Abstract
An emerging pattern of similarity in medical case reports led to a project to compare the phylogenetic affinities of two well-known tropical fungal opportunistic pathogens, Cylindrocarpon lichenicola and Acremonium falciforme, to members of the Fusarium solani species complex. C. lichenicola and A. falciforme, despite their deviating conidial morphologies, were shown via sequencing of the ribosomal large subunit to be well instituted within a clade mainly consisting of typical F. solani strains and other species until recently considered variants of F. solani. The original name Fusarium lichenicola C. B. Massalongo is reestablished, and the new combination F. falciforme is made. Recognition of these species as fusaria is necessary for correct interpretation of current and future molecular diagnostic tests. Reevaluation of species morphology in light of the molecular findings showed that certain features, especially elongate filiform conidiophores with integrated terminal phialides, facilitate correct microscopic classification of these atypical Fusarium species. There is a strong and underrecognized overlap in the spectra of cases caused by members of the F. solani clade, particularly ocular infections, mycetomas, and, in the neutropenic host, disseminated and other serious systemic infections. A novel synthesis of case reports shows that patients from areas with warm climates may develop a distinctive fusarial intertrigo caused by F. solani, Fusarium lichenicola, or Fusarium oxysporum.
The fungal genus Fusarium in recent years has been shown to include several well-distinguished major phylogenetic clades (40, 44, 51). In general, each of the clades comprehended within this large anamorph (separately named asexual reproductive form) genus corresponds to one or, exceptionally, two teleomorph (separately named sexual phenotype) genera. At least two of the clades contain medically important members: the clade corresponding to the teleomorph genus Gibberella and another unified clade corresponding to two teleomorphs, Neocosmospora and the recently delineated Haematonectria (51). The first clade, Gibberella, includes the important opportunists Fusarium oxysporum and Fusarium verticillioides (Fusarium moniliforme), as well as several infrequently etiologically significant fungi such as Fusarium proliferatum, Fusarium napiforme, and Fusarium incarnatum (Fusarium semitectum) (44). It also includes a large number of species not known to be connected with human or animal pathogenicity; many of these have close associations with specific plant hosts. Among members of the second clade, Neocosmospora vasinfecta is known rarely to cause opportunistic infections (5), but most medically important isolates in the clade are anamorphic fungi that are referred to as the “Fusarium solani species complex” (41). Teleomorphs of these fungi, where known, correspond to the genus Haematonectria. In keeping with long-standing tradition, these are all reported under the aggregate name F. solani, even though the existence of at least seven separate, noninterbreeding biological species within this group has been known since the early 1960s (34). Fungi named F. solani are now known to belong to at least 26 separate phylogenetic species (41), most of which are unnamed or named only as plant-pathogenic formae speciales. How many of these species may be associated with mammalian infection is not known. In addition, F. solani isolates belonging to as yet incompletely characterized groups may raise the number of phylogenetic species in this group to more than 50 (51).
While reviewing medical case literature, we noticed that there was a striking correspondence between many of the cases whose causes were attributed to Cylindrocarpon lichenicola, a mainly tropical agent of human opportunistic infection, and cases described to be caused by the F. solani complex. This led to an investigation to determine whether this apparent correspondence reflected a close phylogenetic relationship or merely an ecological convergence. At the same time, consideration of other human opportunists that might be related to Fusarium species led to investigation of the uncommon tropical mycetoma agent Acremonium falciforme, a species with curved and sometimes pointed conidia morphologically reminiscent of Fusarium. The possible affinity of this species with the genus Fusarium had previously been noted by Gams (10). The phylogenetic studies presented here show that both C. lichenicola and A. falciforme belong to the clade containing F. solani, Haematonectria, and Neocosmospora (collectively referred to hereafter as “the F. solani clade”). This recognition improves our overall epidemiological understanding of these fungi and facilitates and clarifies both morphological and molecular laboratory identification.
MATERIALS AND METHODS
The strains studied were obtained from the collection of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands.
DNA extraction and amplification.
DNA was extracted with a FastDNA kit (Qbiogene, Heidelberg, Germany) from mycelium grown for 3 to 5 days in liquid Complete Medium (46). The large-subunit (LSU) region of ribosomal DNA (rDNA) was amplified with primers V9G (7) and LR5 (57). The components for the PCR were used as described by Schroers (52). The PCR program was 60 s at 94°C (initial denaturation); 35 cycles of 35 s at 94°C (denaturation), 50 s at 55°C (annealing), and 120 s at 72°C (elongation); and 6 min at 72°C (final elongation) followed by chilling to 4°C. The PCR products were purified with a GFX purification kit (Amersham Pharmacia Biotech Inc., Roosendaal, The Netherlands) and visualized on an electrophoresis gel after ethidium bromide staining. The rDNA was sequenced with a BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and analyzed on an ABI Prism 3700 instrument (Applied Biosystems) by using the standard conditions recommended by the vendor. The primers used in the sequence reaction were ITS1 and ITS4 (58), NL1 and NL4 (40), and LR5.
DNA data analysis.
Sequence chromatographs were assembled and edited with SeqmanII software (DNAStar, Inc., Madison, Wis.) and aligned with sequences downloaded from GenBank (http://www.ncbi.nlm.nih.gov/), National Center for Biotechnology Information, Bethesda, Md. (Table 1). The alignment was initially performed with the ClustalX program (version 1.8; ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX) and adjusted manually with the Megalign program (DNAStar). The phylogenetic analysis was performed with a part of the LSU rDNA available for all accessions. This part is flanked by positions 116 and 615 in Fig. 1 in the report by Guadet et al. (16), including domain D1 and most of domain D2. A parsimony analysis was performed with the software package PAUP (version 4.0b8) (54). Gaps were coded as missing data; characters were defined as unordered and equally weighted; characters not informative to the parsimony analysis were excluded; heuristic searches of parsimonious trees were performed for all sequences with random sequence addition and 1,000 replicates, using the starting trees from the stepwise addition, tree bisection-reconnection as the swapping algorithm, and all optimal trees for the next swapping round; and branch robustness was tested by use of 100 replications of such searches based on bootstrapped data sets with random sequence addition and 10 replicates per search.
TABLE 1.
