solvent-exposed prospective exit vectors. Surface lysines are shown in cyan. (C) Structure of ISOX-DUAL 1 and 2 with representative structural design of the proposed phenolic ether and piperazine exit vectors.
Results and Discussion
BDOIA383, 2, an early chemical probe with the same core phenotype as ISOX-DUAL, 1, provided a convenient starting point for determining suitable exit vectors from the ISOX-DUAL scaffold. Cocrystal structures of the first bromodomain of BRD4 (BRD4 BD1) and CBP bromodomains in complex with 2(43,45) revealed two potential solvent-facing vectors for linker attachment: the phenolic ether and morpholine groups (Figure 1A,B). We proposed modification to enable linker attachment at either position: dealkylation of the ether, to reveal a phenol, and replacement of the morpholine group by a piperazine (Figure 1C).
We first explored the phenolic ether exit vector strategy, synthesizing a small panel of analogues to assess the feasibility of functionalizing this part of the molecule while retaining balanced binary affinity to both target bromodomains, BRD4 and CBP (Scheme 1). Starting with the nitro analogue 3, known intermediate 5 was subjected to coupling/cyclization procedures previously developed for related analogues.44 A series of products, 6–10, were synthesized. The biochemical binary binding affinities of selected compounds to both BRD4 and CBP were measured using a fluorescence resonance energy transfer (FRET) assay (Table 1) using compounds 1, 2 as controls. Phenol 6 had a similar affinity to both bromodomains as the parent compounds 1 and 2, and affinities toward BRD4 were improved upon the incorporation of an acetyl (7), alkyl ester (8), and longer amide–ether linkages (10), the latter two representing model compounds for exit vectors and potential degraders. Overall, the phenol ether exit vector represented a promising platform for degrader synthesis because biological activity, in biochemical assays, was not unduly compromised.
Scheme 1. Synthetic Approach to ISOX-DUAL Analogues for the Validation of the Phenolic Exit Vector.

Reagents & conditions: [a] 4-(2-aminoethyl)morpholine, Et3N, dimethyl sulfoxide (DMSO), 80 °C, 90%; [b] 3.5-dimethylisoxazole-4-boronic acid pinacol ester, K3PO4, PdCl2(dppf).CH2Cl2, 1,4-dioxane, water, reflux, 94%; [c] (i) 1 M Na2S2O4 (aq), EtOH, 80 °C; (ii) 10% NH3 (aq), 81%; [d] (i) HATU, Et3N, N,N-dimethylformamide (DMF); (ii) AcOH, reflux 30%; [e] Ac2O, DCM, pyridine, rt, 80%; [f] K2CO3, methyl-4-bromobutyrate, MeCN, reflux, 70%; [g] LiOH, tetrahydrofuran (THF), water, rt, 94%; [h] 2-methoxyethylamine, NEt3, DMF, HATU, rt, 79%.
Table 1. Structure–Activity Relationships for BRD4 BD1 and CBP Binding as Determined by FRET for the Phenolic Exit Vector Analoguesa.
| compound | R | BRD4 IC50 (μM) | CBP IC50 (μM) |
|---|---|---|---|
| 6 | H | 3.0 ± 0.1 | 0.3 ± 0.1 |
| 7 | Ac | 1.3 ± 0.1 | 0.17 ± 0.04 |
| 8 | (CH2)3CO2Me | 1.55 ± 0.01 | 0.5 ± 0.1 |
| 10 | (CH2)3C(O)NH(CH2)2OMe | 1.62 ± 0.01 | 0.33 ± 0.05 |
| 2 | Me | 2.4b | 0.12b |
| 1 | (CH2)3NMe2 | 1.5b | 0.65b |
Values given as mean ± SD (n = 3).
Values from literature.43
We proceeded to synthesize a small panel of candidate degraders based on this exit vector strategy, using 5 as a key intermediate. Routine cyclization chemistry, involving the ester-acid lithium salt 11, led to the important precursor 12, which underwent amide couplings with a small range of amine-linker-E3 ligase analogues to afford candidate degraders 13–16 (Scheme 2). Affinities of the degraders to both BRD4 and CBP were again assessed biochemically using a FRET assay (Table 2). Good balances of affinity were observed for degraders 13, 14, and 16, albeit with a marked increase in CBP IC50 values, whereas compound 15 was less active toward both bromodomains.
Scheme 2. Synthesis of ISOX-DUAL Phenolic Ether Degraders.
Reagents & Conditions: [a] (i) HATU, Et3N, DMF; (ii) AcOH, reflux; (iii) HCl (4 M in 1,4-dioxane), 46%; [b] H2N-LINKER-E3 ligand, HATU, Et3N, DMF; [c] tert-butylbromoacetate, K2CO3, MeCN, reflux, 75%; [d] 1 M LiOH (aq), THF, 94%.
Table 2. Structure–Activity Relationships for BRD4 BD1 and CBP Binding as Determined by FRET for the Phenolic Exit Vector Degradersa.
| compound (yield, %) | n | E3 ligase recruited | BRD4 IC50 (μM)b | CBP IC50 (μM)b |
|---|---|---|---|---|
| 13 (60) | 3 | CRBN | 2.0 ± 0.1 | 1.4 ± 0.1 |
| 14 (55) | 4 | CRBN | 1.84 ± 0.04 | 1.5 ± 0.2 |
| 15 (75) | 3 | VHL | 4.6 ± 0.1 | 3.4 ± 0.3 |
| 16 (65) | 4 | VHL | 1.08 ± 0.03 | 1.5 ± 0.5 |
| ISOX-DUAL | N/A | N/A | 1.5b | 0.65b |
Values given as mean ± SD (n = 3).
Values from literature.43
Given the significant reduction in CBP affinities observed with our initial set of candidate degraders, we next explored the piperazine exit vector, aiming to obtain degrader molecules with more potent binary affinities to both targets. As before, a small panel of analogues was first synthesized to validate this position as a suitable exit vector and, in this instance, the ISOX-DUAL dimethylamino side chain was retained. Standard reduction chemistry delivered diamine 19, which was cyclized with known 20(44) to give benzimidazoles 21 and 23. Reacting 21 in the presence of Boc anhydride afforded 22 (Scheme 3).
Scheme 3. Synthesis of ISOX-DUAL Analogues for Validation of the Piperazine Exit Vector.

Reagents & conditions: [a] 4-(2-aminoethyl)-1-Boc-piperazine, Et3N, MW, 125 °C, 10 min, 98%; [b] 3,5-dimethylisoxazole-4-boronic acid pinacol ester, PdCl2(dppf)·CH2Cl2 (5 mol %), K3PO4, 1,4-dioxane, water, reflux, 80%; [c] (a) 1 M Na2S2O4 (aq), EtOH, 80 °C; (b) 10% NH3 (aq), 83%; [d] Br(CH2)3NMe2, N,N-diisopropylethylamine (DIPEA), DMF, 87%; [e] 1 M LiOH (aq), THF, quant. [f] (i) HATU, Et3N, DMF; (ii) 4 N HCl dioxane, MeOH, reflux, 37%; [g] Boc2O, DMAP, NEt3, DCM, rt, 43%; [h] (i) HATU, Et3N, DMF; (ii) AcOH, reflux, 34%.
Instead of attempting to perform the derivatization of the piperazine late into the synthesis of our degrader precursor, the strategy was redesigned (Scheme 4); deprotecting intermediate 17 with trifluoroacetic acid (TFA) afforded free piperazine 24, which was alkylated with tert-butylbromoacetate to afford 25. Subsequent reduction of the nitro moiety afforded 26, which was treated with the standard amide coupling and cyclization chemistry performed previously to afford our precursor compound 27. From here we expanded our analogue library to methyl ester 28 and 2-methoxyethylamide 29. Compounds 21–23, 28 and 29 were analyzed in biochemical assays (Table 3) where the reference values for ISOX-DUAL were found to be around 2x higher than the literature value. Here, a significant loss of affinity toward both targets was observed in most cases, except for 29, which had a good dual affinity toward both targets and was a preferable exit vector for degrader synthesis. Encouraged by the binary affinities observed with compound 29, based on this exit vector strategy and utilizing key intermediate 27, we designed a library of degraders (30–45).
Scheme 4. Synthesis of ISOX-DUAL Piperazine Analogues and Degraders.

Reagents & conditions: [a] TFA, DCM, 99%; [b] tert-butylbromoacetate, DIPEA, DCM, 80%; [c] (a) 1 M Na2S2O4 (aq), EtOH, 80 °C; (b) 10% NH3 (aq), 70%; [d] (i) HATU, Et3N, DMF; (ii) AcOH, reflux; (iii) HCl (4 M in 1,4-dioxane), 36%; [e] H2SO4, MeOH, reflux, 88%; [f] NEt3, 2-methoxyethylamine, HATU. DMF, rt, 77%; [g] H2N-LINKER-E3 Ligand, HATU, Et3N, DMF.
Table 3. Structure–Activity Relationships for CBP and BRD4 BD1 Binding as Determined by FRET for the Piperazine Exit Vector Analoguesa.
| compound (yield, %) | Y | BRD4 IC50 (μM) | CBP IC50 (μM) |
|---|---|---|---|
| ISOX-DUAL | O | 3.6 ± 0.6 (1.5)b | 1.20 (0.65)b |
| 21 (37%) | NH | 5 ± 1 | 3.5 ± 0.1 |
| 22 (43%) | NBoc | 3.2 ± 0.6 | 1.2 ± 0.1 |
| 23 (34%) | NAc | 8 ± 3 | 2.1 ± 0.1 |
| 28 (88%) | NCH2CO2Me | 6 ± 2 | 2.1 ± 0.1 |
| 29 (77%) | NCH2C(O)NH(CH2)2OMe | 1.5 ± 0.4 | 0.83 ± 0.04 |
Values given as mean ± SD (n = 2).
Literature values.
A disappointing drop in CBP affinity, in biochemical assays, was observed for all compounds in this series with a surprising gain in affinity for BRD4 (Table 4), with candidate degraders displaying BRD4 affinities in the 60–210 nM range. It was unclear why addition of the linker and E3 ligase ligand results in such a clear drop in CBP binary affinity, given the strong validation of the exit vector at this position (Table 3). We decided to further test some of the candidate degraders to assess whether, despite the unbalanced potency, they might be able to induce productive ternary complex formation.
Table 4. Structure–Activity Relationships for BRD4 and CBP Binding as Determined by FRET for the Piperazine Exit Vector Degradersa.
| compound (yield, %) | linker type | n | E3 ligase recruited | BRD4 IC50 (nM) | CBP IC50 (μM) | BRD4/CBP selectivity |
|---|---|---|---|---|---|---|
| 30 (36) | A | 1 | CRBN | 160 ± 10 | >20 | >127 |
| 31 (31) | A | 2 | CRBN | 122 ± 4 | 8.3 ± 2.1 | 71 |
| 32 (44) | A | 3 | CRBN | 83 ± 3 | 4.5 ± 0.9 | 54 |
| 33 (24) | A | 4 | CRBN | 81 ± 4 | 10.0 ± 0.3 | 123 |
| 34 (35) | B | 1 | CRBN | 65 ± 6 | 6.7 ± 0.6 | 104 |
| 35 (37) | B | 2 | CRBN | 88 ± 2 | 14.0 ± 0.1 | 163 |
| 36 (35) | B | 3 | CRBN | 114 ± 4 | >20 | >175 |
| 37 (52) | B | 4 | CRBN | 88 ± 2 | 11.0 ± 1.1 | 121 |
| 38 (16) | C | 1 | CRBN | 74 ± 4 | 6.0 ± 0.39 | 82 |
| 39 (32) | C | 2 | CRBN | 111 ± 3 | 4.6 ± 1.5 | 42 |
| 40 (43) | C | 3 | CRBN | 211 ± 6 | 8.8 ± 4.5 | 42 |
| 41 (20) | C | 4 | CRBN | 122 | 13.0 ± 1.5 | 110 |
| 42 (39) | D | 1 | VHL | 161 ± 6 | 3.6 ± 0.13 | 22 |
| 43 (22) | D | 2 | VHL | 133 ± 14 | 3.7 ± 0.003 | 29 |
| 44 (36) | D | 3 | VHL | 101 ± 3 | 13.0 ± 0.3 | 131 |
| 45 (35) | D | 4 | VHL | 131 ± 8 | 9.1 ± 1.6 | 70 |
| ISOX-DUAL | N/A | N/A | N/A | 1500b | 0.65b | 0.43 |
Values given as mean ± SD (n = 3).
Values from the literature.43
An in vitro ubiquitination assay was employed for an initial assessment of the ability for ISOX-DUAL-based degraders to induce productive ternary complex formation. This assay follows the ubiquitination of the target proteins in a cell-free system, removing potentially confounding factors such as degrader cell permeability and efflux.19 We first tested a subset of VHL-recruiting degraders (compounds 42–45) and observed clear dose-dependent ubiquitination of BRD4, but not CBP, with all four degraders tested (Figure 2). A hook effect was evident for BRD4 ubiquitination, with structure–activity relationship (SAR) suggesting a greater degree of ubiquitination at lower compound concentration with increased linker length.
Figure 2.
In vitro ubiquitination assays for VHL-recruiting degraders 42–45 with BRD4 (left panel) and CBP (right panel). FLAG-BRD4 and GST-CREBBP were detected using capillary electrophoresis (Simple Western, Wes) and anti-FLAG and anti-GST antibodies.
A small panel of CRBN-recruiting degraders (compounds 34–36) were also tested for their ability to ubiquitinate BRD4 and CBP in a cell-free environment (Figure 3). Limited ubiquitination of BRD4, but not CBP, was observed with the longest linker tested (compound 36), with no observable ubiquitination for the degraders with shorter linkers (compounds 34 and 35).
Figure 3.
In vitro ubiquitination assays for CRBN-recruiting degraders 34–36 with BRD4 (left panel) and CBP (right panel). FLAG-BRD4 and GST-CREBBP were detected using capillary electrophoresis (Simple Western, Wes) and anti-FLAG and anti-GST antibodies.
We sought to rationalize the different affinities and ubiquitination patterns through the cocrystallization of representative degraders with BRD4 and the evaluation of the binding modes. Several high-resolution crystal structures (1.1–1.9 Å) of BRD4-degrader complexes were determined (Supporting Table S2). Degrader 14 bound with a similar pose to the parent scaffold BDOIA383, with the isoxazole oxygen forming the typical hydrogen bond with the highly conserved Asn140 at the bottom of the binding site, and the benzimidazole moiety packing between Pro82 in the WPF shelf and Leu92. The linker-thalidomide portion protruded into the solvent and was not resolved in the crystal structure (Figure 4B). We can therefore rationalize the similar BRD4 binding affinities for phenolic ether degrader 14 and BDOIA383 (2.4 and 1.8 μM, respectively). The piperazine-based degrader 34, however, adopted a surprising binding mode in the cocrystal structure. There was excellent electron density for the entire degrader molecule in this example, and the thalidomide moiety was found to fold back onto the protein, packing against the side chain of Trp81, which had flipped relative to its orientation in the complex with BDOIA383 and the thalidomide-free parent molecule 29 (Figure 4C,D). Also, the central benzimidazole ring of 34 was rotated by about 180° and was slightly tilted compared with the orientation seen in the other complexes with this core scaffold (Figure 4E). This orientation of 34 was also stabilized via an interesting intramolecular interaction of the thalidomide piperidine-2,6-dione with the aromatic ring of the phenol ether moiety of the inhibitor. The packing of the thalidomide moiety against the Trp81 side chain is likely to contribute to the overall activity observed for this compound against BRD4 (Table 4) but could also rationalize the lack of ability to induce ubiquitination (Figure 3) since the thalidomide binding moiety is sequestered by BRD4 BD1 Trp81 and therefore not freely available for binding to form a ternary complex. The ‘collapse’ of degraders leading to E3 ligase ligand-target protein binding interactions has also been observed for VHL-recruiting degraders46 and may represent an under-reported mechanism by which some putative degrader molecules are inactive with respect to target protein degradation.47 Degrader 34 displayed a nanomolar (65 nM) activity against BRD4 BD1, similar to (+)-JQ1, and this prompted us to compare the binding poses of both. Indeed, 34 mimics (+)-JQ1 by flipping Trp81 and interacting with it; the packing against Trp81, however, occurs from two different sides of the indole ring (Figure 4A,D and Supporting Figure S1). The crystal structure of the complex with 34 also showed an interesting stacking interaction of thalidomide moieties from symmetry-related molecules (Supporting Figure S2).
Figure 4.
Crystal structures of the first bromodomain of human BRD4 in complex with JQ1 and ISOX-DUAL-based degraders. The ligand in each structure is shown as a stick model and the protein as a ribbon diagram, with selected side chains in the binding site highlighted as stick models. The binding mode of the isoxazole moiety of all degraders is conserved, forming a hydrogen bond with Asn140 at the bottom of the binding site (highlighted as a magenta dashed line) and hydrophobic interactions with Phe83, Val87, Leu94, and the gatekeeper residue Ile146. The central benzimidazole moiety is sandwiched between Pro82 and Leu92, with its relative orientation depending on the substitution pattern and degrader warhead. (A) BRD4 BD1 with bound (+)-JQ1 (PDB entry 3MXF). (B) BRD4 BD1 with thalidomide-based degrader 14. The thalidomide moiety was not resolved in the crystal structure. (C) BRD4 BD1 with ISOX-DUAL inhibitor derivative 29. (D) BRD4 BD1 with thalidomide-based degrader 34. The thalidomide moiety was fully resolved in the structure, folding back onto Trp81 in the WPF-shelf region, thereby increasing binding affinity. (E) Superimposition of the core ISOX-DUAL scaffold in the BRD4 BD1 complexes with 29, 34, 44, and 45, highlighting the thalidomide-induced flip of the central benzimidazole scaffold upon binding of degrader 34. (F) Superimposition of the binding modes of VHL-based degraders 44 (chain D) and 45 (chain B). The aliphatic linker packed against the surface patch between Phe79 and Leu148, whereas the VHL moiety was largely unresolved in the crystal structure, indicating high flexibility. For clarity, only the protein chain for the complex with 44 is shown.
Cocrystal structures for VHL-based degraders 44 and 45 were solved with structures determined in a crystal form with four molecules in the asymmetric unit. The VHL-binding moiety was always disordered in these structures, but the linker was visible in most of the chains. When the linker was visible, it always packed against the hydrophobic surface patch between Phe79 and Leu148 (Figure 4F), although this may be influenced by crystal packing. The increased affinity compared with the parent scaffold may be due to such a hydrophobic interaction of the linker with a hydrophobic surface patch. The E3 ligase ligand is clearly solvent-exposed (as opposed to 35) and this may also explain its ability to ubiquitinate BRD4.
Evaluation of the panel of degraders in cell-based assays led to ambiguous results, likely due to the cytotoxicity of the compounds confounding the degradation results at high treatment concentrations. Nonetheless, many of these compounds do serve as useful tools for biochemical investigation.
Conclusions
We sought to rationally design degraders with dual activity against BRD4 and CBP/p300, using the parent inhibitor compound ISOX-DUAL. X-ray cocrystal structure informed the selection of two potential exit vectors, which were explored for degrader design. Several compounds displayed dual inhibitory activity, albeit in many cases with reduced affinity for one or both target proteins, in biochemical assays. Structural studies furthered our understanding of compound activity, with high-resolution X-ray cocrystal structures revealing an unexpected interaction between the E3 ligase recruiting ligand, thalidomide, and Trp81 on BRD4 BD1, resulting from the tryptophan side chain flipping in its relative orientation and effectively sequestering thalidomide, thereby preventing its binding to CRBN and abrogating degrader activity. Such a “degrader collapse” might be a hitherto under-represented mechanism for the lack of action in prototypical degrader design and may merit reinvestigation of past failures or prompt structural evaluation of binary complexes in future cases where the experimental results fail to align with biochemical binding results.
Experimental Section
General Methods
All reagents and solvents were purchased from commercial sources and used without further purification. Nuclear magnetic resonance spectra were recorded on Varian NMR machines operating at 600, 500, or 400 MHz for 1H NMR and at 151 or 126 MHz for 13C NMR, or on a Bruker Advance III HD spectrometer operating at 400 MHz for 1H NMR and 100 MHz 13C NMR. 1H NMR and 13C NMR chemical shifts (δ) are reported in parts per million (ppm) and are referenced to residual protium in solvent and to the carbon resonances of the residual solvent peak, respectively. DEPT and correlation spectra were run in conjunction to aid the assignment. Coupling constants (J) are quoted in Hertz (Hz), and the following abbreviations were used to report multiplicity: s = singlet, d = doublet, dd = doublet of doublets, ddd = double doublet of doublets, t = triplet, q = quartet, m = multiplet, br s = broad singlet. Purification by flash column chromatography was carried out using Teledyne ISCO purification systems. Analytical thin-layer chromatography was performed on commercial glass plates precoated with silica gel with visualization being achieved using UV light (254 nm) and/or by staining with alkaline potassium permanganate dip. Reaction monitoring LCMS analyses and purity determinations were conducted using a Shimadzu 2020 Mass Directed Automated Purification (MDAP) system or an Agilent Infinity Lab LC/MSD system. All final compounds are >95% pure by HPLC analysis except for 7 (92%), 28 (91%) and 45 (87%). HRMS analyses were conducted by Dr. Alaa Abdul-Sada in the laboratories of the University of Sussex Chemistry Department using a Bruker Daltonics Apex III, using Apollo ESI as the ESI source. For EI mass spectra, a Fissions VG Autospec instrument was used at 70 eV. Analyses are for the molecular ion peak [M]+ and are given in m/z, mass to charge ratio. Alphascreen assays were carried out following literature protocols.48
Synthesis of Compounds
4-Bromo-N-(2-morpholinoethyl)-2-nitroaniline (3)

To a stirred solution of 4-bromo-1-fluoro-2-nitrobenzene (2) (29.9 g, 136 mmol) in DMSO (300 mL) at ambient temperature was added triethylamine (56 mL, 408 mmol, 3 equiv) followed by 4-(2-aminoethyl)morpholine (18.7 mL, 143 mmol,) in a dropwise fashion. The reaction mixture was then heated to 80 °C for 2 h. Upon completion of the reaction, the mixture was cooled to ambient temperature and partitioned between ethyl acetate (500 mL) and water (500 mL). The organic layer was collected and the aqueous was extracted with ethyl acetate (3 × 750 mL). The combined organic extracts were washed successively with sodium hydrogen carbonate solution (sat. aq.) (1L) and brine (1 L), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give the title compound as an orange solid (40.6 g, 90%). 1H NMR (400 MHz CDCl3): δ 8.53 (br s, 1H), 8.33 (d, J = 2 Hz, 1H), 7.49 (dd, J = 9, 2 Hz, 1H), 6.73 (d, J = 9 Hz, 1H), 3.79–3.72 (m, 4H), 3.34 (q, J = 6 Hz, 2H), 2.72 (t, J = 6 Hz, 2H), 2.56–2.48 (m, 4H); LCMS (5–95% MeCN over 5 min) tR = 3.178, Purity >99%; m/z (ES+): 332.1 [M+H+]+.
4-(3,5-Dimethylisoxazol-4-yl)-N-(2-morpholinoethyl)-2-nitroaniline (4)

A stirred solution of 4-bromo-N-(2-morpholinoethyl)-2-nitroaniline (3) (38 g, 115 mmol), potassium phosphate (63.5 g, 299 mmol), and 3,5-dimethylisoxazole boronic acid pinacol ester (25.6 g, 115 mmol) in 1,4-dioxane (1.2 L) and water (120 mL) was degassed with argon (×3) before the addition of PdCl2(dppf)·DCM (4.7 g, 5.75 mmol). The reaction mixture was then degassed and refilled with argon once, heated to reflux, and stirred overnight under a stream of nitrogen (g). The reaction mixture was then cooled to ambient temperature and filtered through a pad of Celite before concentrating under reduced pressure to approximately 300 mL. The residue was then partitioned between water (600 mL) and ethyl acetate (600 mL), the organic phase was collected, and the aqueous phase was extracted with ethyl acetate (3 × 250 mL). The combined organic extracts were washed with brine (3 × 400 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–80% ethyl acetate in hexane, afforded the title compound as an orange solid (37.4 g, 94%). 1H NMR (400 MHz, CDCl3): δ 8.58 (s, 1H), 8.09 (d, J = 2 Hz, 1H), 7.34 (dd, J = 9, 2 Hz, 1H), 6.91 (d, J = 9 Hz, 1H), 3.80–3.73 (m, 4H), 3.43 (q, J = 5.5 Hz, 2H), 2.75 (t, J = 5.5 Hz, 2H), 2.57–2.50 (m, 4H), 2.40 (s, 3H), 2.26 (s, 3H); LCMS (5–95% MeCN over 5 min) tR = 3.266, Purity >99%; m/z (ES+): 347.2 [M+H+]+.
4-(3,5-Dimethylisoxazol-4-yl)-N1-(2-morpholinoethyl)benzene-1,2-diamine (5)

To a stirred suspension of 4-(3,5-dimethylisoxazol-4-yl)-N-(2-morpholinoethyl)-2-nitroaniline (4) (17.4 g, 50 mmol) in EtOH (800 mL) was added 1 M aqueous sodium dithionite solution (800 mL), and the resulting mixture was heated at 80 °C for 1 h. The reaction mixture was then cooled and partitioned between 10% aqueous ammonia solution (800 mL) and ethyl acetate (400 mL). The organic phase was separated, and the aqueous phase was extracted with ethyl acetate (4 × 400 mL). The combined organic extracts were washed with brine (2 × 500 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to afford the title compound as a beige solid (12.70 g, 81%). 1H NMR (400 MHz, CDCl3): δ 6.68 (s, 2H), 6.59 (s, 1H), 4.08 (br s, 1H), 3.76–3.69 (m, 4H), 3.45 (br s, 2H), 3.23–3.17 (m, 2H), 2.71 (t, J = 5.9 Hz, 2H), 2.54–2.46 (s, 4H), 2.38 (s, 3H), 2.25 (s, 3H); LCMS (5–95% MeCN over 5 min) tR = 2.364, Purity >99%; m/z (ES+): 317.2 [M+H+]+.
4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenol (6)

To a solution of 4-hydroxyphenyl propionic acid (686 mg, 4.13 mmol) and HATU (1.99 g, 5.25 mmol) in DMF (30 mL) was added triethylamine (1.6 mL, 11.3 mmol) followed by a solution of 5 (1.3 g, 3.75 mmol.) in DMF (5 mL). The stirring solution was left to stir overnight at ambient temperature. The reaction mixture was partitioned between dichloromethane (100 mL) and water (100 mL). The aqueous phase was then extracted with dichloromethane (3 × 25 mL). The combined organic phases were washed with saturated aqueous sodium hydrogen carbonate solution (150 mL) and brine (200 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in acetic acid (50 mL) and heated to reflux for 2 h. The reaction mixture was then cooled, concentrated under reduced pressure and dichloromethane (50 mL) was added before neutralization with saturated aqueous sodium hydrogen carbonate solution. The organic phase was separated, and the aqueous component was extracted with dichloromethane (4 × 50 mL), before being combined and washed with brine (200 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% methanol (with 0.5% NH4OH) in dichloromethane afforded the title compound as a colorless solid. (502 mg, 30%). 1H NMR (600 MHz, CDCl3): δ 8.07 (br s, 1H), 7.58 (s, 1H), 7.36 (d, J = 8.2 Hz, 1H), 7.13 (d, J = 8.2 Hz, 1H), 7.00 (d, J = 7.8 Hz, 2H), 6.73 (d, J = 7.8 Hz, 2H), 4.17 (t, J = 6.8 Hz, 2H), 3.72–3.66 (m, 4H), 3.23–3.13 (m, 4H), 2.65 (t, J = 6.8 Hz, 2H), 2.52–2.45 (m, 4H), 2.39 (s, 3H), 2.26 (s, 3H); 13C NMR (151 MHz, CDCl3): δ 165.2, 159.1, 155.6, 155.4, 142.6, 134.2, 131.8, 129.5, 124.6, 123.7, 119.8, 117.1, 115.9, 109.7, 66.9, 57.7, 54.2, 41.7, 33.3, 29.9, 11.7, 11.0; LCMS (5–95% MeCN over 20 min) tR = 3.23 min, Purity >97%; m/z (ES+): 447.05 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C29H31N4O3, 447.2391; found, 447.2367.
4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenyl Acetate (7)

To a stirred solution of 6 (87 mg, 0.195 mmol) in dichloromethane (5 mL), was added pyridine (0.032 mL, 0.39 mmol) and acetic anhydride (0.037 mL, 0.39 mmol) at ambient temperature, and the mixture was left to stir for 1 h. The reaction mixture was then quenched with saturated aqueous NH4Cl (10 mL) and extracted with DCM (10 mL). The organic layer was collected, washed with brine (10 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–10% methanol (with 0.5% NH4OH) in dichloromethane afforded the title compound as a clear oil. (76 mg, 80%).1H NMR (600 MHz, CDCl3): δ 7.63 (s, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.24 (d, J = 7.9 Hz, 2H), 7.12 (d, J = 8.2 Hz, 1H), 7.01 (d, J = 7.7 Hz, 2H), 4.10 (t, J = 6.8 Hz, 2H), 3.68–3.64 (m, 4H), 3.33–3.25 (m, 2H), 3.21–3.15 (m, 2H), 2.61 (t, J = 6.7 Hz, 2H), 2.48–2.43 (m, 4H), 2.42 (3H s), 2.30–2.26 (m, 6H). 13C NMR (151 MHz, CDCl3): 169.7, 165.1, 159.1, 155.2, 149.4, 143.1, 138.3, 134.4, 129.5, 124.4, 123.6, 121.9, 120.0, 117.2, 109.5, 66.9, 57.7, 54.1, 41.6, 33.3, 29.7, 21.2, 11.7, 11.0. LCMS (5–95 MeCN in 20 min) tR = 4.10 min, Purity = 92%, HRMS-ESI (m/z): [M+H+]+ calculated for C28H33N4O4, 489.2496; found, 489.2477.
Methyl-4-(4-(2-(5-(3,5-dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)butanoate (8)
To a stirred solution of 6 (1.4 g, 3.1 mmol) in acetonitrile (50 mL) was added potassium carbonate(s) (0.857 g, 6.2 mmol), followed by methyl-4-bromobutyrate (1.1224 g, 6.2 mmol) before leaving to stir overnight at reflux. The reaction was cooled and partitioned between ethyl acetate (50 mL) and water (50 mL). The organic phase was collected, and the aqueous layer was extracted with ethyl acetate (3 × 25 mL). The organics were combined and washed with brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–5% methanol in dichloromethane with 0.5% NH4OH, afforded the title compound as a clear oil (1.12 g, 70%). 1H NMR (500 MHz, CDCl3): δ 7.62 (s,1H), 7.35 (d, J = 8 Hz, 1H), 7.15–7.10 (m, 3H), 6.83 (d, J = 8 Hz, 2H), 4.12 (t, J = 7 Hz, 2H), 3.99 (t, J = 6 Hz, 2H), 3.7–3.64 (m, 7H), 3.25–3.12 (m, 4H), 2.60 (t, J = 7 Hz, 2H), 2.54 (t, J = 7 Hz, 2H), 2.48–2.41 (m, 7H), 2.30 (s, 3H), 2.12–2.08 (m, 2H); 13C NMR (126 MHz, CDCl3): δ 173.8, 165.2, 159.2, 157.6, 155.6, 143.2, 134.4, 133.1, 129.5, 124.3, 123.5, 120.0, 117.2, 114.8, 110.1, 109.5, 67.0, 66.8, 57.7, 54.2, 51.8, 41.6, 33.2, 30.7, 30.1, 24.7, 15.4, 11.7, 11.0; LCMS (5–95% MeCN over 20 min) tR = 12.30 min, Purity >96%; m/z (ES+): 547.20 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C31H39N4O5, 547.2915; found, 547.2892.
Lithium-4-(4-(2-(5-(3,5-dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)butanoate (9)
To a stirred solution of 8 (1.1 g, 2.5 mmol) in THF (100 mL) and water (20 mL) was added lithium hydroxide monohydrate (0.115 g, 2.75 mmol) and was left to stir at ambient temperature overnight. Upon reaction completion, the resultant solution was concentrated under reduced pressure, and reconcentrated from THF (5 × 50 mL), to give the title compound as a colorless solid, which was used directly in the subsequent reaction without further purification (1.0 g, 94%). LCMS (5–95% MeCN over 20 min) tR = 11.04, Purity >95%; m/z (ES-): 531.15 [M-Li+]−.