Taxa of the members of the families Nectriaceae (Hypocreales) and Phyllachoraceae (Phyllachorales) analyzed
| Taxona | Natural affiliation (major generic or sub- generic clade level) | GenBank accession no. | Reference for sequence (per GenBank record) | Strain no.b | Substratum strain was isolated from (new sequences only) | Geographic origin (new sequences only) |
|---|---|---|---|---|---|---|
| F. napiforme Marasas et al. | G. fujikuroi complex | U34541 | 44 | NRRL 13604 | ||
| Fusarium brevicatenulatum Nirenberg, O'Donnell, Kroschel et Andrianaivo | G. fujikuroi complex | U61649 | 44 | NRRL 25446 | ||
| F. verticillioides (Sacc.) Nirenberg | G. fujikuroi complex | U34526 | 44 | NRRL 22172 | ||
| Fusarium nygamai Burgess et Trimboli | G. fujikuroi complex | U34539 | 42 | NRRL 13448 | ||
| Fusarium fujikuroi Nirenberg | G. fujikuroi complex | U34528 | 42 | NRRL 13566 | ||
| Fusarium phyllophilum Nirenberg et O'Donnell | G. fujikuroi complex | U34545 | 42 | NRRL 13617 | ||
| F. proliferatum (Mats.) Nirenberg ex Gerlach et Nirenberg | G. fujikuroi complex | AJ271215 | M. Y. Abdalla et al. (unpublished) | Item 2386 | ||
| F. oxysporum Schlecht.: Fr. | Gibberella clade, F. oxysporum complex | AF060383 | E. Cigelnik (unpublished) | NRRL 26409 | ||
| F. oxysporum | U34542 | 42 | NRRL 13307 | |||
| F. oxysporum | U34537 | 42 | NRRL 22902 | |||
| Fusarium nisikadoi T. Aoki et Nirenberg | G. fujikuroi complex | U61659 | 44 | NRRL 25179 | ||
| Fusarium redolens Wollenw. | Gibberella clade, F. oxysporum complex | U34536 | 42 | NRRL 22901 | ||
| Fusarium camptoceras Wollenw. et Reinking | Gibberella clade, Fusarium section Fusarium (formerly section Arthrosporiella) | U88102 | 40 | NRRL 13382 | ||
| Fusarium culmorum (W. G. Smith) Sacc. | Gibberella clade, Fusarium section Fusarium (formerly section Arthrosporiella) | AF006322 | 43 | NRRL 25475 | ||
| Fusarium flocciferum Corda | Gibberella clade, Fusarium section Fusarium (formerly section Arthrosporiella) | AF006323 | 43 | NRRL 25471 | ||
| Fusarium sporotrichioides Sherbakoff | Gibberella clade, Fusarium section Fusarium (formerly section Arthrosporiella) | AF006328 | 43 | NRRL 25479 | ||
| Albonectria rigidiuscula (Berk. et Br.) Rossman et Samuels | Albonectria | U88104 | 40 | NRRL 13412 | ||
| Albonectria albosuccinea (Pat.) Rossman & Samuels | Albonectria | U34554 | 40 | NRRL 20459 | ||
| “Nectria ventricosa” C. Booth, anamorph Fusarium ventricosum Appel et Wollenw. | Fusarium section Ventricosum | U88118 | 40 | NRRL 13953 | ||
| “N. ventricosa” | L36613 | 40 | NRRL 20846 | |||
| Cosmospora episphaeria (Tode: Fr.) Rossman et Samuels, anamorph F. aquaeductuum (Radlk. et Rabenh.) Lagerh. var. medium Wollenw. | Cosmospora/Fusarium section of Eupionnotes | U88100 | 40 | NRRL 20687 | ||
| Cosmospora vilior (Starbäck) Rossman et Samuels, anamorph Acremonium berkeleyanum (P. Karsten) W. Gams | Cosmospora | U57348 | A. E. Glenn and C. W. Bacon (unpublished) | ATCC 16217 | ||
| Cosmospora flammea (Tul. et C. Tul.) Rossman et Samuels, anamorph F. coccophilum (Desm.) Wollenw. et Reink. | Cosmospora/Fusarium section Eupionnotes | U88103 | 40 | NRRL 20441 | ||
| Fusarium merismoides Corda var. violaceum Gerlach | Cosmospora/Fusarium section Eupionnotes | U88112 | 40 | NRRL 20896 | ||
| F. merismoides | Cosmospora/Fusarium section Eupionnotes | U88111 | 40 | NRRL 20895 | ||
| F. merismoides var. crassa Wollenw. | Cosmospora/Fusarium section Eupionnotes | U88110 | 40 | NRRL 20894 | ||
| F. martiiphaseoli Burk (F. solani (Mart.) Sacc. f. sp. phaseoli (Burk.) Snyd. et Hans.) | Haematonectria/Fusarium section Martiella/F. solani complex | L36629 | 45 | NRRL 22292/PICK> | ||
| F. martiiphaseoli | L36632 | 45 | NRRL 22678 | |||
| F. solani (Mart.) Sacc. | Haematonectria/Fusarium section Martiella/F. solani complex | AY097316c | CBS 490.63, received as Cephalosporium keratoplasticum Morikawa | Human, causing intertrigo | Japan | |
| F. solani | AY097318c | CBS 109696 | Human, causing eye infection | Ontario, Canada | ||
| H. haematococca (Berk. & Br.) Samuels et Nirenberg mating population V, anamorph F. solani var. petroliphilum Chen (F. solani f. sp. cucurbitae race 2) | Haematonectria/Fusarium section Martiella/F. solani complex | L36623 | 45 | NRRL 22141 | ||
| H. haematococca mating population VI, anamorph Fusarium lathyri Taub. (F. solani f. sp. pisi (F. R. Jones) Snyd. et Hans.) | Haematonectria/Fusarium section Martiella/F. solani complex | L36622 | 45 | NRRL 22278 | ||
| H. ipomoeae (Halst.) Samuels et Nirenberg, anamorph F. striatum Sherb. | Haematonectria/Fusarium section Martiella/Fusarium solani complex | U88106 | 40 | NRRL 13952 | ||
| F. solani (Mart.) Sacc. | Haematonectria/Fusarium section Martiella/F. solani complex | AY097317c | CBS 102256 | Human, blood | Germany | |
| F. solani (Mart.) Sacc. | AY097325d | CBS 115.40, ex-type strain of Cylindrocarpon tonkinense Bugnicourt | Musa sapientum | Vietnam | ||
| A. falciforme (Carrión) W. Gams | Haematonectria/Fusarium section of the Martiella/F. solani complex | AY097326d | CBS 101427 | Human, white-grain mycetoma | United States (patient from India) | |
| A. falciforme | AY097319c | CBS 475.67, ex-type strain of A. falciforme | Human, white-grain mycetoma | Puerto Rico | ||
| N. vasinfecta E. F. Sm. | Neocosmospora/Fusarium section Martiella/F. solani complex | U47836 | J. W. Spatafora (unpublished) | RSA 1898 | ||
| N. vasinfecta | U17406 | 47 | JP 963 | |||
| C. lichenicola (C. B. Massalongo) D. Hawksw. | Haematonectria/Fusarium section Martiella/F. solani complex | AY097320d | CBS 483.96 | Soil | Japan | |
| C. lichenicola | AY097324d | CBS 109048 (31) | Human, cornea | Argentina | ||
| C. lichenicola | AY097322d | CBS 238.58 | Soil | Tahiti | ||
| C. lichenicola | AY097321d | CBS 623.92 | Human, necrotic wounds on foot of patient under chemotherapy | Germany | ||
| C. lichenicola | AY097323d | CBS 279.34 | Human, skin | Somalia | ||
| F. buxicola Sacc. | Fusarium section Macroconia | U88125 | 40 | NRRL 20474 | ||
| Nectria pseudotrichia Berk. et M. A. Curtis, anamorph Tubercularia lateritia (Berk.) Seif. | Nectria sensu stricto | U17410 | 47 | AR 1755 | ||
| Nectria cinnabarina (Tode: Fr.) Fr., anamorph Tubercularia vulgaris Tode: Fr. | Nectia sensu stricto | U00748 | 47 | GJS2 89-107 | ||
| Cylindrocladium floridanum Sobers et C. P. Seymour | Calonectria | U17408 | 47 | ATCC 22677 | ||
| Calonectria morganii Crous, Alfenas et Wingfield | Calonectria | U17409 | 47 | ATCC 11614 | ||
| Calonectria pyrochroa (Desm.) Sacc., anamorph Cylindrocladium ilicicola (Hawley) Boedijn et Reitsma | Calonectria | U88097 | 40 | NRRL 13941 | ||
| Leuconectria clusiae (Samuels et C. T. Rog.) Rossm. et al., anamorph Gliocephalotrichum bulbilium, J. J. Ellis et Hesseltine | Leuconectria | U17412 | 47 | AR 2706 | ||
| N. radicicola (Gerlach et L. Nilsson) Mantiri et Samuels, anamorph Cylindrocarpon destructans (Zins.) Scholt | Neonectria | U17415 | 47 | AR 2553 | ||
| V. dahliae Klebahn | Sordariomycetes, Phyllachorales, Phyllachoraceae (outgroup) | U17425 | 47 | ATCC 16535 | ||
| P. cucumerina (Lindfors) W. Gams, anamorph Plectosporium tabacinum (van Beyma) M. E. Palm, W. Gams et Nirenberg | Sordariomycetes, Phyllachorales, Phyllachoraceae (outgroup) | U17399 | 47 | ATCC 96328 |
The arrangement of the isolates is as in the dendrogram in Fig. 1.
NRRL, USDA Northern Regional Research Laboratory, Peoria, Ill.; ATCC, American Type Culture Collection, Manassas, Va.; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; other acronyms represent the collection designations of particular researchers or institutions and do not represent public collections.
Newly reported sequence including a partial sequence of the 28S RNA gene.
Newly reported sequence, including the complete sequences of internal transcribed spacer 1, the 5.8S rRNA gene, and internal transcribed spacer 2 and the partial sequence of the 28S rRNA gene.
FIG. 1.
Phylogenetic relationships within the genus Fusarium showing the positions of the members of the Fusarium solani clade and its substituents Acremonium falciforme and Cylindrocarpon lichenicola in relation to those of other pathogenic and nonpathogenic species. One of 60 most parsimonious trees derived from parsimony analysis of positions 116 to 615 of the 28S rDNA is shown. See the text for methods, scores, and other details. Abbreviations for genus names: Alb., Albonectria; Acr., Acremonium; Cal., Calonectria; Ccl., Cylindrocladium; Cos., Cosmospora; Cyl., Cylindrocarpon; F., Fusarium; H., Haematonectria; L., Leuconectria; Nec., Nectria; Nen., Neonectria; Neo., Neocosmospora; P., Plectosphaerella; V., Verticillium.
Morphological analysis was conducted with an Olympus BX50 microscope equipped with an Olympus DP10 digital camera unit (Olympus Optical Co. Europe, Hamburg, Germany). Colonies were grown on synthetic nutrient agar (38), oatmeal agar (11), and malt extract agar (Oxoid, Basingstoke, United Kingdom) and were prepared for photography after 7 to 10 days at room temperature (22°C).
RESULTS
Heuristic searches of the shortest trees resulted in 60 trees of equal length. The trees, one of which is shown in Fig. 1, differed by minor changes mainly within the Gibberella fujikoroi group. Tree scores are as follows: length = 343; consistency index = 0.434; retention index = 0.794; rescaled consistency index = 0.345; homoplasy index = 0.566. Of the 493 alignment positions chosen, 114 were informative to the parsimony analysis and were included in the analysis. Two multiple gaps were encountered in the data set; both were caused by sequences of the two related taxa used as the outgroup, Verticillium dahliae and Plectosphaerella cucumerina.