4-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-N-(2-methoxyethyl)butanamide (10)
To a stirred solution of 9 (100 mg, 0.19 mmol, 1 equiv) and HATU (83.7 mg, 0.22 mmol, 1.2 equiv) in DMF (mL) was added triethylamine (26.5 μL, 0.19 mmol, 1.2 equiv) followed by 2-methoxyethylamine (14.3 mg, 0.19 mmol, 1 equiv) before leaving the reaction to stir at ambient temperature overnight. The mixture was partitioned between dichloromethane (25 mL) and water (25 mL). The organic layer was collected and washed with saturated aqueous sodium hydrogen carbonate solution (25 mL) and brine (3 × 10 mL), dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–10% methanol in dichloromethane with 0.5% NH4OH, afforded the title compound as a colorless oil (88 mg, 79%). 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 7.34 (d, J = 9 Hz, 1H), 7.15–7.10 (m, 3H), 6.82 (d, J = 9 Hz, 2H), 5.94 (s, 1H), 4.11 (t, J = 7 Hz, 2H), 3.98 (t, J = 6 Hz, 2H), 3.68–3.63 (m, 4H), 3.45–3.42 (m, 4H), 3.32 (s, 3H), 3.24–3.19 (m 2H), 3.18–3.14 (m, 2H), 2.59 (t, J = 6.9 Hz, 2H), 2.48–2.43 (m, 4H), 2.42 (s, 3H), 2.39 (t, J = 7.3 Hz, 2H), 2.29 (s, 3H), 2.15–2.08 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 172.4, 165.2, 159.1, 157.6, 155.5, 143.1, 134.4, 133.1, 129.5, 124.3, 123.5, 120.0, 117.2, 114.8, 109.5, 71.3, 67.0, 66.9, 58.9, 57.7, 54.1, 41.6, 39.3, 33.1, 33.0, 30.0, 11.7, 11.0; LCMS (5–95% MeCN over 20 min) tR = 11.08 min, Purity >99%; m/z (ES+): 590.20 [M+H+]+; HRMS-ESI (m/z): [M+Na+]+ calculated for C33H43N5O5Na, 612.3156; found, 612.3143.
tert-Butyl 4-(4-(3-Methoxy-3-oxopropyl)phenoxy)butanoate (11a)
To a solution of methyl 3-(4-hydroxyphenyl)propionate (20 g, 111 mmol) in acetonitrile (200 mL) was added potassium carbonate (30.6 g, 222 mmol), followed by the dropwise addition of a solution of tert-butyl 4-bromobutanoate (25 g, 111 mmol.) in acetonitrile (100 mL). The reaction mixture was then heated to reflux overnight. Upon cooling the reaction mixture was filtered and the filtrate was and partitioned between dichloromethane (700 mL) and water (500 mL). The organic phase was separated and washed with 1 M (aq) potassium carbonate solution (10 × 250 mL) and brine (2 × 300 mL) before being dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification by flash column chromatography and elution with 5–20% ethyl acetate in petroleum ether (40–60), afforded the title compound as a colorless oil, which crystallized upon standing (27 g, 75%). 1H NMR (400 MHz, CDCl3): δ 7.10 (d, J = 9 Hz, 2H), 6.81 (d, J = 9 Hz, 2H), 3.96 (t, J = 7 Hz, 2H), 3.66 (s, 3H), 2.88 (t, J = 8 Hz, 2H), 2.59 (t, J = 8 Hz, 2H), 2.41 (t, J = 7 Hz, 2H), 2.08–2.01 (m, 2H), 1.45 (s, 9H); LCMS (5–95% MeCN over 5 min) tR = 5.879, Purity >96%; m/z (ES+): 289.2 [M-tBu+Na+]+.
4-(4-(3-Methoxy-3-oxopropyl)phenoxy)butanoic Acid (11)
To a stirred solution of tert-butyl 4-(4-(3-methoxy-3-oxopropyl)phenoxy)butanoate (27 g, 87.7 mmol) in THF (200 mL) was added lithium hydroxide (200 mL, 1 M aqueous solution). The reaction mixture was left to stir until completion by TLC. The reaction was then acidified to pH 2 with 2 M HCl and extracted with ethyl acetate (4 × 200 mL). The combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure affording the title compound as a colorless solid (24.3 g, mmol, 94%). 1H NMR (400 MHz, CDCl3): δ 12.09 (br s, 1H), 7.11 (d, J = 8.5 Hz, 2H), 6.81 (d, J = 8.5 Hz, 2H), 3.94–3.90 (m, 2H), 2.74 (t, J = 7.5 Hz, 2H), 2.46 (t, J = 7.5 Hz, 2H), 2.34 (t, 7.5 Hz, 2H), 1.93–1.87 (m, 2H), 1.40 (s, 9H).
4-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)butanoic Acid (12)
To a suspension of compound 11 (12.38 g, 40.14 mmol) in DMF (200 mL), was added triethylamine (16.8 mL, 120 mmol) and HATU (19.84 g, 15.1 mmol). The reaction mixture was degassed with argon and stirred for 1 h before the addition of a solution of compound 5 (12.7 g, 40.14 mmol) in DMF (150 mL). After stirring at ambient temperature overnight, the reaction mixture was partitioned between ethyl acetate (500 mL) and water (2 L). The organic phase was separated, and the aqueous component was extracted with ethyl acetate (3 × 300 mL). The organic extracts were combined and successively washed with saturated aqueous sodium hydrogen carbonate solution (150 mL) and brine (150 mL), before being dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification by flash column chromatography and elution with 1–4% 7N methanolic ammonia solution in dichloromethane afforded a pale brown solid. This solid was dissolved in acetic acid and heated to reflux for 2 h, after which the reaction mixture was cooled, concentrated under reduced pressure, and successively reconcentrated from ethyl acetate (100 mL) and then heptane (3 × 300 mL). The residue was dissolved in ethyl acetate (300 mL) and poured into a saturated aqueous sodium hydrogen carbonate solution (400 mL). The organic phase was collected and washed with brine (500 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 3–7% 7N methanolic ammonia solution in dichloromethane afforded a beige solid. This solid was dissolved in 1,4-dioxane (100 mL) and hydrogen chloride (50 mL, 200 mmol, 4 M solution in 1,4-dioxane) was added before leaving to stir for 4 h, after which the reaction mixture was concentrated and triturated overnight with acetonitrile. The resulting precipitate was filtered and washed with diethyl ether and dried under vacuum, affording the title compound as a colorless solid (10.5 g, 46%).
1H NMR (400 MHz, DMSO-d6): δ 12.57 (br s, 1H), 8.29 (d, 8.5 Hz, 1H), 7.81, (s, 1H), 7.60 (d, 8.6 Hz, 1H), 7.32 (d, 8.6 Hz, 2H), 6.88 (d, 8.6 Hz, 2H), 5.05–4.97 (m, 2H), 4.07–3.97 (m, 2H), 3.95 (t, J = 6.4 Hz, 2H), 3.91–3.82 (m, 2H), 3.58–3.45 (m, 6H), 3.26–3.15 (m, 4H), 2.43 (s, 3H), 2.37 (t, J = 7.3 Hz, 2H), 2.24 (s, 3H), 1.95–1.88 (m, 2H); 13C NMR (151 MHz, DMSO-d6): 174.1, 165.7, 158.2, 157.3, 155.0, 131.8, 131.2, 131.0, 129.7, 127.7, 126.5, 115.5, 114.7, 114.5, 113.4, 66.6, 66.4, 63.2, 51.9, 51.1, 40.1, 38.5, 31.3, 30.1, 27.4, 24.3, 11.3, 10.5. LCMS (30–95 MeCN over 20 min) tR = 7.27 min, Purity >95%, m/z (ES+): 533.55 [M+H+]+. HRMS-ESI (m/z): [M+H+]+ calculated for C30H37N4O5, 533.2764; found, 533.2785.
General Procedure A for Degrader Synthesis
A solution of 12 (1 equiv) in DMF (typically 5 mL) was treated with the relevant commercially available amine-reactive degrader building block (E3 ligase ligand functionalized with a linker with amine termini) (1 equiv; typically 25 mg), triethylamine (3 equiv) and HATU (1.3 equiv) and stirred overnight at ambient temperature. The reaction mixture was partitioned between dichloromethane (25 mL) and water (50 mL), and the organic phase was separated. The aqueous component was extracted with further dichloromethane (4 × 10 mL), and the combined organics were then washed with saturated aqueous sodium hydrogen carbonate solution (50 mL) and brine (2 × 50 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure.
4-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-N-(1-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)-2-oxo-6,9,12-trioxa-3-azatetradecan-14-yl)butanamide (13)
Degrader 13 was synthesized according to General Procedure A, using thalidomide 4′-oxyacetamide-PEG3-amine. Purification by flash column chromatography and elution with 0–20% methanol (with 0.5% NH4OH) in dichloromethane over 20 column volumes (cvs), afforded the title compound 13 as a colorless oil. (29.6 mg, 60%). 1H NMR (500 MHz, CDCl3): δ 9.64 (br s, 1H), 7.75–7.66 (m, 2H), 7.63 (d, J = 0.8 Hz, 1H), 7.53 (d, J = 7.3 Hz, 1H), 7.36 (d, J = 8.3 Hz, 1H), 7.16 (d, J = 8.3 Hz, 1H), 7.13 (d, J = 1.3 Hz, 1H), 7.11 (d, J = 8.5 Hz, 2H), 6.79 (d, J = 8.5 Hz, 2H), 6.73–6.68 (m, 1H), 5.60–5.40 (m, 2H), 4.93 (dd, J = 12.3, 5.4 Hz, 1H), 4.62 (s, 2H), 4.13 (t, J = 6.9 Hz, 2H), 3.95 (t, J = 6.1 Hz, 2H), 3.69–3.36 (m, 18H), 3.23–3.15 (m, 4H), 2.88–2.83 (m, 1H), 2.77–2.68 (m, 2H), 2.60 (t, J = 6.9 Hz, 2H), 2.49–2.45 (m, 3H), 2.42 (s, 3H), 2.37 (t, J = 7.3 Hz, 2H), 2.29 (s, 3H), 2.17–2.05 (m, 4H); 13C NMR (126 MHz, CDCl3): δ 172.8, 171.7, 171.7, 168.7, 166.9, 166.7, 166.0, 165.2, 159.1, 157.7, 155.49, 154.4, 137.1, 134.1, 133.7, 132.9, 129.5, 124.5, 123.7, 119.8, 119.4, 188.1, 117.4, 117.1, 114.8, 110.1, 109.7, 70.3, 70.3, 70.2, 70.2, 69.6, 67.8, 67.2, 66.8, 57.6, 54.1, 49.3, 41.4, 39.1, 33.1, 32.8, 31.5, 29.9, 25.3, 22.9, 22.8, 11.7, 11.0; LCMS (5–95% MeCN over 20 min) tR = 11.77 min, purity >99%; m/z (ES+): 1043.40 [M+Na+]+; HRMS-ESI (m/z): [M+Na+]+ calculated for C53H64N8NaO13, 1043.4491; found, 1043.4453.
4-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-N-(1-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)-2-oxo-6,9,12,15-tetraoxa-3-azaheptadecan-17-yl)butanamide (14)
Degrader 14 was synthesized according to General Procedure A, using thalidomide 4′-oxyacetamide-PEG4-amine. Purification by flash column chromatography and elution with 0–20% methanol (with 0.5% NH4OH) in dichloromethane over 20 cvs, afforded the title compound as a colorless oil (25 mg, 55%). 1H NMR (600 MHz, CDCl3): δ 9.51 (br s, 1H), 7.75–7.62 (m, 3H), 7.53 (d, J = 7.3 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.17–7.09 (m, 3H), 6.79 (d, J = 8.1 Hz, 2H), 6.65–6.60 (m, 1H), 5.55–5.35, (m, 2H), 4.97–4.93 (m, 1H), 4.62 (s, 2H), 4.17–4.10 (m, 2H), 3.95 (t, J = 6 Hz, 2H), 3.71–3.52 (m, 18H), 3.45–3.40 (m, 2H), 3.23–3.15 (m, 4H), 2.89–2.68 (m, 4H), 2.61 (t, J = 7 Hz, 2H), 2.52–2.46 (m, 4H), 2.42 (s, 3H), 2.37 (t, J = 7 Hz, 2H), 2.29 (s, 3H), 2.17–2.03 (m, 5H). 13C NMR (151 MHz, CDCl3): δ 172.8, 171.5, 168.6, 166.9, 166.8, 166.0, 165.2, 159.1, 157.7, 155.5, 154.6, 137.12, 134.2, 133.8, 132.9, 129.5, 124.6, 123.7, 119.9, 119.5, 118.1, 117.4, 117.1, 114.8, 109.6, 70.6, 70.5, 70.45, 70.43, 70.3, 70.1, 70.1, 69.9, 67.9, 67.2, 66.8, 57.6, 54.7, 49.5, 39.3, 39.1, 33.2, 32.8, 31.6, 29.9, 25.4, 22.8, 22.7, 11.7, 11.0; LCMS (5–95% MeCN over 20 min) tR = 11.92, Purity >95%, m/z(ES+): 1087.35 [M+Na+]+; HRMS (m/z): [M+Na+]+ calculated for C55H68N8NaO14, 1087.4753; found, 1087.4664.
(2S,4R)-1-((S)-2-(tert-Butyl)-19-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-4,16-dioxo-6,9,12-trioxa-3,15-diazanonadecan-1-oyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (15)
Degrader 15 was synthesized according to General Procedure A, using VH 032 amide-PEG3-amine. Purification by flash column chromatography and elution with 0–20% methanol (with 0.5% NH4OH) in dichloromethane over 20 cvs, afforded the title compound as a colorless oil (34 mg, 75%). 1H NMR (600 MHz, CDCl3): δ 8.65 (s, 1H), 7.60 (s, 1H), 7.48–7.43 (m, 1H), 7.36–7.32 (m, 4H), 7.29 (d, J = 9 Hz, 1H), 7.13–7.09 (m, 3H), 6.80 (d, J = 8 Hz, 2H), 6.58–6.52 (m, 1H), 5.71–5.50 (m, 2H), 4.67 (t, J = 8 Hz, 1H), 4.58–4.51 (m, 3H), 4.36–4.31 (m, 1H), 4.11 (t, J = 7 Hz, 2H), 4.05–3.97 (m, 3H), 3.95 (t, J = 6 Hz, 2H), 3.70–3.43 (m, 13H), 3.41–3.36 (m, 2H), 3.21–3.13 (m, 4H), 2.59 (t, J = 7 Hz, 2H), 2.49 (s, 3H), 2.47–2.43 (m, 3H), 2.41 (s, 3H), 2.35 (t, J = 7 Hz, 2H), 2.30–2.24 (m, 4H), 2.14–2.03 (m, Hz, 3H), 1.19 (t, J = 7 Hz, 2H), 0.95 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 172.8, 171.1, 170.3, 165.1, 159.1, 157.6, 155.5, 150.4, 148.5, 143.1, 138.3, 134.4, 133.1, 131.7, 131.0, 129.6, 129.4, 128.2, 124.3, 123.5, 120.0, 117.2, 114.8, 109.6, 70.9, 70.6, 70.5, 70.1, 70.0, 67.2, 66.9, 65.9, 58.8, 57.7, 57.0, 56.9, 54.1, 43.3, 41.6, 39.4, 36.5, 35.5, 33.1, 32.8, 30.0, 26.5, 25.3, 16.1, 15.4, 11.7, 11.0. LCMS (5–95% MeCN over 20 min) tR = 7.18, Purity >95%, m/z (ES+): 1134.50 [M+H+]+; HRMS (m/z): [M+Na+]+ calculated for C60H79N9NaO11S+, 1156.5512; found, 1156.5388.