The F. solani clade appears in Fig. 1 as a moderately well supported branch, with 67% bootstrap support, compared to 77 and 54% bootstrap supports for the well-established Cosmospora and G. fujikoroi clades, respectively, in the same data set. Included within the F. solani clade, with bootstrap support in the same range, are the opportunistic pathogens C. lichenicola and N. vasinfecta. The LSU sequences of the ex-type strain of A. falciforme and a recently obtained strain were identical, and the two strains were on a branch well separated from other species. The small number of F. solani comparison strains from humans with confirmed cases of infection all clustered into “F. solani species complex clade 3” of O'Donnell (41); the sequences of two strains (strains CBS 102256 and CBS 109696) were identical to those of members of Haematonectria haematococca mating group V, also called F. solani var. petroliphilum, a group containing pathogens of cucurbits (41) as well as numerous isolates from oily water (22). Confirmatory work on other sequences and characters is necessary, however, before the medical isolates can be fully evaluated as possible representatives of this taxon. H. haematococca mating group VI from peas was also closely related to the medical isolates, as was Haematonectria ipomoeae, a species isolated from Passiflora edulis and various other mostly tropical plants (51). None of the medical isolates were related to the morphologically similar but phylogenetically distinct isolates in the clade formerly referred to as F. solani f. sp. phaseoli, a group of isolates pathogenic for beans. This clade, which forms part of “F. solani species complex clade 2” of O'Donnell (41), has recently been segregated as Fusarium martiiphaseoli (22). A typical Cylindrocarpon species, the anamorph of Neonectria radicicola (32), was strongly phylogenetically distinct from C. lichenicola and other members of the F. solani clade. Typical Acremonium species such as Acremonium alternatum and Acremonium kiliense, already known mainly to belong to the phylogenetically separate family Bionectriaceae (47, 51) and to be well separated phylogenetically from Fusarium species (14), were not included in the dendrogram. A less prototypical Acremonium species of nectriaceous affinity, Acremonium berkeleyanum (Acremonium butyri), is included as the anamorph of Cosmospora vilior.
Human-pathogenic Fusarium species outside the F. solani clade, e.g., F. oxysporum and F. verticillioides, were clustered in the clade corresponding to the teleomorph genus Gibberella. Several other distinct clades containing Fusarium anamorphs not known to be associated with human and animal disease, e.g., Fusarium sections Eupionnotes and Spicarioides, as well as Fusarium buxicola, were also well distinguished.
Strain CBS 115.40, the ex-type strain of Cylindrocarpon tonkinense, a name long considered synonymous with C. lichenicola (21), yielded a sequence compatible with that of F. solani, not C. lichenicola. Morphological investigation showed that, while atypical for F. solani, this strain was also completely distinct from typical C. lichenicola isolates, with tapering and often curved macroconidia rather than the straight and club-shaped conidia seen in the latter species. It also differed strongly from its own morphological description and illustrations in Bugnicourt's (3) original publication describing C. tonkinense, suggesting that the strain currently held as CBS 115.40 may have been derived from a strain transposition error. The matter is being further investigated. Other strains described in literature as C. tonkinense, e.g., the strain from a patient with keratitis reported by Matsumoto et al. (33) (available as ATCC 42055 but not examined in the present study), can be clearly recognized from their descriptions and photographs as typical C. lichenicola isolates.
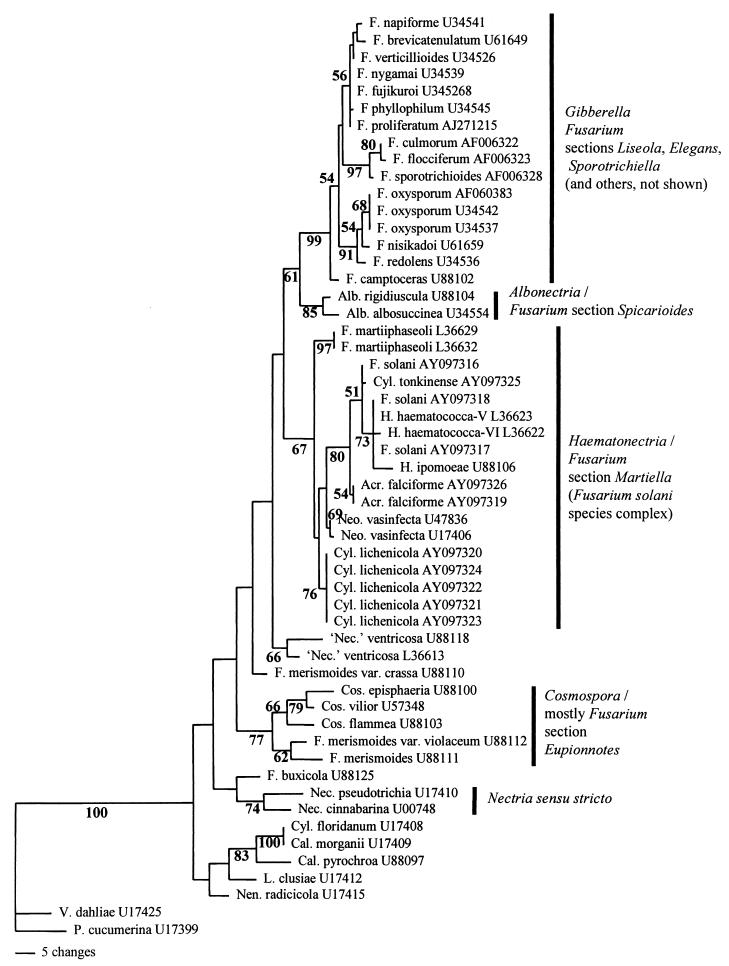
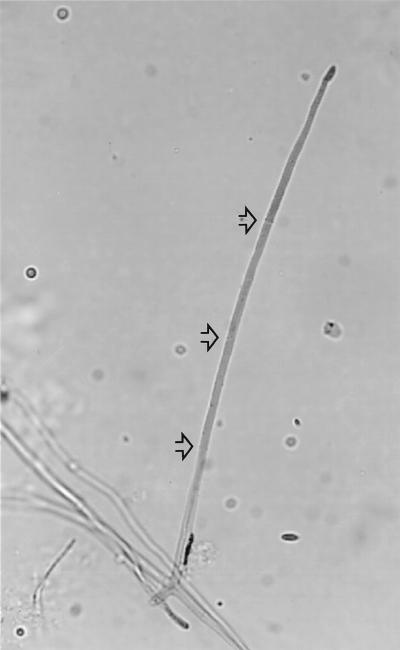
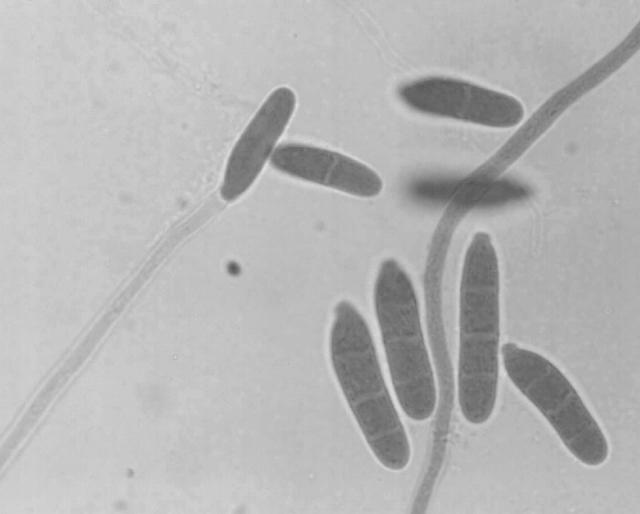
Morphological analysis of pathogenic species and strains in the F. solani clade showed that members of this group diverged considerably in conidial morphology, although the typical curved macroconidia with foot cells were found in isolates that had been identified as F. solani. The main micromorphological factor found in common among the members of the group was the production of elongate, filiform, often several-celled conidiophores incorporating terminal monophialides up to 40 μm in length, with a relatively broad, blunt apex. In F. solani isolates per se, these elongate conidiophores (Fig. 2) were mainly associated with microconidia; conidiophores forming macroconidia were somewhat shorter. In C. lichenicola, however, conidiophores forming macroconidia were typically elongate (Fig. 3). The conidiophores in A. falciforme tended to be similar in shape to the conidiophores of other isolates in the F. solani clade but were relatively strongly septate (Fig. 4); the terminal phialidic cell was frequently less than 20 μm in length, although longer phialides were also seen. Other species-specific characters were as described previously (2, 8, 10, 12).