(2S,4R)-1-((S)-2-(tert-Butyl)-22-(4-(2-(5-(3,5-dimethylisoxazol-4-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-4,19-dioxo-6,9,12,15-tetraoxa-3,18-diazadocosan-1-oyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (16)
Degrader 16 was synthesized according to General Procedure A, using VH 032 amide-PEG4-amine. Purification by flash column chromatography and elution with 0–20% methanol (with 0.5% NH4OH) in dichloromethane over 20 cvs, afforded the title compound as a colorless oil (28 mg, 65%). 1H NMR (600 MHz, CDCl3): δ 8.65 (s, 1H), 7.60 (s, 1H), 7.39 (t, J = 6 Hz, 1H), 7.36–7.30 (m, 4H), 7.26 (s, 2H), 7.09–7.13 (m, 3H), 6.80 (d, J = 8 Hz, 2H), 6.50 (br s, 1H), 5.75–5.45 (m, 2H), 4.70 (t, J = 8 Hz, 1H), 4.55–4.50 (m, 3H), 4.37–4.31 (m, 1H), 4.12 (d, J = 7 Hz, 2H), 4.03–3.88 (m, 4H), 3.70–3.50 (m, 18H), 3.41 (q, J = 5 Hz, 2H), 3.19–3.11 (m, 4H), 2.59 (t, J = 7 Hz, 2H), 2.49 (s, 3H), 2.48–2.42 (m, 4H), 2.41 (s, 3H), 2.36 (t, J = 7 Hz, 2H), 2.28 (s, 3H), 2.15–2.04 (m, 4H), 0.94 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 172.7, 171.3, 171.1, 170.2, 165.1, 159.1, 157.6, 155.5, 150.4, 148.5, 143.1, 138.3, 134.4, 133.1, 131.7, 131.0, 129.6, 129.4, 128.2, 124.3, 123.5, 120.0, 117.2, 114.8, 109.6, 71.0, 70.6, 70.6, 70.5, 70.2, 70.1, 70.0, 67.2, 66.9, 58.7, 57.7, 57.1, 56.9, 54.1, 43.3, 41.6, 39.3, 36.3, 35.4, 33.1, 32.8, 30.0, 26.5, 25.3, 16.1, 15.4, 11.7, 11.0. LCMS (5–95% MeCN over 20 min) tR = 7.16, Purity >96%, m/z (ES+): 1178.55 [M+H+]+; HRMS (m/z): [M+Na+]+ calculated for C62H83N9NaO12S+, 1200.5780; found, 1200.5796.
tert-Butyl 4-(2-((4-Bromo-2-nitrophenyl)amino)ethyl)piperazine-1-carboxylate (17)

A microwave vial was equipped with a magnetic flea and flushed with argon. 4-(2-aminoethyl)-1-Boc-piperazine (5.05 g, 22 mmol) was added followed by triethylamine (15 mL). This was stirred for 3 min before the addition of 4-fluoro-3-nitrobromobenzene (4.40 g, 20 mmol). Following the addition, the vial was sealed and heated using the dynamic heating method, with max power set to 300 W, max pressure 300 psi, max temperature 125 °C, high stirring throughout, and power max turned off. This method was used to hold the temperature at 125 °C for 10 min. After cooling, the reaction mixture was transferred to a separating funnel where it was partitioned between water (250 mL) and ethyl acetate (200 mL). The organic phase was separated, and the aqueous component was extracted with ethyl acetate (3 × 75 mL). The combined organic extracts were washed with saturated aqueous sodium hydrogen carbonate solution (200 mL) and brine (200 mL), and then dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to afford the title compound as an orange solid (8.40 g, 98%). 1H NMR (600 MHz,CDCl3): δ 8.51 (s, 1H), 8.32 (d, J = 2 Hz, 1H), 7.49 (dd, J = 9, 2 Hz, 1H), 6.73 (d, J = 9 Hz, 1H), 3.50–3.45 (m, 4H), 3.34 (q, J = 6 Hz, 2H), 2.73 (t, J = 6 Hz, 2H), 2.50–2.4 (m, 4H), 1.46 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 154.9, 144.3, 139.0, 132.5, 129.1, 115.9, 106.4, 79.9, 55.6, 52.7, 39.8, 28.6. LCMS (5–95% MeCN over 20 min) tR = 7.94 min, Purity >99%, m/z (ES+): 429.00 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C17H26BrN4O4, 429.1132; found, 429.1132.
tert-Butyl-4-(2-((4-(3,5-dimethylisoxazol-4-yl)-2-nitrophenyl)amino)ethyl)piperazine-1-carboxylate (18)

A mixture of 17 (8.40 g, 19.60 mmol), potassium phosphate (10.82 g, 50.96 mmol), PdCl2(dppf)•DCM (0.80 g, 0.98 mmol), and 3,5-dimethylisoxazol-4-boronic acid pinacol ester (4.90 g, 21.95 mmol) in 1,4-dioxane (200 mL) was degassed and backfilled with argon. The reaction was then heated to reflux and stirred overnight. After cooling, the reaction mixture was filtered through diatomaceous earth and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–100% ethyl acetate in hexane, afforded the title compound as an orange oil (6.99 g, 80%).1H NMR (600 MHz, CDCl3): δ 8.58 (s, 1H), 8.08 (d, J = 2 Hz, 1H), 7.34 (dd, J = 8.8, 2 Hz, 1H), 6.91 (d, J = 8.8 Hz, 1H), 3.52–3.46 (m, 4H), 3.41 (q, J = 5.8 Hz, 2H), 2.76 (t, J = 6.1 Hz, 2H), 2.52–2.45 (m, 4H), 2.40 (s, 3H), 2.26 (s, 3H), 1.47 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 165.5, 158.7, 154.9, 144.6, 136.9, 132.1, 127.2, 117.5, 115.1, 114.9, 79.9, 55.7, 52.7, 44.4, 43.4, 39.8, 28.6, 11.7, 10.9. LCMS (5–95% MeCN over 20 min) tR = 7.61 min, Purity >99%, m/z (ES+): 446.50 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C22H32N5O5, 446.2398; found, 446.2421.
tert-Butyl-4-(2-((2-amino-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)ethyl)piperazine-1-carboxylate (19)

To a solution of 18 (1.50 g, 3.47 mmol) in EtOH (55 mL) was added 1 M aqueous sodium dithionite solution (55 mL), and the reaction was heated to 80 °C for 1 h. Upon cooling, the reaction mixture was treated with 10% ammonia solution (55 mL) and ethyl acetate (75 mL). The organic phase was separated, and the aqueous component was extracted with ethyl acetate (3 × 30 mL). The combined organics were washed with brine (3 × 100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to afford the title compound as a yellow oil, which was used directly in the next step without further purification or manipulation (1.20 g, 83%).
3-(4-(2-(5-(3,5-Dimethylisoxazol-4-yl)-1-(2-(piperazin-1-yl)ethyl)-1H-benzo[d]imidazol-2-yl)ethyl)phenoxy)-N,N-dimethylpropan-1-amine (21)
To a suspension of 20(44) (0.818 g, 3.18 mmol) in DMF (10 mL), was added triethylamine (0.8 mL, 5.78 mmol) and HATU (1.43 g, 3.76 mmol). The reaction vessel was flushed with argon and left to stir for 1 h before the addition of 19 (1.20 g, 3.76 mmol) in DMF (10 mL). The reaction was then left to stir at ambient temperature overnight. The reaction mixture was then partitioned between ethyl acetate (50 mL) and water (50 mL). The organic phase was separated and washed with water (4 × 150 mL), saturated aqueous sodium hydrogen carbonate solution (100 mL) and brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% 7N methanolic ammonia solution in dichloromethane, afforded the diamine intermediate. This was dissolved in methanol (20 mL) before the addition of HCl (2.6 mL, 10.5 mmol, 4 M solution in 1,4-dioxane), and the reaction was heated at reflux overnight. The reaction mixture was cooled and concentrated under reduced pressure before addition of dichloromethane (50 mL) and saturated aqueous sodium hydrogen carbonate solution (50 mL) solution. After stirring vigorously for 10 min, the organic phase was separated. The aqueous component was extracted with dichloromethane (3 × 50 mL), and the combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% 7N methanolic ammonia solution in dichloromethane, afforded the title compound as a beige solid (564 mg, 37%). 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.15–7.09 (m, 3H), 6.84 (d, J = 8.2 Hz, 2H), 4.11 (t, J = 6.9 Hz, 2H), 3.99 (d, J = 6.4 Hz, 2H), 3.25–3.15 (m, 4H), 2.88–2.83 (m, 4H), 2.59 (t, J = 6.9 Hz, 2H), 2.49–2.43 (m, 5H), 2.42 (s, 3H), 2.33–2.39 (m, 4H), 2.27 (s, 6H), 2.01 (br s, 1H), 1.98–1.93 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 165.1, 159.2, 157.8, 155.7, 143.2, 134.5, 133.0, 129.4, 124.3, 123.5, 120.0, 117.3, 114.8, 109.6, 66.4, 58.0, 56.5, 55.0, 46.1, 45.5, 41.7, 33.2, 30.1, 27.6, 11.7, 11.0. LCMS (5–95% MeCN over 20 min) tR = 7.34 min, Purity >99%, m/z (ES+): 531.4 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C31H43N6O2, 531.3447; found, 531.3443.
tert-Butyl-4-(2-(2-(4-(3-(dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazine-1-carboxylate (22)
To a solution of di-tert-butyl dicarbonate (58 mg, 0.265 mmol) and DMAP (4.6 mg, 0.038 mmol) in dichloromethane (5 mL) was added a dropwise a solution of 21 (100 mg, 0.189 mmol) in dichloromethane (3 mL) and triethylamine (0.131 mL, 0.945 mmol), and the resulting mixture was stirred overnight. After this time, water (10 mL) was added, and the organic phase was separated. The aqueous component was extracted with dichloromethane (5 × 10 mL), and the combined organic extracts were washed with brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–10% (7N ammonia in methanol) in dichloromethane, afforded the title compound as a colorless oil (52 mg, 43%). 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.15–7.09 (m, 3H), 6.83 (d, J = 8.1 Hz, 2H), 4.11 (d, J = 6.8 Hz, 2H), 4.00 (t, J = 6 Hz, 2H), 3.45–3.33 (m, 4H), 3.24–3.18 (m, 2H), 3.18–3.13 (m, 2H), 2.70–2.59 (m, 4H), 2.45–2.35 (m, 13H), 2.29 (s, 3H), 2.07 (q, J = 7 Hz, 2H), 1.44 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 165.1, 159.1, 157.5, 155.5, 154.7, 143.1, 134.4, 133.1, 129.5, 129.4, 124.3, 123.5, 120.0, 117.2, 114.7, 114.5, 109.5, 80.0, 65.9, 57.3, 56.4, 45.0, 41.7, 33.1, 31.7, 30.1, 30.1, 28.5, 26.9, 22.8, 11.7, 11.1. LCMS (5–95% MeCN over 20 min) tR = 7.38 min, Purity >99%, m/z (ES+): 631.45 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C36H51N6O4, 631.3972; found, 631.3998.
1-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)ethanone (23)
To a suspension of 19 (0.918 g, 3.57 mmol) in DMF (20 mL), was added triethylamine (1.36 mL, 9.75 mmol) and HATU (1.61 g, 4.23 mmol). The reaction vessel was degassed and backfilled with argon and left to stir for 1 h before the addition of a solution of 20 (1.35 g, 3.25 mmol) in DMF (30 mL). The reaction was then left to stir at ambient temperature overnight before being partitioned between ethyl acetate (50 mL) and water (50 mL). The organic phase was separated and washed with water (4 × 150 mL), saturated aqueous sodium hydrogen carbonate solution (100 mL), and brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% (7N ammonia in methanol) in dichloromethane, afforded the diamine intermediate, which was dissolved in acetic acid and heated at reflux overnight. The reaction mixture was cooled, concentrated, and suspended in dichloromethane before neutralizing with saturated aqueous sodium hydrogen carbonate solution. The organic phase was separated, and the aqueous component was extracted with dichloromethane (4 × 50 mL). The combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% (7N ammonia in methanol) in dichloromethane, afforded the title compound as a pale brown oil (640 mg, 34%). 1H NMR (600 MHz, CDCl3): δ 7.64–7.61 (m, 1H), 7.35–7.31 (m, 1H), 7.15–7.09 (m, 3H), 6.85–6.80 (m, 2H), 4.13–4.09 (m, 2H), 4.01–3.97 (m, 2H), 3.56–3.48 (m, 2H), 3.39–3.30 (m, 2H), 3.24–3.13 (m, 4H), 2.63–2.61 (m, 2H), 2.46–2.41 (m, 7H), 2.32–2.28 (m, 12H), 2.00–1.94 (m, 4H); 13C NMR (151 MHz, CDCl3): δ 165.2, 160.8, 159.1, 157.8, 155.5, 143.2, 134.3, 133.0, 129.5, 129.4, 124.4, 123.6, 120.1, 117.2, 114.8, 114.5, 109.5, 66.3, 57.2, 56.4, 54.3, 52.9, 45.6, 45.4, 41.8, 39.9, 33.2, 30.2, 27.4, 11.8, 11.1; LCMS (5–95% MeCN over 20 min) tR = 6.97 min, purity >96%; m/z (ES+): 573.55 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C33H45N6O3, 573.3553; found, 573.3546.
4-(3,5-Dimethylisoxazol-4-yl)-2-nitro-N-(2-(piperazin-1-yl)ethyl)aniline (24)

To a stirred solution of 17 (2.8 g, 6.3 mmol) in dichloromethane (200 mL) was added TFA (20 mL, 26.2 mmol), and the reaction was left to stir at ambient temperature overnight. The reaction mixture was concentrated under reduced pressure, and reconcentrated from dichloromethane (5 × 50 mL), affording the crude product as the TFA salt. This was partitioned between dichloromethane (50 mL) and saturated aqueous sodium hydrogen carbonate solution. The organic phase was separated, and the aqueous component was extracted with dichloromethane (5 × 50 mL). The combined organic extracts were washed with brine (2 × 100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure affording the title compound as a red oil (2.2 g, 99%). 1H NMR (600 MHz, CDCl3): δ 8.58 (s, 1H), 8.09 (s, 1H), 7.34 (d, J = 9 Hz, 1H), 6.91 (d, J = 9 Hz, 1H), 3.40 (q, J = 6 Hz, 2H), 3.00–2.93 (m, 4H), 2.75 (t, J = 6.1 Hz, 2H), 2.60–2.50 (m, 4H), 2.40 (s, 3H), 2.35 (br s, 1H), 2.26 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 165.5, 158.7, 144.6, 136.9, 132.1, 117.4, 115.1, 114.9, 56.2, 53.8, 46.1, 39.7, 11.7, 10.9. LCMS (5–95% MeCN over 20 min) tR = 10.56 min, Purity >99%, m/z (ES+): 345.95 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C17H24N5O3, 346.1874; found, 346.1859.
tert-Butyl-2-(4-(2-((4-(3,5-dimethylisoxazol-4-yl)-2-nitrophenyl)amino)ethyl)piperazin-1-yl)acetate (25)
To a stirred solution of 24 (2.1 g, 6.3 mmol) in dichloromethane (100 mL) was added DIPEA (4.40 mL, 25.2 mmol) followed by tert-butylbromoacetate (1.11 mL, 7.56 mmol), and the resulting solution was left to stir overnight at ambient temperature. The reaction mixture was washed with water (50 mL), saturated aqueous sodium hydrogen carbonate solution (50 mL) and brine (100 mL), before being dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–100% ethyl acetate in hexane, afforded the title compound as an orange oil (2.31 g 80%). 1H NMR (600 MHz, CDCl3): δ 8.57 (s, 1H), 8.08 (s, 1H), 7.33 (d, J = 8.5 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 3.39 (q, J = 6 Hz, 2H), 3.13 (s, 2H), 2.76 (t, J = 6 Hz, 2H), 2.73–2.52 (s, 8H), 2.40 (s, 3H), 2.26 (s, 3H), 1.47 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 169.6, 165.3, 158.6, 144.5, 136.7, 131.9, 127.0, 117.2, 115.0, 114.7, 81.1, 59.9, 55.5, 53.1, 52.5, 39.7, 28.2, 11.6, 10.8. LCMS (5–95% MeCN over 20 min) tR = 12.59 min, Purity >99%, m/z (ES+): 460.10 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C23H34N5O5+, 460.2554; found, 460.2542.
tert-Butyl-2-(4-(2-((2-amino-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino) ethyl)piperazin-1-yl)acetate (26)

To a suspension of 25 (2.30 g, 5.01 mmol) in ethanol (75 mL) was added 1 M aqueous sodium dithionite solution (75 mL), and the reaction was heated to 80 °C for 1 h. Upon cooling, the reaction mixture was partitioned between 10% ammonium hydroxide solution (75 mL) and ethyl acetate (75 mL). The organic phase was separated, and the aqueous component was extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give the title compound as a yellow oil, which was used directly in the next step without any further purification (1.36 g, 70%). 1H NMR (600 MHz, CDCl3): δ 6.68 (s, 2H), 6.58 (s, 1H), 3.48 (br s, 2H), 3.20 (t, J = 6 Hz, 2H), 3.12 (s, 2H), 2.71 (t, J = 6 Hz, 2H), 2.65–2.50 (m, 8H), 2.37 (s, 3H), 2.25 (s, 3H), 1.47 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 169.5, 164.4, 159.0, 137.1, 134.6, 121.2, 120.2, 116.8, 116.5, 111.6, 81.1, 59.6, 56.7, 53.00, 52.8, 40.6, 28.1, 11.5, 10.8. LCMS (30–95% MeCN over 20 min) tR = 17.02 min, Purity >95%, m/z (ES+): 430.05 [M+H+]+; HRMS-ESI (m/z): [M+Na+]+ calculated for C23H35N5NaO3+, 452.2632; found, 452.2638.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetic Acid Dihydrochloride (27)
To a stirred suspension of 20 (3.3 g, 12.8 mmol) in DMF (30 mL), was added triethylamine (3.23 mL, 23.2 mmol) and HATU (5.7 g, 15.1 mmol). The reaction vessel was flushed with argon and left to stir for 1 h before the addition of a solution of 26 (5 g, 11.6 mmol) in DMF (30 mL), and the reaction mixture was then left to stir at ambient temperature overnight. The reaction mixture was partitioned between dichloromethane (150 mL) and water (150 mL). The organic phase was separated and washed with water (4 × 150 mL), saturated aqueous sodium hydrogen carbonate solution (150 mL), and brine (150 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 3–5% methanol (with up to 0.5% NH4OH) in dichloromethane, produced a pale brown gum (2.59 g, 3.908 mmol). The residue was then dissolved in acetic acid (50 mL) and heated to reflux for 1.5 h, concentrated under reduced pressure, and reconcentrated from heptane (5 × 75 mL). The brown gum was dissolved in anhydrous ethyl acetate (60 mL) and purged with nitrogen (g). To this stirring solution was added hydrogen chloride (6 mL, 11.72 mmol, 2 M solution in diethyl ether) and a solid immediately formed. Excess diethyl ether was added, and the solution was left to stir overnight at ambient temperature. The precipitate was then collected by filtration, dried under vacuum, and freeze-dried, affording the title compound as an off-white solid (3.04 g, 36%). 1H NMR (600 MHz, d6-DMSO): δ 11.03 (br s, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.79 (s, 1H), 7.57 (d, J = 8.2 Hz, 1H), 7.33 (d, J = 9 Hz, 2H), 6.89 (d, J = 9 Hz, 2H), 4.85 (br s, 2H), 4.10–3.83 (m, 5H), 3.76–3.09 (m, 15H), 2.75–2.70 (m, 6H), 2.42 (s, 3H), 2.24 (s, 3H), 2.10–2.17 (m, 2H). 13C NMR (151 MHz, d6-DMSO): δ 165.7, 158.3, 157.1, 154.8, 131.6, 131.1, 129.7, 127.6, 126.5, 115.5, 114.6, 113.5, 64.9, 55.0, 53.9, 42.0, 31.3, 27.3, 23.9, 11.4, 10.5. LCMS (30–95 MeCN over 20 min) tR = 7.36 min, Purity >95%, m/z (ES+): 589.25 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C33H45N6O4, 589.3502; found, 589.3577.