FIG. 2.
A typical elongate, microconidium-producing, septate conidiophore of F. solani. Septa, which are in slightly different planes of focus, are marked with arrows. Magnification, ×1,400.
FIG. 3.
Macroconidia of Cylindrocarpon lichenicola produced from an elongate phialidic conidiophore (not fully shown). Magnification, ×1,400.
FIG. 4.
Conidiophores of A. falciforme. The conidiophore at the center has two septa (arrows); comparable structures in typical Acremonium species in the family Bionectriaceae (e.g., Acremonium kiliense, Acremonium strictum, Acremonium alternatum) would have no septum or, less commonly, one septum (10). Magnification, ×1,400.
DISCUSSION
Sequencing of the LSU of rDNA, a sensitive indicator of major clade relationships within the order Hypocreales (16, 41, 44, 50, 52), indicates that C. lichenicola and A. falciforme are members of the F. solani clade. They are not the first morphologically divergent anamorphs shown to be associated with the F. solani clade: O'Donnell (41) showed, using sequencing of the LSU, internal transcribed spacer, and elongation factor 1α sequences, that members of the ascomycetous genus Neocosmospora, which have unnamed anamorphs that produce no macroconidia and that otherwise resemble a microconidial state of Fusarium, are also nested deeply within the F. solani clade. It is clear, especially in the context of the whole range of Fusarium species, that the typical F. solani morphology is a symplesiomorphy, a shared ancestral character, and that several divergent and reduced forms have evolved from it.
Gams (10) recognized that A. falciforme was an atypical addition to the already polymorphous genus Acremonium. Its long, septate conidiophores and curved, often two-celled conidia suggested a possible relationship to Fusarium. It was not, however, the only recognized Acremonium species elaborating some didymoconidia, and its slow growth rate was consistent with placement as an Acremonium species. Growth rate was strongly emphasized in the taxonomy of Gams (10), as it was one of the few discrete characters that could unfailingly be used to separate the highly variable fusaria, with their frequent elaboration of Acremonium-like, mostly microconidial strains and species, from the even more diverse acremonia. Even the isolation of an A. falciforme strain with sharply pointed, very Fusarium-like conidia from a patient with mycetoma in Vanuatu (35) did not cause the generic placement of this fungus to be questioned. (Unfortunately, this interesting strain, which was well illustrated and described in the case report, is no longer available for study.) In light of the results of the present study, however, the link between A. falciforme and Acremonium can no longer be maintained. The type species of Acremonium, A. alternatum, is a member of the family Bionectriaceae, a group phylogenetically quite distinct from, although related to, the family Nectriaceae, which contains Fusarium and its relatives (47, 50, 51). The overall maintenance of biological predictiveness (e.g., for predictions like “all Fusarium species will [tend to be] specifically detected by appropriate genus-specific PCR primers”) entails that A. falciforme be correctly associated with its true biological relatives.
While A. falciforme was always an anomaly in its genus, C. lichenicola is morphologically typical of the historic concept of the genus Cylindrocarpon, as per the monograph of Booth (2). Its relatively long and narrow, several-celled, cylindrical conidia with typical rounded apices deviate only slightly from the macroconidia of other Cylindrocarpon species with straight conidia by having a conspicuously protuberant, symmetrical, truncate base. It is now clear that the type species of the genus Cylindrocarpon, Cylindrocarpon cylindroides (teleomorph Neonectria neomacrospora), to which the generic name attaches, is phylogenetically distinct from C. lichenicola and members of the genus Fusarium (32). The most extensively studied Cylindrocarpon, the medically important (60) and plant-pathogenic Cylindrocarpon destructans (teleomorph Neonectria radicicola), also clusters phylogenetically in a group of organisms well segregated from the F. solani and Gibberella clades (Fig. 1).
A previous study on the potential of sequencing of the LSU of rDNA for the identification of medically important Fusarium species (21) showed that two C. lichenicola isolates (recorded as C. tonkinense) clustered near F. solani; however, this study, as its authors stated, was “not a phylogenetic re-evaluation,” and insufficient comparison organisms were tested to determine, for example, that C. lichenicola was more closely related to F. solani than to other Cylindrocarpon species.