Methyl 2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetate Hydrochloride (28)
A solution of 27 (100 mg, 0.151 mmol) in methanol (5 mL) was treated with sulfuric acid (1 drop) and heated to reflux overnight. Upon cooling, the reaction mixture was concentrated under reduced pressure, partitioned between ethyl acetate (10 mL) and water (10 mL), and the biphasic mixture was treated with saturated aqueous sodium hydrogen carbonate solution (10 mL). The organic phase was separated and washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–20% methanol in dichloromethane, afforded a colorless oil, which was dissolved in anhydrous ethyl acetate (10 mL) and purged with nitrogen (g). To this stirring solution was added hydrogen chloride (0.132 mL, 0.27 mmol, 2 M solution in diethyl ether), whereupon a solid immediately formed, excess diethyl ether was added and the solution was left to stir overnight at ambient temperature. The precipitate was then collected by filtration, affording the title compound as a beige solid (85 mg, 88%). 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 7.34 (d, J = 8.5 Hz, 1H), 7.16–7.10 (m, 3H), 6.83 (d, J = 8.5 Hz, 2H), 4.10 (t, J = 7.0 Hz, 2H), 4.00 (t, J = 6.3 Hz, 2H), 3.72 (s, 3H), 3.24–3.19 (m, 4H), 3.18–3.14 (m, 2H), 2.62 (t, J = 7.0 Hz, 2H), 2.60–2.52 (m, 10H), 2.43 (s, 3H), 2.33 (s, 6H), 2.30 (s, 3H), 2.03–1.97 (m, 2H).13C NMR (151 MHz, CDCl3): δ 170.8, 165.2, 159.2, 157.7, 155.6, 143.2, 134.4, 133.1, 129.5, 124.3, 123.5, 120.0, 117.3, 114.8, 109.6, 66.2, 59.4, 57.3, 56.5, 53.5, 53.0, 51.9, 45.4, 41.7, 33.2, 30.1, 27.3, 11.8, 11.1. LCMS (30–95 MeCN over 20 min) tR = 7.03 min, Purity >91%, m/z (ES+): 603.25 [M+H+]; HRMS-ESI (m/z): [M+H+]+ calculated for C34H47N6O4, 603.3659; found, 603.3651.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(2-methoxyethyl)acetamide Hydrochloride (29)
To a stirred solution of 27 (150 mg, 0.227 mmol) and HATU (112.2 mg, 0.295 mmol) in DMF (5 mL) was added triethylamine (95 μL, 0.681 mmol) followed by 2-methoxyethylamine (40 μL, 0.453 mmol) before leaving the reaction to stir at ambient temperature overnight. The mixture was then partitioned between dichloromethane (25 mL) and water (25 mL). The organic phase was separated and washed with saturated aqueous sodium hydrogen carbonate solution (25 mL), brine (3 × 10 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by flash column chromatography and elution with 0–10% (7N ammonia in methanol) in dichloromethane, afforded the free base as a colorless oil. This was dissolved in anhydrous ethyl acetate (10 mL) and purged with nitrogen (g). To this stirring solution was added hydrogen chloride (0.17 mL, 0.33 mmol, 2 M solution in diethyl ether), whereupon a solid immediately formed. Excess diethyl ether was added, and the mixture was left to stir overnight at ambient temperature. The precipitate was collected by filtration to afford the title compound as a colorless solid (120 mg, 77%). 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 1.5 Hz, 1H), 7.34 (d, J = 8.2 Hz, 2H), 7.15 (d, J = 8.6 Hz, 2H), 7.10–7.12 (m, 1H), 6.83 (d, J = 8.6 Hz, 2H), 4.11 (t, J = 6.7 Hz, 2H), 4.04 (t, J = 6.0 Hz, 2H), 3.48–3.42 (m, 4H), 3.34 (s, 3H), 3.23 (t, J = 7.0 Hz, 2H), 3.15–3.19 (m, 2H), 2.98 (s, 2H), 2.9–2.83 (m, 2H), 2.64 (t, J = 6.7 Hz, 2H), 2.57 (s, 6H), 2.55–2.45 (s, 8H), 2.42 (s, 3H), 2.29 (s, 3H), 2.20–2.12 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 170.0, 165.0, 159.0, 157.2, 155.5, 143.1, 134.3, 133.4, 129.4, 124.2, 123.4, 119.9, 117.1, 114.6, 109.3, 71.3, 65.4, 61.4, 58.7, 57.1, 56.1, 53.6, 53.2, 44.3, 41.7, 38.6, 32.8, 29.8, 26.0, 22.6, 11.6, 10.9. LCMS (5–95% MeCN over 20 min) tR = 8.69 min, Purity >96%, m/z (ES+): 646.30 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C36H52N7O4, 646.4081; found, 646.4094.
General Procedure B for Degrader Synthesis
A stirred suspension of 27 (150 mg, 0.227 mmol) in DMF (10 mL) was treated with the relevant commercially available amine-reactive degrader building block (E3 ligase ligand functionalized with a linker with amine termini) (1 equiv), triethylamine (221 μL, 1.587 mmol, 7 equiv) and HATU (112 mg, 0.295 mmol, 1.3 equiv) and stirred overnight at ambient temperature. The reaction was then diluted with water (100 mL) and extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine (50 mL), saturated aqueous sodium hydrogen carbonate solution (50 mL), and brine (2 × 50 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)ethyl)acetamide (30)
Degrader 30 was synthesized according to General Procedure B using pomalidomide 4′-alkylC2-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a yellow solid (72 mg, 36%). 1H NMR (600 MHz, d6-DMSO): δ 11.11 (br s, 1H), 7.90 (t, J = 6 Hz, 1H), 7.58–7.54 (m, 2H), 7.21 (d, J = 9 Hz, 2H), 7.16 (dd, J = 8, 2 Hz, 1H), 7.02 (d, J = 7 Hz, 1H), 6.84 (d, J = 9 Hz, 2H), 6.71–6.66 (m, 1H), 5.05 (dd, J = 13, 5 Hz, 1H), 4.24 (t, J = 6 Hz, 2H), 3.93 (t, J = 6 Hz, 2H), 3.40–3.27 (m, 12H), 3.14–3.18 (m, 4H), 2.90–2.80 (m, 3H), 2.57–2.50 (m, 4H), 2.47–2.39 (m, 4H), 2.37–2.30 (m, 4H), 2.23 (s, 3H), 2.12 (s, 6H), 1.82–1.78 (m, 2H).13C NMR (151 MHz, d6-DMSO): δ 172.8, 170.1, 169.7, 168.7, 167.3, 164.5, 158.4, 157.0, 155.6, 146.4, 142.6, 136.2, 134. 5, 133.0, 132.2, 129.4, 122.7, 122.6, 122.6, 118.9, 117.3, 116.7, 114.3, 110.6, 110.3, 109.2, 65.7, 61.2, 57.1, 55.7, 52.9, 52.8, 48.5, 45.2, 41.3, 40.7, 40.0, 37.7, 31.8, 31.0, 28.6, 27.0, 22.2, 11.4, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.212 min, Purity = 99%, m/z (ES+): 887.40 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C48H59N10O7, 887.4568; found, 887.4599.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(4-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)butyl)acetamide (31)
Degrader 31 was synthesized according to General Procedure B using pomalidomide 4′-alkylC4-amine. Purification by flash column chromatography and elution with 2–6% 7N (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a yellow solid (65 mg, 31%). 1H NMR (600 MHz, d6-DMSO): δ 11.11 (br s, 1H), 7.73–7.68 (t, J = 6 Hz, 1H), 7.57–7.53 (m, 3H), 7.21 (d, J = 9 Hz, 2H), 7.16 (dd, J = 8, 2 Hz, 1H), 7.08 (d, J = 9 Hz, 1H), 7.00 (d, J = 7 Hz, 1H), 6.84 (d, J = 9 Hz, 2H), 6.57 (t, J = 6 Hz, 1H), 5.04 (dd, J = 13, 5 Hz, 1H), 4.24 (t, J = 6 Hz, 2H), 3.94 (t, J = 6 Hz, 2H), 3.30 (q, J = 7 Hz, 4H), 3.09–3.19 (m, 6H), 2.90–2.82 (m, 4H), 2.59–2.51 (m, 6H), 2.45–2.39 (m, 5H), 2.35–2.30 (m, J = 7 Hz, 4H), 2.23 (s, 3H), 2.13 (s, 6H), 1.97–2.02 (m, 1H), 1.83–1.78 (m, 2H), 1.45–1.52 (m, 3H). 13C NMR (151 MHz, d6-DMSO): δ 172.9, 170.1, 169.0, 167.3, 164.6, 158.4, 157.0, 155.6, 146.4, 142.6, 136.3, 134.5, 133.1, 132.2, 129.4, 122.7, 122.6, 118.9, 117.2, 116.7, 114.3, 110.4, 110.3, 109.0, 65.7, 61.3, 57.1, 55.7, 52.9, 52.8, 48.5, 45.2, 41.5, 40.1, 37.8, 31.8, 31.0, 28.6, 26.9, 26.7, 26.2, 22.2, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.212 min, Purity >99%, m/z (ES+): 915.5 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C50H63N10O7, 915.4881; found, 915.4950.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(6-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)hexyl)acetamide (32)
Degrader 32 was synthesized according to General Procedure B using pomalidomide 4′-alkylC6-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a yellow solid (95 mg, 44%). 1H NMR (600 MHz, DMSO-d6): δ 11.11 (br s, 1H), 7.63 (t, J = 5.9 Hz, 1H), 7.57–7.53 (m, 3H), 7.20 (d, J = 8.6 Hz, 2H), 7.16 (dd, J = 8.2, 1.5 Hz, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.99 (d, J = 7.0 Hz, 1H), 6.83 (d, J = 8.6 Hz, 2H), 6.53 (t, J = 5.7 Hz, 1H), 5.04 (dd, J = 12.9, 5.7 Hz, 1H), 4.24 (t, J = 6.3 Hz, 2H), 3.93 (t, J = 6.4 Hz, 2H), 3.34–3.24 (m, 3H), 3.20–3.01 (m, 6H), 2.89–2.82 (m, 3H), 2.60–2.30 (m, 19H), 2.12 (s, 6H), 2.01–1.97 (m, 1H), 1.83–1.78 (m, 2H), 1.57–1.53 (m, 2H), 1.43–1.23 (m, 7H).13C NMR (151 MHz, DMSO-d6): δ 172.8, 170.1, 168.9, 168.8, 167.3, 164.5, 158.4, 157.0, 155.6, 146.4, 142.6, 136.3, 134.5, 133.0, 132.2, 129.4, 122.7, 122.6, 118.9, 117.2, 116.7, 114.3, 112.8, 110.4, 110.3, 110.1, 109.0, 65.7, 61.3, 57.1, 55.7, 52.9, 52.8, 48.5, 45.2, 41.8, 40.0, 38.1, 31.8, 30.9, 29.1, 28.6, 26.9, 26.1, 26.0, 22.1, 11.3, 10.5. LCMS (5–95% MeCN over 5 min) tR = 3.681 min, Purity >97%, m/z (ES+): 943.6 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C52H67N10O7, 943.5194; found, 943.5203.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(8-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)octyl)acetamide (33)
Degrader 33 was synthesized according to General Procedure B using pomalidomide 4′-alkylC8-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a yellow solid (53 mg, 24%). 1H NMR (600 MHz, DMSO-d6): δ 11.11 (br s, 1H), 7.61 (t, J = 6 Hz, 1H), 7.53–7.56 (m, 3H), 7.20 (d, J = 9 Hz, 2H), 7.16 (dd, J = 8, 2 Hz, 1H), 7.07 (d, J = 9 Hz, 1H), 7.00 (d, J = 7 Hz, 1H), 6.83 (d, J = 9 Hz, 2H), 6.52 (t, J = 6 Hz, 1H), 5.04 (dd, J = 13, 6 Hz, 1H), 4.24 (t, J = 6 Hz, 2H), 3.93 (d, J = 6 Hz, 2H), 3.27 (d, J = 7 Hz, 2H), 3.14–3.17 (m, 2H), 3.09–3.12 (m, 2H), 3.05 (d, J = 7 Hz, 2H), 2.82–2.90 (m, 4H), 2.51–2.62 (m, 4H), 2.48–2.30 (m, 10H), 2.23 (s, 3H), 2.12 (s, 6H), 1.99–2.03 (m, 1H), 1.80 (d, J = 6 Hz, 2H), 1.55 (t, J = 7 Hz, 2H), 1.36–1.39 (m, 2H), 1.20–1.33 (m, 10H). 13C NMR (151 MHz, DMSO-d6): δ 172.9, 170.2, 168.8, 164.6, 158.4, 157.1, 155.6, 146.4, 142.6, 136.3, 134.5, 133.1, 132.2, 129.4, 122.7, 122.6, 118.9, 117.2, 116.7, 114.3, 110.4, 110.3, 65.7, 61.3, 57.1, 55.7, 53.0, 52.8, 48.5, 45.3, 41.8, 40.1, 38.1, 31.8, 31.0, 29.2, 28.7, 28.7, 27.0, 26.3, 26.3, 22.2, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.94 min, Purity = 97%, m/z (ES+): 971.5 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C54H71N10O7, 971.5507; found, 971.5601.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)ethyl)acetamide (34)
Degrader 34 was synthesized according to General Procedure B using thalidomide 4′-ether-alkylC2-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (70 mg, 35%). 1H NMR (600 MHz, DMSO-d6): δ 11.11 (br s, 1H), 7.82 (t, J = 6 Hz, 1H), 7.78 (dd, J = 8, 7 Hz, 1H), 7.54–7.51 (m, 3H), 7.43 (d, J = 7 Hz, 1H), 7.17 (d, J = 9 Hz, 2H), 7.14 (dd, J = 8, 2 Hz, 1H), 6.81 (d, J = 9 Hz, 2H), 5.05 (dd, J = 13, 5 Hz, 1H), 4.27–4.18 (m, 4H), 3.91 (t, J = 6 Hz, 2H), 3.49 (q, J = 5 Hz, 2H), 3.15–3.05 (m, 4H), 2.77–2.87 (m, 4H), 2.52–2.45 (m, 4H), 2.42–2.27 (m, 12H), 2.21 (s, 3H), 2.11 (s, 6H), 1.92–1.97 (m, 1H), 1.84–1.78 (m, 2H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 169.9, 169.6, 166.8, 165.2, 164.6, 158.4, 157.0, 155.6, 155.6, 142.6, 137.1, 134.5, 133.3, 133.1, 129.4, 122.7, 122.6, 120.0, 118.9, 116.7, 116.4, 115.6, 114.3, 110.3, 67.3, 65.7, 61.1, 57.2, 55.7, 52.9, 52.8, 48.8, 45.2, 40.1, 37.5, 31.8, 30.9, 28.6, 26.9, 22.1, 11.4, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.15 min, Purity >99%, m/z (ES+): 888.4 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C48H58N9O8, 888.4408; found, 888.4467.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(4-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)butyl)acetamide (35)
Degrader 35 was synthesized according to General Procedure B using thalidomide 4′-ether-alkylC4-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (75 mg, 37%). 1H NMR (600 MHz, DMSO-d6): δ 11.10 (br s, 1H), 7.77 (dd, J = 9, 7 Hz, 1H), 7.71 (t, J = 6 Hz, 1H), 7.53 (d, J = 8 Hz, 2H), 7.47 (d, J = 9 Hz, 1H), 7.40 (d, J = 7 Hz, 1H), 7.18 (d, J = 9 Hz, 2H), 7.13 (dd, J = 8, 2 Hz, 1H), 6.81 (d, J = 9 Hz, 2H), 5.04 (dd, J = 13, 5 Hz, 1H), 4.23–4.17 (m, 4H), 3.91 (t, J = 6 Hz, 2H), 3.14 (dd, J = 8, 5 Hz, 4H), 3.09–3.06 (m, 2H), 2.87–2.81 (m, 3H), 2.56–2.48 (m, 5H), 2.44–2.27 (m, 12H), 2.20 (s, 3H), 2.11 (s, 6H), 2.00–1.95 (m, 1H), 1.84–1.79 (m, 2H), 1.76–1.70 (m, 2H), 1.62–1.56 (m, 2H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 170.0, 169.0, 166.9, 165.4, 164.6, 158.4, 157.0, 155.9, 155.6, 142.6, 137.1, 134.5, 133.3, 133.1, 129.4, 122.7, 122.6, 119.8, 118.9, 116.7, 116.2, 115.2, 114.3, 110.3, 68.5, 65.7, 61.3, 57.1, 55.7, 52.9, 52.9, 48.7, 45.2, 40.1, 37.7, 31.8, 31.0, 28.6, 26.9, 25.9, 25.8, 22.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.19 min, Purity >96%, m/z (ES+): 916.4 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C50H62N9O8, 916.4721; found, 916.4725.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(6-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)hexyl)acetamide (36)
Degrader 36 was synthesized according to General Procedure B using thalidomide 4′-ether-alkylC6-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (74 mg, 35%). 1H NMR (600 MHz, DMSO-d6): δ 11.11 (br s, 1H), 7.76 (dd, J = 8, 7 Hz, 1H), 7.60 (t, J = 6 Hz, 1H), 7.57–7.54 (m, 2H), 7.47 (d, J = 8 Hz, 1H), 7.40 (d, J = 7 Hz, 1H), 7.19 (d, J = 9 Hz, 2H), 7.14 (dd, J = 8, 2 Hz, 1H), 6.82 (d, J = 9 Hz, 2H), 5.06 (dd, J = 13, 5 Hz, 1H), 4.23 (t, J = 6 Hz, 2H), 4.16 (t, J = 6 Hz, 2H), 3.92 (t, J = 6 Hz, 2H), 3.16–3.12 (m, 2H), 3.11–3.07 (m, 2H), 3.04 (q, J = 7 Hz, 2H), 2.88–2.81 (m, 3H), 2.59–2.50 (m, 4H), 2.48–2.38 (m, 5H) 2.38–2.30 (m, 4H), 2.21 (s, 3H), 2.11 (s, 6H), 2.04–1.97 (m, 1H), 1.84–1.78 (m, 2H), 1.77–1.70 (m, 2H), 1.42–1.35 (m, 4H), 1.34–1.19 (m, 6H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 170.0, 168.8, 166.9, 165.3, 164.6, 158.4, 157.1, 156.0, 155.6, 142.6, 137.1, 134.5, 133.3, 133.1, 129.4, 122.7, 122.6, 119.8, 118.9, 116.7, 116.2, 115.2, 114.3, 110.3, 68.8, 65.7, 61.3, 57.1, 55.7, 53.0, 48.7, 45.2, 40.7, 40.1, 38.1, 31.8, 31.0, 29.2, 28.7, 28.4, 26.9, 26.3, 25.3, 22.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.42 min, Purity >99%, m/z (ES+): 944.5 [M+H+]+; HRMS-ESI (m/z) [M+H+]+ calculated for C52H66N9O8, 944.5034; found, 944.5126.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(8-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)octyl)acetamide (37)
Degrader 37 was synthesized according to General Procedure B using thalidomide 4′-ether-alkylC8-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (114 mg, 52%). 1H NMR (600 MHz, DMSO-d6): δ 11.12 (br s, 1H), 7.77 (t, 7 Hz, 1H), 7.64 (t, J = 6 Hz, 1H), 7.57–7.54 (m, 2H), 7.49 (d, J = 9 Hz, 1H), 7.42 (d, J = 7 Hz, 1H), 7.20 (d, J = 9 Hz, 2H), 7.16 (dd, J = 8, 2 Hz, 1H), 6.84 (d, J = 9 Hz, 2H), 5.07 (dd, J = 13, 5 Hz, 1H), 4.24 (t, J = 6 Hz, 2H), 4.18 (t, J = 6 Hz, 2H), 3.93 (t, J = 6 Hz, 2H), 3.19–3.14 (t, J = 7 Hz, 2H), 3.12–3.06 (m, 4H), 2.90–2.81 (m, 3H), 2.59–2.51 (m, 4H), 2.49–2.30 (m, 16H), 2.23 (s, 3H), 2.12 (s, 6H), 2.02–1.98 (m, 2H), 1.80–1.76 (m, 2H), 1.75–1.68 (m, 2H), 1.46–1.40 (m, 4H), 1.33–1.27 (m, 2H).13C NMR (151 MHz, DMSO-d6): δ 172.8, 170.0, 168.9, 166.9, 165.3, 164.6, 158.4, 157.1, 156.0, 155.6, 142.6, 137.1, 134.5, 133.3, 133.1, 129.4, 122.7, 122.6, 119.8, 118.9, 116.7, 116.2, 115.2, 114.3, 110.3, 109.6, 68.7, 65.7, 61.3, 57.1, 55.7, 52.9, 52.8, 48.7, 45.2, 40.7, 40.1, 38.1, 31.8, 31.0, 29.2, 28.6, 28.4, 27.0, 26.1, 25.0, 22.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.69 min, Purity >97%, m/z (ES+): 972.5 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C54H70N9O8, 972.5347; found, 972.5416.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(2-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamido)ethyl)acetamide (38)
Degrader 38 was synthesized according to General Procedure B using thalidomide 4′-oxyacetamide-alkylC2-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (34 mg, 16%). 1H NMR (600 MHz, DMSO-d6): δ 11.14 (br s, 1H), 8.05 (t, J = 6 Hz, 1H), 7.80–7.76 (m, 1H), 7.57–7.53 (m, 2H), 7.47 (d, J = 7 Hz, 1H), 7.37 (d, J = 8 Hz, 1H), 7.23–7.14 (m, 4H), 6.84 (d, J = 8 Hz, 2H), 5.11 (dd, J = 13, 5 Hz, 1H), 4.75 (s, 2H), 4.25–4.20 (m, 2H), 3.94 (t, J = 6 Hz, 2H), 3.24–3.08 (m, 10H), 2.91–2.78 (m, 4H), 2.62–2.53 (m, 2H), 2.45–2.22 (m, 12H), 2.22–2.18 (m, 8H), 2.03–1.90 (m, 2H), 1.85–1.79 (m, 2H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 169.9, 169.5, 167.2, 166.8, 165.5, 164.6, 158.4, 157.0, 155.6, 155.1, 142.6, 137.0, 134.5, 133.1, 133.1, 129.5, 129.4, 122.7, 122.6, 118.9, 116.8, 116.7, 116.1, 114.4, 114.3, 110.3, 67.6, 65.6, 65.4, 61.2, 57.1, 55.5, 52.9, 52.9, 48.8, 44.9, 40.1, 38.3, 38.1, 31.8, 31.0, 28.6, 22.0, 11.4, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.17 min, Purity >99%, m/z (ES+): 945.4 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C50H61N10O9, 945.4623; found, 945.4614.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(4-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamido)butyl)acetamide (39)
Degrader 39 was synthesized according to General Procedure B using thalidomide 4′-oxyacetamide-alkylC4-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (72 mg, 32%). 1H NMR (600 MHz, DMSO-d6): δ 11.12 (br s, 1H), 7.96 (t, J = 6 Hz, 1H), 7.79–7.75 (m, 1H), 7.63 (t, J = 6 Hz, 1H), 7.53 (m, 2H), 7.45 (d, J = 7 Hz, 1H), 7.34 (d, J = 9 Hz, 1H), 7.19 (d, J = 8 Hz, 2H), 7.14 (d, J = 8 Hz, 1H), 6.82 (d, J = 9 Hz, 2H), 5.09 (dd, J = 13, 5 Hz, 1H), 4.74 (s, 2H), 4.66–4.51 (m, 2H), 4.25–4.19 (m, 2H), 3.92 (t, J = 6 Hz, 2H), 3.17–2.94 (m, 11H), 2.89–2.79 (m, 4H), 2.58–2.49 (m, 4H), 2.49–2.25 (m, 10H), 2.21–2.18 (m, 7H), 2.02–1.92 (m, 2H), 1.85–1.80 (m, 2H), 1.37 (d, J = 3 Hz, 4H). 13C NMR (151 MHz, DMSO-d6): δ 173.0, 172.8, 169.9, 169.0, 166.8, 166.7, 165.5, 164.6, 158.4, 157.0, 155.6, 155.1, 142.6, 137.0, 134.5, 133.1, 133.1, 129.4, 122.7, 122.6, 120.4, 118.9, 116.8, 116.7, 116.1, 114.3, 110.3, 67.6, 65.6, 61.3, 57.1, 55.5, 52.9, 52.9, 48.8, 44.8, 40.1, 38.1, 37.9, 31.8, 31.0, 28.6, 26.7, 26.5, 22.0, 11.4, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.262 min, Purity >99%, m/z (ES+): 973.5 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C52H65N10O9, 973.4936; found, 973.5108.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-i-(6-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamido)hexyl)acetamide (40)
Degrader 40 was synthesized according to General Procedure B using thalidomide 4′-oxyacetamide-alkylC6-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (97 mg, 43%). 1H NMR (600 MHz, DMSO-d6): δ 11.14 (br s, 1H), 7.94 (t, J = 6 Hz, 1H), 7.81–7.78 (m, 1H), 7.63 (t, J = 6 Hz, 1H), 7.55–7.52 (m, 2H), 7.48 (d, J = 7 Hz, 1H), 7.37 (d, J = 9 Hz, 1H), 7.21 (d, J = 9 Hz, 2H), 7.16 (dd, J = 8, 2 Hz, 1H), 6.84 (d, J = 9 Hz, 2H), 5.09 (dd, J = 13, 5 Hz, 1H), 4.76 (s, 2H), 4.65–4.45 (m, 1H), 4.25–4.20 (m, 2H), 3.94 (t, J = 6 Hz, 2H), 3.15–3.05 (m, 6H), 3.05–3.00 (m, 2H), 2.90–2.80 (m, 3H), 2.58–2.52 (m, 4H), 2.49–2.30 (m, 12H), 2.23 (s, 3H), 2.16 (s, 6H), 2.05–2.00 (m, 1H), 1.85–1.79 (m, 2H), 1.45–1.33 (m, 4H), 1.27–1.18 (m, 4H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 169.9, 168.9, 166.8, 166.6, 165.5, 164.6, 158.4, 157.0, 155.6, 155.1, 142.6, 137.0, 134.5, 133.1, 133.0, 129.4, 122.7, 122.6, 120.4, 118.9, 116.8, 116.7, 116.1, 114.3, 110.3, 67.6, 65.6, 61.3, 57.1, 55.6, 52.9, 52.8, 48.8, 45.0, 40.7, 40.1, 38.2, 38.1, 31.8, 31.0, 29.2, 29.0, 28.6, 26.8, 26.1, 26.0, 22.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.341 min, Purity >99.9%, m/z (ES+): 1001.2 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C54H69N10O9, 1001.5249; found, 1001.5238.
2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)-N-(8-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamido)octyl)acetamide (41)
Degrader 41 was synthesized according to General Procedure B using thalidomide 4′-oxyacetamide-alkylC8-amine. Purification by flash column chromatography and elution with 2–6% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (48 mg, 20%). 1H NMR (600 MHz, DMSO-d6): δ 11.12 (br s, 1H), 7.92 (t, J = 6 Hz, 1H), 7.79–7.75 (m, 1H), 7.62–7.57 (t, J = 6 Hz, 1H), 7.54 (dd, J = 5, 3 Hz, 2H), 7.47 (d, J = 7 Hz, 1H), 7.36 (d, J = 9 Hz, 1H), 7.19 (d, J = 9 Hz, 2H), 7.14 (dd, J = 8, 2 Hz, 1H), 6.82 (d, J = 8 Hz, 2H), 5.10 (dd, J = 13, 5 Hz, 1H), 4.75 (s, 2H), 4.65–4.47 (m, 1H), 4.23 (t, J = 6 Hz, 2H), 3.92 (t, J = 6 Hz, 2H), 3.20–3.08 (m, 6H), 3.06–3.01 (m, 2H), 2.92–2.82 (m, 3H), 2.62–2.52 (m, 4H), 2.48–2.26 (m, 10H), 2.21 (s, 3H), 2.16 (s, 6H), 2.03–1.98 (m, 1H), 1.86–1.80 (m, 2H), 1.44–1.33 (m, 4H), 1.25–1.18 (m, 8H). 13C NMR (151 MHz, DMSO-d6): δ 172.8, 169.9, 168.8, 166.8, 166.6, 165.5, 164.6, 158.4, 157.0, 155.6, 155.1, 142.6, 136.9, 134.5, 133.1, 133.0, 129.4, 122.7, 122.6, 120.4, 118.9, 116.8, 116.7, 116.1, 114.3, 110.3, 67.6, 65.6, 61.3, 57.1, 55.6, 53.0, 52.8, 48.8, 45.0, 40.1, 38.3, 38.1, 31.8, 31.0, 29.2, 29.0, 28.7, 28.6, 26.7, 26.3, 22.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.478 min, Purity >95%, m/z (ES+): 1029.5 [M+H+]+.HRMS-ESI (m/z): [M+H+]+ calculated for C56H73N10O9, 1029.5562; found, 1029.5603.
(2S,4R)-1-((S)-2-(3-(2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetamido)propanamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (42)
Degrader 42 was synthesized according to General Procedure B using VH 032 amide-alkylC2-amine. Purification by flash column chromatography and elution with 3–7% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (96 mg, 39%). 1H NMR (600 MHz, DMSO-d6): δ 8.96 (s, 1H), 8.57 (t, J = 6 Hz, 1H), 8.00 (d, J = 9 Hz, 1H), 7.62 (t, J = 6 Hz, 1H), 7.59–7.54 (m, 2H), 7.45–7.35 (m, 4H), 7.19 (d, J = 9 Hz, 2H), 7.14 (d, J = 8 Hz, 1H), 6.82 (d, J = 9 Hz, 2H), 5.18–5.14 (m, 1H), 4.52 (d, J = 9 Hz, 1H), 4.44–4.39 (m, 2H), 4.32 (br s, 1H), 4.28–4.17 (m, 3H), 3.92 (t, J = 6 Hz, 2H), 3.68–3.59 (m, 2H), 3.29–3.22 (m, 2H), 3.15 (t, J = 7 Hz, 2H), 3.11–3.07 (m, 2H), 2.85–2.77 (m, 2H), 2.55–2.51 (m, 3H), 2.49–2.40 (m, 10H), 2.40–2.30 (m, 7H), 2.21 (s, 3H), 2.16 (s, 6H), 2.05–2.00 (m, 1H), 1.90–1.85 (m, 1H), 1.82–1.79 (m, 2H), 0.90 (s, 9H). 13C NMR (151 MHz, DMSO-d6): δ 172.0, 170.5, 169.5, 168.9, 164.6, 158.4, 157.1, 155.6, 151.5, 147.7, 142.6, 139.5, 134.5, 133.1, 131.2, 129.7, 129.4, 128.7, 127.4, 122.7, 122.6, 118.9, 116.7, 114.3, 110.3, 68.9, 65.7, 61.2, 58.7, 57.1, 56.4, 55.7, 53.0, 52.9, 45.2, 41.7, 40.8, 40.1, 38.0, 35.3, 35.0, 34.7, 31.8, 28.6, 27.0, 26.4, 16.0, 11.4, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.405 min, Purity >98%, m/z (ES+): 1072.6 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C58H78N11O7S, 1072.5806; found, 1072.5883.