Before A. falciforme and C. lichenicola can comfortably be removed from their current genera and associated with Fusarium, some questions about this genus must also be considered. Its validity as currently delimited might be questioned, since Fusarium anamorphs are associated with several teleomorph genera, now known roughly to correspond to several related phylogenetic clades. The type species of Fusarium, Fusarium sambucinum Fuckel, belongs to the clade associated with the teleomorph genus Gibberella, making this clade the central group of Fusarium species and the one that would bear the name if Fusarium were split up. As mentioned previously, this clade contains several medically important species such as F. oxysporum and F. verticillioides (44). The F. solani clade has Haematonectria or Neocosmospora teleomorphs. Other Fusarium species have teleomorphs in the genera Cosmospora and Albonectria (51). There is therefore considerable temptation for phylogenetic systematists to split Fusarium itself into multiple parallel anamorph genera corresponding to the clades or to the teleomorph genera. Although a poorly resolved monophyletic clade appears to encompass all fusaria, some branches bearing disparate anamorphs appear to emerge from within this clade in the dendrogram based on the sequences of the LSU of rDNA (Fig. 1), particularly a branch bearing Cylindrocladium floridanum and N. radicicola/C. destructans. Despite these factors, no move has yet been made to split up Fusarium. This may be in part because of the desirability of maintaining as much nomenclatural stability as possible among the widely used, sometimes nearly 150-year-old Fusarium species names and in part because there are many morphological and general biological characters that make the historic, unitary generic concept of Fusarium useful. Such characters are diverse, e.g., possession of curved, pointed macroconidia with a differentiated foot cell, manifestation of plant-pathogenic ability, and manifestation of a high degree of amphotericin B resistance in opportunistic infections in humans. If Fusarium were to be divided, by default the names of the species in the F. solani complex would likely be used in combination with the long unused generic name Lachnidium, based on a seemingly unnecessary synonym coined for F. solani isolates obtained from infected crickets in 1891 (13). To take such a step would be seen by many practical users of fungal anamorph names to be counterproductive. Establishing the link between Fusarium and C. lichenicola, despite the deviating phragmoconidial morphology of the latter, scarcely attenuates the biological and morphological unity of the Fusarium clades. We therefore refrain from sundering the genus Fusarium on the basis of our findings.
Even anamorph classifications, while something of an artifice (15), function best if they reflect underlying biological reality to a reasonable degree. In keeping with this practical need, then, we hereby formally reinstate the original name Fusarium lichenicola C. B. Massalongo and make the following new formal combination: Fusarium falciforme (Carrión) Summerbell et Schroers, comb. nov. Basionym: Cephalosporium falciforme Carrión (in Mycologia 43:523, 1951).
The epidemiologic links between F. lichenicola and medically implicated isolates of F. solani are clearly suggested by a review of case literature pertaining to these fungi. F. lichenicola, for example, is most frequently medically significant as an agent of relatively aggressive keratitis subsequent to ocular trauma (1, 29, 31, 33), just as F. solani is (18, 19, 26, 30, 59). Other well-documented cases of infection caused by F. lichenicola include disseminated infection (25) and local soft tissue infection (24) in leukemia patients and peritonitis related to chronic ambulatory peritoneal dialysis (53). F. solani is well known as a more commonly occurring cause of similar infections (8, 18, 37). Most intriguingly, an unusual type of intertrigo, an infection almost never definitively shown to be caused by any fungi other than dermatophytes and Scytalidium species (older literature contains many inadequately substantiated case reports in which other species are mentioned, probably based on misattribution of the infection to contaminants isolated when a causal dermatophyte failed to grow), appears to be caused exclusively by opportunistic members of the F. solani and Gibberella clades. F. solani has been reported from two well-documented cases of this intertrigo (6, 49). The cause of a third such case, probably valid but not as rigorously documented (the putative agent was not reisolated on successive occasions in order to rule out dermatophytosis), was ascribed to the invalid name Cephalosporium keratoplasticum Morikawa by Harada and Usui (20); the case isolate, preserved as CBS 490.63, is F. solani and was sequenced in the present study. Only F. oxysporum (48) and a “Cylindrocarpon sp.” (27) that can be clearly recognized from its published description and photos as F. lichenicola have been well documented as causes of similar cases of intertrigo. Three of these fusarial intertrigo cases were described from patients of recent West African origin living in Europe (27, 48, 49), while in a fourth case (6), the patient's geographic history was not discussed, but he was described both as a French resident and as a “practicing Muslim…who washes his feet five times per day [prior to] his prayers.” The fifth, incompletely confirmed case was from Japan (20). It is possible that, at least in areas with warm climates, the regular exposure of feet for any reason, including hygiene, to water or other materials containing an inoculum of opportunistic Fusarium species, including F. lichenicola, may rarely be conducive to development of fusarial intertrigo.
F. falciforme has long been known to be a rarely occurring agent of mycetoma in tropical and subtropical areas (17). While F. solani is not principally thought of as a cause of mycetoma, it has been reported from at least four such cases worldwide (18); the United Kingdom National Collection of Pathogenic Fungi contains isolates from patients with additional confirmed cases (18). In recent years, there has been evidence that F. falciforme is emerging as an agent of localized (28, 36, 56) and disseminated (39) infections in immunocompromised patients, thus overlapping more broadly in pathogenic potential with F. solani. An F. falciforme endophthalmitis has also been documented (4). The apparent distinction between F. falciforme and F. solani in terms of their potential to cause opportunistic infections may relate more to their ecological and geographic distributions and the way in which they are contracted by patients than to virulence factors. Nothing is known about the natural habitat of F. falciforme, but this species is certainly not a common fungus of food, household plant material, garden soil, and domestic water, as F. solani is. By virtue of this factor alone, F. falciforme would be less likely than F. solani to cause nosocomial infections in temperate-region medical facilities even if both species were identical in virulence.
When F. lichenicola and F. falciforme are recognized as members of the F. solani clade, certain morphological correspondences facilitating recognition in the laboratory become discernible. The long, slender, cylindrical conidiophores and integrated, terminal phialides seen in all these species clearly reflect their biological unity. In contrast, other Fusarium groups tend to have strongly different phialides. Many species form shorter, discrete monophialides (phialides with only a single fertile opening) that are subulate (awl or candle shaped, i.e., rigid looking, tapered, and narrow at the apex) or inflated, while some species form polyphialides, in which each phialide has several fertile openings. Monophialides tend to be strongly visibly differentiated with respect to the subtending cells they are attached to; they seldom occur as scarcely differentiated, integrated end cells on extended filiform conidiophores, as is common with phialides in the F. solani clade. In conidial morphology, F. solani shows a classical fusarial macroconidial form (curved phragmoconidia with somewhat pointed apices and basal foot cells), while F. lichenicola does not; and F. falciforme may vary from strain to strain in this regard. Above and beyond these differences, however, all these species have two- to several-celled conidia that are significantly blunter at the apex and proportionately broader than the macroconidia of fusaria in the Gibberella clade. The macroconidia of F. lichenicola have lost their curving aspect and their heel-and-toe-shaped fusarial foot cell, but F. solani itself is only moderately curved and the foot cells of many isolates are minimally differentiated. While all these species are variable in terms of their colony colorations, similar chestnut red-brown to purplish reverse pigments are seen in many representatives of all three species (2, 8, 10, 12). The violet-purple reverse color that may be seen in F. falciforme overlaps with that seen in some F. solani isolates.