(2S,4R)-1-((S)-2-(5-(2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetamido)pentanamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (43)
Degrader 43 was synthesized according to General Procedure B using VH 032 amide-alkylC4-amine. Purification by flash column chromatography and elution with 3–7% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (55 mg, 22%). 1H NMR (600 MHz, DMSO-d6): δ 8.96 (s, 1H), 8.57 (t, J = 6 Hz, 1H), 7.86 (d, J = 9 Hz, 1H), 7.64 (t, J = 6 Hz, 1H), 7.55–7.53 (m, 2H), 7.40 (d, J = 8 Hz, 2H), 7.36 (d, J = 8 Hz, 2H), 7.19 (d, J = 9 Hz, 2H), 7.14 (dd, J = 8, 2 Hz, 1H), 6.82 (d, J = 9 Hz, 2H), 5.17–5.13 (m, 1H), 4.52 (d, J = 9 Hz, 1H), 4.46–4.39 (m, 2H), 4.32 (br s, 1H), 4.23 (t, J = 6 Hz, 2H), 4.19 (dd, J = 16, 5 Hz, 1H), 3.92 (t, J = 6 Hz, 2H), 3.66–3.59 (m, 2H), 3.15 (t, J = 7 Hz, 2H), 3.10 (d, J = 8 Hz, 2H), 3.04 (q, J = 7 Hz, 2H), 2.82 (s, 2H), 2.53 (t, J = 6 Hz, 2H), 2.48–2.24 (m, 15H), 2.21 (s, 3H), 2.10 (s, 6H), 2.05–2.09 (m, 1H), 2.05–2.00 (m, 1H), 1.90–1.85 (m, 1H), 1.82–1.78 (m, 2H), 1.50–1.33 (m, 4H), 0.90 (s, 9H). 3H signal hidden behind residual water signal. 13C NMR (151 MHz, DMSO-d6): δ 172.0, 169.7, 168.9, 164.6, 158.4, 157.1, 155.6, 151.5, 147.7, 142.6, 139.5, 134.5, 133.1, 131.2, 129.6, 129.4, 128.7, 127.4, 122.7, 122.6, 118.9, 116.7, 114.3, 110.3, 68.9, 65.7, 61.3, 58.7, 57.1, 56.4, 56.3, 55.7, 53.0, 52.9, 45.3, 41.6, 40.1, 38.0, 37.9, 35.3, 35.0, 34.6, 31.8, 29.6, 28.6, 27.0, 26.4, 22.9, 16.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.562 min, Purity >96%, m/z (ES+): 1100.6 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C60H82N11O7S, 1100.6119; found, 1100.6189.
(2S,4R)-1-((S)-2-(7-(2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetamido)heptanamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (44)
Degrader 44 was synthesized according to General Procedure B using VH 032 amide-alkylC6-amine. Purification by flash column chromatography and elution with 3–7% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (93 mg, 36%). 1H NMR (600 MHz, DMSO-d6): δ 8.96 (s, 1H), 8.57 (t, J = 6 Hz, 1H), 7.85 (d, J = 9 Hz, 1H), 7.61 (t, J = 6 Hz, 1H), 7.54 (dd, J = 5, 3 Hz, 2H), 7.40 (d, J = 8 Hz, 2H), 7.36 (d, J = 8 Hz, 2H), 7.19 (d, J = 9 Hz, 2H), 7.14 (dd, J = 8, 1.5 Hz, 1H), 6.82 (d, J = 9 Hz, 2H), 5.12 (s, 1H), 4.52 (d, J = 9 Hz, 1H), 4.45–4.39 (m, 2H), 4.32 (br s, 1H), 4.24 (t, J = 6 Hz, 2H), 4.19 (dd, J = 16, 5 Hz, 1H), 3.92 (t, J = 6 Hz, 2H), 3.59–3.66 (m, 2H), 3.13–3.16 (m, 2H), 3.09 (t, J = 7 Hz, 2H), 3.02 (q, J = 7 Hz, 2H), 2.82 (s, 2H), 2.52 (t, J = 6 Hz, 2H), 2.49–2.24 (m, 14H), 2.21 (s, 3H), 2.10 (s, 6H), 2.05–2.09 (m, 1H), 1.99 (d, J = 8 Hz, 1H), 1.85–1.89 (m, 1H), 1.79–1.75 (m, 2H), 1.49–1.39 (m, 2H), 1.35 (t, J = 7 Hz, 2H), 1.20 (d, J = 4 Hz, 4H), 0.90 (s, 9H). 3H signal hidden behind residual water signal. 13C NMR (151 MHz, DMSO-d6): δ 172.1, 172.0, 169.7, 168.9, 164.6, 158.4, 157.1, 155.6, 151.5, 147.7, 142.6, 139.5, 134.5, 133.1, 131.2, 129.6, 129.4, 128.7, 127.4, 122.7, 122.6, 118.9, 116.7, 114.3, 110.3, 68.9, 65.7, 61.3, 58.7, 57.1, 56.4, 56.3, 55.7, 53.0, 52.8, 45.3, 41.7, 40.1, 38.2, 38.0, 35.2, 34.8, 31.8, 29.1, 28.6, 28.4, 27.0, 26.4, 26.2, 25.4, 16.0, 11.3, 10.6. LCMS (5–95% MeCN over 5 min) tR = 3.930 min, Purity = 97%, m/z (ES+): 1128.6 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C62H86N11O7S, 1128.6432; found, 1128.6401.
(2S,4R)-1-((S)-2-(9-(2-(4-(2-(2-(4-(3-(Dimethylamino)propoxy)phenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1H-benzo[d]imidazol-1-yl)ethyl)piperazin-1-yl)acetamido)nonamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (45)
Degrader 45 was synthesized according to General Procedure B using VH 032 amide-alkylC8-amine. Purification by flash column chromatography and elution with 3–7% (7N ammonia in methanol) in dichloromethane, followed by lyophilization, afforded the title compound as a colorless solid (91 mg, 35%). 1H NMR (600 MHz, DMSO-d6): δ 8.98 (s, 1H), 8.58 (t, J = 6 Hz, 1H), 7.86 (d, J = 9 Hz, 1H), 7.62 (t, J = 6 Hz, 1H), 7.54–7.57 (m, 2H), 7.41 (d, J = 8 Hz, 2H), 7.38 (d, J = 8 Hz, 2H), 7.20 (d, J = 8 Hz, 2H), 7.16 (d, J = 8 Hz, 1H), 6.84 (d, J = 9 Hz, 2H), 5.14 (d, J = 3 Hz, 1H), 4.53 (d, J = 9 Hz, 1H), 4.39–4.45 (m, 2H), 4.34 (s, 1H), 4.27–4.18 (m, 3H), 3.94 (t, J = 6 Hz, 2H), 3.61–3.67 (m, 2H), 3.16 (t, J = 8 Hz, 2H), 3.11 (d, J = 9 Hz, 2H), 3.04 (q, J = 7 Hz, 2H), 2.83 (s, 2H), 2.54 (t, J = 6 Hz, 3H), 2.45 (s, 2H), 2.44 (s, 3H), 2.40 (s, 3H), 2.32 (t, J = 7 Hz, 6H), 2.23 (s, 4H), 2.12 (s, 6H), 2.00–2.04 (m, 1H), 1.93–1.86 (m, 1H), 1.83–1.78 (m, 2H), 1.52–1.35 (m, 5H), 1.22 (s, 9H), 0.92 (s, 9H). 13C NMR (151 MHz, DMSO-d6): δ 172.1, 172.0, 169.7, 168.8, 164.6, 158.4, 157.1, 155.6, 151.5, 147.7, 142.6, 139.5, 134.5, 133.1, 131.2, 129.6, 129.4, 128.7, 127.4, 122.7, 122.6, 118.9, 116.7, 114.3, 110.3, 68.9, 65.7, 61.3, 58.7, 57.1, 56.4, 56.3, 55.7, 53.0, 52.8, 45.3, 41.6, 40.1, 38.2, 38.0, 35.2, 34.9, 31.8, 29.2, 28.7, 28.7, 27.0, 26.4, 25.5, 16.0, 11.3, 10.6.LCMS (5–95% MeCN over 5 min) tR = 4.271 min, Purity = 87%, m/z (ES+): 1156.6 [M+H+]+; HRMS-ESI (m/z): [M+H+]+ calculated for C64H90N11O7S, 1156.6745; found, 1156.6660.
AlphaScreen assays were performed at the University of Oxford, with minor modifications from the manufacturer’s protocol (PerkinElmer, USA). Briefly, all reagents were diluted in the recommended buffer (50 mM HEPES, 100 mM NaCl, 0.1% BSA; pH = 7.4) supplemented with 0.05% CHAPS and allowed to equilibrate to ambient temperature prior to addition to plates. Concentrations of the various proteins, peptides, solvents, and compounds are given in the relevant results sections and are expressed as the final concentrations after the addition of all assay components. Four μL of HIS-tagged protein was added to low-volume 384-well plates (ProxiPlatet-384 Plus, PerkinElmer, USA), followed by 4 μL of either buffer, nonbiotinylated peptide, solvent, or compound. Plates were sealed and incubated at ambient temperature for 30 min, before the addition of 4 μL of biotinylated peptide, resealing, and incubation for a further 30 min. Four μL of streptavidin-coated donor beads (25 mg mL–1) and 4 μL of nickel chelate acceptor beads (25 μg/mL) were then added under low light conditions. Plates were foil sealed to protect from light, incubated at ambient temperature for 60 min, and read on a PHERAstar FS plate reader (BMG Labtech, Germany) using an AlphaScreen 680 excitation/570 emission filter set. IC50s were calculated in GraphPad Prism 5 (GraphPad Software, USA). Results for compounds dissolved in DMSO were normalized against corresponding DMSO controls prior to IC50 determination, which are given as the final concentration of the compound in the 20 μL reaction volume. Cell lines. HAP1 cells [male] were obtained from Horizon Discovery and grown in IMDM media supplemented with 10% fetal bovine serum (FBS). All cells were cultured at 37 °C in a 5% CO2 atmosphere.
Protein Expression and Purification
The N-terminal bromodomain of BRD4 used for structural studies was expressed and purified as previously described.49 Briefly, human BRD4 BD1 (residues N44-E168) was subcloned into a pNIC28-Bsa4 vector (N-terminal His6-tag, followed by a TEV protease cleavage site). The expression plasmid was transformed into Escherichia coli BL21(D3)-R3-pRARE2 Rosetta cells. Cells were cultured in Terrific Broth (TB) media at 37 °C to an optical density (OD) of 2.8–3.0, and then expression was induced with 0.5 mM IPTG at 18 °C overnight. Cells were harvested and resuspended in a buffer containing 50 mM HEPES, pH 7.5, 500 mM NaCl, 0.5 mM TCEP, 5% glycerol and subsequently lysed by sonication. The first purification step of the recombinant protein was by Ni2+-affinity chromatography. The hexahistidine tag was then removed by TEV protease cleavage overnight, and the cleaved protein was separated by reverse Ni2+-affinity purification. The protein was further purified by size exclusion chromatography and elution with a HiLoad 16/600 Superdex 75 column with SEC buffer containing 25 mM HEPES, 150 mM NaCl, 0.5 mM TCEP, and 5% glycerol. Quality control was performed by SDS-polyacrylamide gel electrophoresis and ESI-MS.
Crystallization and Structure Determination
Crystals of BRD4 BD1 in complex with degraders were grown using the sitting-drop vapor-diffusion technique at 277 K utilizing a mosquito crystallization robot (TTP Labtech, Royston, U.K.). BRD4 BD1 protein (10 mg/mL in SEC buffer) was incubated with inhibitors at a final concentration of 1–2 mM prior to setting up crystallization trials. Detailed crystallization conditions for each inhibitor/degrader are listed in Supporting Information Table S1. Crystals were cryo-protected with mother liquor supplemented with 23% ethylene glycol and flash-frozen in liquid nitrogen. X-ray diffraction data sets were collected at 100 K at beamline X06DA of the Swiss Light Source, Villigen, Switzerland, and at beamline I03 of the Diamond Light Source, Oxford, United Kingdom. The obtained diffraction data were integrated with either XDS50 or autoPROC51 and scaled with AIMLESS,52 which is part of the CCP4 package.53 Depending on the space group, the structures were then solved by molecular replacement using PHASER54 or by difference Fourier analysis using PHENIX55 with PDB entry 8P9H(56) as a starting model. Structure refinement was performed using iterative cycles of manual model building with COOT57 and refinement with PHENIX. Dictionary files for the compounds were generated using the Grade Web Server (http://grade.globalphasing.org). X-ray data collection and refinement statistics are listed in Supporting Information Table S2.
In Vitro Ubiquitination
50 μL in vitro reactions included the following components and concentrations: UBE1 (E-304–050, R&D Systems), 100 nM; UBE2D1 (E2–616, R&D Systems), 1 μM; CRBN/DDB1/CUL4A(neddylated)/RBX1 complex (E3–441 and E3–500, premixed), 50 nM; FLAG-BRD4 (residues 49–460, SP-600, R&D Systems), 2 μM; ubiquitin (U-100H, R&D Systems), 50 μM; ATP (B-20, R&D Systems), 10 mM; degrader compound, variable concentrations. The buffer used was HEPES, 50 mM, pH 7.5; NaCl, 50 mM; TCEP, 1 mM. A premix containing all components other than degrader and ATP was added to individual wells in a 96-well plate. Degrader was then added at concentrations from 40 nM–40 μM (a no-compound control was also included). The plate was left for 10 min at room temperature and then brought to 37 °C for 5 min before initiating reactions with ATP addition. (dH2O was substituted for ATP in a negative control). Reactions were carried out for 60 min then terminated with reducing SDS sample buffer. The same assay was performed for VHL-recruiting degraders, replacing the CRBN/DDB1/CUL4A(neddylated)/RBX1 complex with VHL/Elongin B/Elongin C/CUL2/RBX1 complex (E3–600 and E3–420, R&D Systems, premixed), 100 nM. Analysis was on a WES (ProteinSimple) with 12–230 kDa, 25 capillary module (SM-W004, ProteinSimple): diluted samples 1:60 and used 3 μL per analysis (<4 ng FLAG-BRD4 per capillary). Anti-FLAG primary (MAB8529, R&D Systems) and Anti-Rabbit Detection Module (DM-001, ProteinSimple).
Acknowledgments
A.K.E., J.S., T.J.T.C., and S.M. thank EPSRC and Bio-Techne (Tocris) for an iCASE PhD award (EP/N509784/1). M.B.R. and J.S. thank Blood Cancer UK (grant # 23015) for funding. A.C.J. acknowledges funding by the German Research Foundation (DFG) grant JO 1473/1-3 and the German Cancer Aid grant TACTIC. D.-I.B., J.B., O.F., and A.C.J. are grateful for support by the Structural Genomics Consortium (SGC), a registered charity (No: 1097737) that received funds from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Genome Canada through Ontario Genomics Institute, [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA, Pfizer, and Takeda. We thank the staff at SLS beamline X06DA and DLS beamline I03 for help and advice during data collection.
Glossary
Abbreviations Used
- BRD4 BD1
Bromodomain-containing protein 4 first bromodomain
- CBP/EP300
CREB-binding protein/E1A-binding protein P300
- CRBN
Cereblon
- FRET
Fluorescence resonance energy transfer
- IRF4
Interferon regulatory factor 4
- c-MYC
Myelocytomatosis oncogene cellular homologue
- VHL
Von Hippel–Lindau
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c00395.
The ESI contains 1H, 13C NMR, HPLC purity, and mass spectral data of final compounds (PDF)
Molecular Formula Strings (CSV)
The atomic coordinates and structure factors of the first bromodomain of human BRD4 in complex with different degraders have been deposited in the Protein Data Bank (PDB) under accession codes 9F1J (14), 9F1K (29), 9F1L (34), 9F1M (44), and 9F1N (45) (PDB)
Author Contributions
H.J.M. and J.S. devised the project, performed data analysis and supervised, along with G.P.M., the practical chemistry aspects of this work by A.K.E., who contributed to manuscript preparation alongside H.J.M., G.P.M. and J.S. X-ray studies were performed by A.C.J. and D.-I.B. All other authors contributed to biochemical and cell-based biological assays. All authors offered critical comments.
The authors declare no competing financial interest.
Supplementary Material
References
- Das S. K.; Lewis B. A.; Levens D. MYC: a complex problem. Trends Cell Biol. 2023, 33 (3), 235–246. 10.1016/j.tcb.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V. MYC on the Path to Cancer. Cell 2012, 149 (1), 22–35. 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M.; Arvanitis C.; Chu K.; Dewey W.; Leonhardt E.; Trinh M.; Sundberg C. D.; Bishop J. M.; Felsher D. W. Sustained Loss of a Neoplastic Phenotype by Brief Inactivation of MYC. Science 2002, 297 (5578), 102–104. 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- Soucek L.; Whitfield J.; Martins C. P.; Finch A. J.; Murphy D. J.; Sodir N. M.; Karnezis A. N.; Swigart L. B.; Nasi S.; Evan G. I. Modelling Myc inhibition as a cancer therapy. Nature 2008, 455 (7213), 679–683. 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L.; Helmer-Citterich M.; Sacco A.; Jucker R.; Cesareni G.; Nasi S. Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene 1998, 17 (19), 2463–2472. 10.1038/sj.onc.1202199. [DOI] [PubMed] [Google Scholar]
- Madden S. K.; de Araujo A. D.; Gerhardt M.; Fairlie D. P.; Mason J. M. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20 (1), 3 10.1186/s12943-020-01291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore J. E.; Issa G. C.; Lemieux M. E.; Rahl P. B.; Shi J.; Jacobs H. M.; Kastritis E.; Gilpatrick T.; Paranal R. M.; Qi J.; et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell 2011, 146 (6), 904–917. 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P.; Qi J.; Picaud S.; Shen Y.; Smith W. B.; Fedorov O.; Morse E. M.; Keates T.; Hickman T. T.; Felletar I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468 (7327), 1067–1073. 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer A. L.; Emre N. C. T.; Lamy L.; Ngo V. N.; Wright G.; Xiao W.; Powell J.; Dave S.; Yu X.; Zhao H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454 (7201), 226–231. 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conery A. R.; Centore R. C.; Neiss A.; Keller P. J.; Joshi S.; Spillane K. L.; Sandy P.; Hatton C.; Pardo E.; Zawadzke L.; et al. Bromodomain inhibition of the transcriptional coactivators CBP/EP300 as a therapeutic strategy to target the IRF4 network in multiple myeloma. eLife 2016, 5, e10483 10.7554/eLife.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara H.; Sasaki M.; Mitachi T.; Oike T.; Higuchi S.; Tominaga Y.; Kohno T. Targeting p300 Addiction in CBP-Deficient Cancers Causes Synthetic Lethality by Apoptotic Cell Death due to Abrogation of MYC Expression. Cancer Discovery 2016, 6 (4), 430–445. 10.1158/2159-8290.CD-15-0754. [DOI] [PubMed] [Google Scholar]
- Agnarelli A.; Mitchell S.; Caalim G.; Wood C. D.; Milton-Harris L.; Chevassut T.; West M. J.; Mancini E. J. Dissecting the impact of bromodomain inhibitors on the Interferon Regulatory Factor 4-MYC oncogenic axis in multiple myeloma. Hematol. Oncol. 2022, 40 (3), 417–429. 10.1002/hon.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Békés M.; Langley D. R.; Crews C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discovery 2022, 21 (3), 181–200. 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.; Crews C. M. PROTACs: past, present and future. Chem. Soc. Rev. 2022, 51 (12), 5214–5236. 10.1039/D2CS00193D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M. J.; Crews C. M. Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chem. Biol. 2021, 2 (3), 725–742. 10.1039/D1CB00011J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.; Saboo S.; Zhou L.; Askin S.; Bak A. Early evaluation of opportunities in oral delivery of PROTACs to overcome their molecular challenges. Drug Discovery Today 2024, 29 (2), 103865 10.1016/j.drudis.2023.103865. [DOI] [PubMed] [Google Scholar]
- Li M.; Zhi Y.; Liu B.; Yao Q. Advancing Strategies for Proteolysis-Targeting Chimera Design. J. Med. Chem. 2023, 66 (4), 2308–2329. 10.1021/acs.jmedchem.2c01555. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Wang S.; Han S.; Zhao Y.; Yu C.; Liu H.; Li N. Targeted protein degrader development for cancer: advances, challenges, and opportunities. Trends Pharmacol. Sci. 2023, 44 (5), 303–317. 10.1016/j.tips.2023.03.003. [DOI] [PubMed] [Google Scholar]
- Maple H. J.; Clayden N.; Baron A.; Stacey C.; Felix R. Developing degraders: principles and perspectives on design and chemical space. MedChemComm 2019, 10 (10), 1755–1764. 10.1039/C9MD00272C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike A.; Williamson B.; Harlfinger S.; Martin S.; McGinnity D. F. Optimising proteolysis-targeting chimeras (PROTACs) for oral drug delivery: a drug metabolism and pharmacokinetics perspective. Drug Discovery Today 2020, 25 (10), 1793–1800. 10.1016/j.drudis.2020.07.013. [DOI] [PubMed] [Google Scholar]
- Kelm J. M.; Pandey D. S.; Malin E.; Kansou H.; Arora S.; Kumar R.; Gavande N. S. PROTAC’ing oncoproteins: targeted protein degradation for cancer therapy. Mol. Cancer 2023, 22 (1), 62 10.1186/s12943-022-01707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein V. G.; Bond A. G.; Craigon C.; Lokey R. S.; Ciulli A. Amide-to-Ester Substitution as a Strategy for Optimizing PROTAC Permeability and Cellular Activity. J. Med. Chem. 2021, 64 (24), 18082–18101. 10.1021/acs.jmedchem.1c01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares A.; Miah A. H.; Smith I. E. D.; Rackham M.; Thawani A. R.; Cryan J.; Haile P. A.; Votta B. J.; Beal A. M.; Capriotti C.; et al. Extended pharmacodynamic responses observed upon PROTAC-mediated degradation of RIPK2. Commun. Biol. 2020, 3 (1), 140 10.1038/s42003-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.; To C.; De Clercq D. J. H.; Park E.; Ponthier C. M.; Shin B. H.; Mushajiang M.; Nowak R. P.; Fischer E. S.; Eck M. J.; et al. Mutant-Selective Allosteric EGFR Degraders are Effective Against a Broad Range of Drug-Resistant Mutations. Angew. Chem., Int. Ed. 2020, 59 (34), 14481–14489. 10.1002/anie.202003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson D. P.; Smith B. E.; Burslem G. M.; Buhimschi A. D.; Hines J.; Jaime-Figueroa S.; Wang J.; Hamman B. D.; Ishchenko A.; Crews C. M. Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem. Biol. 2018, 25 (1), 78–87 e75. 10.1016/j.chembiol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Wang M.; Wu D.; Bai L.; Xu T.; Metwally H.; Wang Y.; McEachern D.; Zhao L.; Li R.; et al. Discovery of CBPD-268 as an Exceptionally Potent and Orally Efficacious CBP/p300 PROTAC Degrader Capable of Achieving Tumor Regression. J. Med. Chem. 2024, 67, 5275. 10.1021/acs.jmedchem.3c02124. [DOI] [PubMed] [Google Scholar]
- Cheng-Sánchez I.; Gosselé K. A.; Palaferri L.; Kirillova M. S.; Nevado C. Discovery and Characterization of Active CBP/EP300 Degraders Targeting the HAT Domain. ACS Med. Chem. Lett. 2024, 15 (3), 355–361. 10.1021/acsmedchemlett.3c00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q.; Li J.; Deng Y.; Zhou R.; Wang B.; Wang Y.; Zhang M.; Huang X.; Li Y. Discovery of Novel PROTAC Degraders of p300/CBP as Potential Therapeutics for Hepatocellular Carcinoma. J. Med. Chem. 2024, 67 (4), 2466–2486. 10.1021/acs.jmedchem.3c01468. [DOI] [PubMed] [Google Scholar]
- Thomas J. E. II; Wang M.; Jiang W.; Wang M.; Wang L.; Wen B.; Sun D.; Wang S. Discovery of Exceptionally Potent, Selective, and Efficacious PROTAC Degraders of CBP and p300 Proteins. J. Med. Chem. 2023, 66 (12), 8178–8199. 10.1021/acs.jmedchem.3c00492. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Wang Z.; Li Y.; Peng H.; Liu J.; Zhang J.; Xiao X. The Role of CREBBP/EP300 and Its Therapeutic Implications in Hematological Malignancies. Cancers 2023, 15 (4), 1219. 10.3390/cancers15041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannam R.; Sayilgan J.; Ojeda S.; Karakyriakou B.; Hu E.; Kreuzer J.; Morris R.; Herrera Lopez X. I.; Rai S.; Haas W.; et al. Targeted degradation of the enhancer lysine acetyltransferases CBP and p300. Cell Chem. Biol. 2021, 28 (4), 503–514.e512. 10.1016/j.chembiol.2020.12.004. [DOI] [PubMed] [Google Scholar]
- Ding M.; Shao Y.; Sun D.; Meng S.; Zang Y.; Zhou Y.; Li J.; Lu W.; Zhu S. Design, synthesis, and biological evaluation of BRD4 degraders. Bioorg. Med. Chem. 2023, 78, 117134 10.1016/j.bmc.2022.117134. [DOI] [PubMed] [Google Scholar]
- Kuchta R.; Heim C.; Herrmann A.; Maiwald S.; Ng Y. L. D.; Sosic I.; Keuler T.; Kronke J.; Gutschow M.; Hartmann M. D.; Steinebach C. Accessing three-branched high-affinity cereblon ligands for molecular glue and protein degrader design. RSC Chem. Biol. 2023, 4 (3), 229–234. 10.1039/D2CB00223J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R. P.; Ragosta L.; Huerta F.; Liu H.; Ficarro S. B.; Cruite J. T.; Metivier R. J.; Donovan K. A.; Marto J. A.; Fischer E. S.; et al. Development of a covalent cereblon-based PROTAC employing a fluorosulfate warhead. RSC Chem. Biol. 2023, 4 (11), 906–912. 10.1039/D3CB00103B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Li B.; Zhang W.; Li Z.; Jiang B.; Hou S.; Ma S.; Qin C. Lethal activity of BRD4 PROTAC degrader QCA570 against bladder cancer cells. Front. Chem. 2023, 11, 1121724 10.3389/fchem.2023.1121724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R. P.; DeAngelo S. L.; Buckley D.; He Z.; Donovan K. A.; An J.; Safaee N.; Jedrychowski M. P.; Ponthier C. M.; Ishoey M.; et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol. 2018, 14 (7), 706–714. 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengerle M.; Chan K.-H.; Ciulli A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem. Biol. 2015, 10 (8), 1770–1777. 10.1021/acschembio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Qian Y.; Altieri M.; Dong H.; Wang J.; Raina K.; Hines J.; Winkler J. D.; Crew A. P.; Coleman K.; Crews C. M. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015, 22 (6), 755–763. 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C.; Stickler S.; Rath B.; Teufelsbauer M.; Weigl L.; Hohenegger M.; Hamilton G. BRD4-targeting PROTACs Synergize With Chemotherapeutics Against Osteosarcoma Cell Lines. Anticancer Res. 2024, 44 (3), 971–980. 10.21873/anticanres.16892. [DOI] [PubMed] [Google Scholar]
- Min J.; Mayasundari A.; Keramatnia F.; Jonchere B.; Yang S. W.; Jarusiewicz J.; Actis M.; Das S.; Young B.; Slavish J.; et al. Phenyl-Glutarimides: Alternative Cereblon Binders for the Design of PROTACs. Angew. Chem., Int. Ed. 2021, 60 (51), 26663–26670. 10.1002/anie.202108848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.; Nikonova A. S.; Zhang P.; Deneka A. Y.; Fitzgerald M. E.; Michael R. E.; Lee L.; Lilly A. C.; Fisher S. L.; Phillips A. J.; et al. Evaluation of the Small-molecule BRD4 Degrader CFT-2718 in Small-cell Lung Cancer and Pancreatic Cancer Models. Mol. Cancer Ther. 2021, 20 (8), 1367–1377. 10.1158/1535-7163.MCT-20-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Jiang Y.; Hong Y.; Chu X.; Zhang Z.; Tao Y.; Fan Z.; Bai Z.; Li X.; Chen Y.; et al. BRD4 PROTAC degrader ARV-825 inhibits T-cell acute lymphoblastic leukemia by targeting ’Undruggable’ Myc-pathway genes. Cancer Cell Int. 2021, 21 (1), 230 10.1186/s12935-021-01908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekler E. L. P.; Pellegrino J. A.; Lanz T. A.; Denny R. A.; Flick A. C.; Coe J.; Langille J.; Basak A.; Liu S.; Stock I. A.; et al. Transcriptional Profiling of a Selective CREB Binding Protein Bromodomain Inhibitor Highlights Therapeutic Opportunities. Chem. Biol. 2015, 22 (12), 1588–1596. 10.1016/j.chembiol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Edmonds A. K.; Oakes C. S.; Hassell-Hart S.; Bruyere D.; Tizzard G. J.; Coles S. J.; Felix R.; Maple H. J.; Marsh G. P.; Spencer J. Scale-up and optimization of the synthesis of dual CBP/BRD4 inhibitor ISOX-DUAL. Org. Biomol Chem. 2022, 20 (19), 4021–4029. 10.1039/D2OB00609J. [DOI] [PubMed] [Google Scholar]
- Brennan P.Isoxazole inhibitors of Bromodomains. In RSC Advances in Synthesis and Medicinal Chemistry Conference BioPark, Welwyn Garden City, UK, 2012.
- Geiger T. M.; Walz M.; Meyners C.; Kuehn A.; Dreizler J. K.; Sugiarto W. O.; Maciel E. V. S.; Zheng M.; Lermyte F.; Hausch F. Discovery of a Potent Proteolysis Targeting Chimera Enables Targeting the Scaffolding Functions of FK506-Binding Protein 51 (FKBP51). Angew. Chem., Int. Ed. 2024, 63 (3), e202309706 10.1002/anie.202309706. [DOI] [PubMed] [Google Scholar]
- Yang J.; Li Y.; Aguilar A.; Liu Z.; Yang C. Y.; Wang S. Simple Structural Modifications Converting a Bona fide MDM2 PROTAC Degrader into a Molecular Glue Molecule: A Cautionary Tale in the Design of PROTAC Degraders. J. Med. Chem. 2019, 62 (21), 9471–9487. 10.1021/acs.jmedchem.9b00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott M.; Yang J.; Tumber T.; Fedorov O.; Uttarkar S.; Filippakopoulos P.; Picaud S.; Keates T.; Felletar I.; Ciulli A.; et al. Bromodomain-peptide displacement assays for interactome mapping and inhibitor discovery. Mol. BioSyst. 2011, 7 (10), 2899–2908. 10.1039/c1mb05099k. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zegar T.; Weiser T.; Hamdan F. H.; Berger B. T.; Lucas R.; Balourdas D. I.; Ladigan S.; Cheung P. F.; Liffers S. T.; et al. Characterization of a dual BET/HDAC inhibitor for treatment of pancreatic ductal adenocarcinoma. Int. J. Cancer 2020, 147 (10), 2847–2861. 10.1002/ijc.33137. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Xds. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66 (Pt 2), 125–132. 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonrhein C.; Flensburg C.; Keller P.; Sharff A.; Smart O.; Paciorek W.; Womack T.; Bricogne G. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67 (Pt 4), 293–302. 10.1107/S0907444911007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. R.; Murshudov G. N. How good are my data and what is the resolution?. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2013, 69 (Pt 7), 1204–1214. 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M. D.; Ballard C. C.; Cowtan K. D.; Dodson E. J.; Emsley P.; Evans P. R.; Keegan R. M.; Krissinel E. B.; Leslie A. G.; McCoy A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67 (Pt 4), 235–242. 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. J.; Grosse-Kunstleve R. W.; Adams P. D.; Winn M. D.; Storoni L. C.; Read R. J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40 (Pt 4), 658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D.; Afonine P. V.; Baker M. L.; Bunkoczi G.; Chen V. B.; Croll T. I.; Hintze B.; Hung L. W.; Jain S.; McCoy A. J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr., Sect. D: Struct. Biol. 2019, 75 (Pt 10), 861–877. 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer N.; Balourdas D.-I.; Schneider J. R.; Zhang X.; Berger L. M.; Berger B.-T.; Schwalm M. P.; Klopp N. A.; Siveke J. T.; Knapp S.; Joerger A. C. Development of Potent Dual BET/HDAC Inhibitors via Pharmacophore Merging and Structure-Guided Optimization. ACS Chem. Biol. 2024, 19 (2), 266–279. 10.1021/acschembio.3c00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. Features and development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66 (Pt 4), 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.