A similar deep blue-purple color is also seen in Fusarium coeruleum, a species formerly called F. solani var. coeruleum and considered to be closely related to or conspecific with F. solani. This species was also once recorded as an agent of mycetoma in Thailand (55). The record, however, is dubious, as molecularly verified isolates of this species are strongly distinct from F. solani and are known to have been isolated only from potatoes (22). The description given for the isolate from the patient with mycetoma is also compatible with a strongly purple colored F. solani isolate, and it is possible that this case represents yet another case of F. solani mycetoma.
For molecular identification, there is a strong need to know which species are and are not true fusaria in order to allow the design of diagnostic primers and probes and to allow meaningful interpretation of related results. For example, the ribosomal primers recently published by Hue et al. (23) are specific for a range of Fusarium species spanning both the Gibberella and F. solani clades and would be strongly predicted to give a positive result with F. falciforme and F. lichenicola. On the other hand, the finding of those investigators that “Fusarium nivale” gave a positive reaction can be deduced as being based on a misidentified isolate, since the species formerly bearing this name, Microdochium nivale, is now known to belong to the family Hyponectriaceae, order Xylariales (9), a fungal group much less closely related to Fusarium (family Nectriaceae) than most of the species that gave negative reactions with the primer set. As an obligate psychrophile not growing at 35°C, M. nivale is almost certainly never pathogenic for mammals. Rationalization of the taxonomy of the fusaria, then, is essential for increasing both diagnostic accuracy and epidemiological understanding of this important group of emerging pathogens.
Acknowledgments
We thank Arien van Iperen for technical assistance and Walter Gams for inspiration and for correcting the manuscript.
REFERENCES
- 1.Affeldt, J. C., H. W. Flynn, R. K. Forster, S. Mandelbaum, J. G. Clarkson, and G. D. Jarus. 1987. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology 94:407-413. [DOI] [PubMed] [Google Scholar]
- 2.Booth, C. 1966. The genus Cylindrocarpon. Mycol. Papers 104:1-58. [Google Scholar]
- 3.Bugnicourt, F. 1939. Les Fusarium et Cylindrocarpon de l'Indochine, p. 1-206. In P. Lechevalier (ed.), Encyclopédie mycologique. P. Lechevalier, Paris, France.
- 4.Cameron, J. A., E. M. Badawi, P. A. Hoffman, and K. F. Tabbara. 1996. Chronic endophthalmitis caused by Acremonium falciforme. Can. J. Ophthalmol. 31:367-368. [PubMed] [Google Scholar]
- 5.Chandenier, J., M. P. Hayette, C. de Bièvre, P. F. Westeal, J. Petit, J. M. Achard, N. Bove, and B. Carme. 1993. Tuméfaction de la jambe à Neocosmospora vasinfecta chez un transplanté rénal. J. Mycol. Med. 3:165-168. [Google Scholar]
- 6.Comparot, S., G. Reboux, H. van Landuyt, L. Guetarni, and T. Barale. 1995. Fusarium solani: un case rebelle d'intertrigo. J. Mycol. Med. 5:119-121. [Google Scholar]
- 7.de Hoog, G. S., and A. H. G. Gerrits van den Ende. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41:183-189. [DOI] [PubMed] [Google Scholar]
- 8.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi. 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 9.Eriksson, O. E. 2000. Notes on Ascomycete systematics. Nos. 2940-3127. Myconet 5:1-35.*** [Online: http://www.umu.se/myconet/M5.html.] [Google Scholar]
- 10.Gams, W. 1971. Cephalosporium-artige Schimmelpilze. Gustav Fischer, Stuttgart, Germany.
- 11.Gams, W., E. S. Hoekstra and A. Aptroot. 1998. CBS course of mycology. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
- 12.Gerlach, W., and H. Nirenberg. 1982. The genus Fusarium—a pictorial atlas. Mitt. Biol. Bundesanst. Land-Forstwirtsch. 209:1-406. [Google Scholar]
- 13.Giard, M. A. 1891. Sur les cladosporiées entomophytes, nouveau groupe de champignons parasites des insectes. C. R. Acad. Sci. 112:1518-1521. [Google Scholar]
- 14.Glenn, A. E., C. W. Bacon, R. Price, and R. T. Hanlin. 1996. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 88:369-383. [Google Scholar]
- 15.Greuter, W., J. McNeill, F. R. Barrie, H. M. Burdet, V. Demoulin, T. S. Filgueiras, D. H. Nicolson, P. C. Silva, J. E. Skog, P. Trehane, N. J. Turland, and D. L. Hawksworth. 2000. International code of botanical nomenclature. Koeltz Scientific Books, Königstein, Germany.
- 16.Guadet, J., J. Julien, J. F. Lafay, and Y. Brygoo. 1989. Phylogeny of some Fusarium species, as determined by large-subunit rRNA sequence comparison. Mol. Biol. Evol. 6:227-242. [DOI] [PubMed] [Google Scholar]
- 17.Guarro, J., W. Gams, I. Pujol, and J. Gené. 1997. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin. Infect. Dis. 25:1222-1229. [DOI] [PubMed] [Google Scholar]
- 18.Guarro, J., and J. Gené. 1995. Opportunistic fusarial infection in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 19.Gugnani, H. C., R. S. Talwar, A. N. U. Njuko-obi, and H. C. Kodilinye. 1976. Mycotic keratitis in Nigeria. Br. J. Ophthalmol. 60:607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada, S., and Y. Usui. 1960. On a species of Cephalosporium isolated from the epidermophytosis between toes. Nagaoa 7:51-55. [Google Scholar]
- 21.Hawksworth, D. L. 1979. The lichenicolous hyphomycetes. Bull. Br. Mus. Nat. Hist. (Bot.) 6:183-300. [Google Scholar]
- 21a.Hennequin, C., E. Abachin, F. Symoens, V. Lavarde, G. Reboux, N. Nolard, and P. Berche. 1999. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J. Clin. Microbiol. 37:3586-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hering, O. 1997. Charakterisierung und Differenzierung bei Fusarium Link mittels RAPD und ITS-RFLP. Mitt. Biol. Bundesanst. Land-Forstwirtsch. 331:1-133. [Google Scholar]
- 23.Hue, F.-X., M. Huerre, M. A. Rouffault, and C. de Bièvre. 1999. Specific identification of Fusarium species in blood and tissue by a PCR technique. J. Clin. Microbiol. 37:2434-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwen, P. C., S. R. Tarantolo, D. A. Sutton, M. G. Rinaldi, and S. H. Hinrichs. 2000. Cutaneous infection caused by Cylindrocarpon lichenicola in a patient with acute myelogenous leukemia. J. Clin. Microbiol. 38:3375-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James, E. A., K. Orchard, P. H. McWhinney, D. W. Warnock, E. M. Johnson, A. B. Mehta, and C. C. Kibbler. 1997. Disseminated infection due to Cylindrocarpon lichenicola in a patient with acute myeloid leukaemia. J. Infect. 34:65-67. [DOI] [PubMed] [Google Scholar]
- 26.Jones, D. B., R. Sexton, and G. Rebell. 1969. Mycotic keratitis in south Florida: a review of thirty-nine cases. Trans. Ophthalmol. Soc. 89:781-797. [PubMed] [Google Scholar]
- 27.Lamey, B., C. Blanc, and J. Lapalu. 1985. Le Cylindrocarpon: nouvel agent d'intertrigo. Bull. Soc. Fr. Mycol. Med. 14:73-76. [Google Scholar]
- 28.Lau, Y. L., K. Y. Yuen, C. W. Lee, and C. F. Chan. 1995. Invasive Acremonium falciforme infection in a patient with severe combined immunodeficiency. Clin. Infect. Dis. 20:197-198. [DOI] [PubMed] [Google Scholar]
- 29.Laverde, S., L. H. Moncada, A. Restrepo, and C. L. Vera. 1973. Mycotic keratitis; 5 cases caused by unusual fungi. Sabouraudia 11:119-123. [DOI] [PubMed] [Google Scholar]
- 30.Liesegang, T. J., and R. K. Forster. 1980. Spectrum of microbial keratitis in south Florida. Am. J. Ophthalmol. 90:38-47. [DOI] [PubMed] [Google Scholar]
- 31.Mangiaterra, M., G. Giusiano, G. Smilasky, L. Zamar, G. Amado, and C. Vicentín. 2001. Keratomycosis caused by Cylindrocarpon lichenicola. Med. Mycol. 39:143-145. [DOI] [PubMed] [Google Scholar]
- 32.Mantiri, F. R., G. J. Samuels, J. E. Rahe, and B. M. Honda. 2001. Phylogenetic relationships in Neonectria species having Cylindrocarpon anamorphs inferred from mitochondrial ribosomal DNA sequences. Can. J. Bot. 79:334-340. [Google Scholar]
- 33.Matsumoto, T., J. Masaki, and T. Okabe. 1979. Cylindrocarpon tonkinense: as a cause of keratomycosis. Trans. Br. Mycol. Soc. 72:503-504. [Google Scholar]
- 34.Matuo, T., and W. C. Snyder. 1973. Use of morphology and mating populations in the identification of formae speciales in Fusarium solani. Phytopathology 63:562-565. [Google Scholar]
- 35.McCormack, J. G., P. B. McIntyre, M. H. Tilse, and D. H. Ellis. 1987. Mycetoma associated with Acremonium falciforme infection. Med. J. Aust. 147:187-188. [DOI] [PubMed] [Google Scholar]
- 36.Miró, O., J. Ferrando, V. Lecha, and J. M. Campistol. 1994. Abscesos subcutáneos por Acremonium falciforme en un trasplantado renal. Med. Clin. (Barcelona) 102:316.. [PubMed] [Google Scholar]
- 37.Nelson, P. J., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nirenberg, H. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt. Biol. Bundesanst. Land-Forstwirtsch. 119:1-117. [Google Scholar]
- 39.Noble, R. C., J. Salgado, S. W. Newell, and N. L. Goodman. 1997. Endophthalmitis and lumbar diskitis due to Acremonium falciforme in a splenectomized patient. Clin. Infect. Dis. 24:277-278. [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell, K. 1993. Fusarium and its near relatives, p. 225-233. In D. R. Reynolds and J. W. Taylor (ed.), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, England.
- 41.O'Donnell, K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92:919-938. [Google Scholar]
- 42.O'Donnell, K., and E. Cigelnik. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell, K., E. Cigelnik, and H. H. Casper. 1998. Molecular phylogenetic, morphological, and mycotoxin data support reidentification of the Quorn mycoprotein fungus as Fusarium venenatum. Fung. Genet. Biol. 23:57-67. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell, K., E. Cigelnik, and H. I. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikoroi species complex. Mycologia 90:465-493. [Google Scholar]
- 45.O'Donnell, K., and L. E. Gray. 1995. Phylogenetic relationships of the soybean sudden death syndrome pathogen Fusarium solani f. sp. phaseoli inferred from rDNA sequence data and PCR primers for its identification. Mol. Plant-Microbe Interact. 8:709-716. [DOI] [PubMed] [Google Scholar]
- 46.Raper, J. R., and C. A. Raper. 1972. Genetic analysis of the life cycle of Agaricus bisporus. Mycologia 64:1088-1117. [Google Scholar]
- 47.Rehner, S. A., and G. J. Samuels. 1995. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Can. J. Bot. 73(Suppl. 1):S816-S823. [Google Scholar]
- 48.Romano, C., C. Miracco, and E. M. Difonzo. 1998. Skin and nail infections due to Fusarium oxysporum in Tuscany, Italy. Mycoses 41:433-437. [DOI] [PubMed] [Google Scholar]
- 49.Romano, C., L. Presenti, and L. Massai. 1999. Interdigital intertrigo of the feet due to therapy-resistant Fusarium solani. Dermatology 199:177-179. [DOI] [PubMed] [Google Scholar]
- 50.Rossman, A. Y., J. M. McKemy, R. A. Pardo-Schultheiss, and H. J. Schroers. 2001. Molecular studies of the Bionectriaceae using large subunit rDNA sequences. Mycologia 93:100-110. [Google Scholar]
- 51.Rossman, A. Y., G. J. Samuels, C. T. Rogerson, and R. Lowen. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 42:1-248. [Google Scholar]
- 52.Schroers, H.-J. 2000. Generic delimitation of Bionectria (Bionectriaceae, Hypocreales) based on holomorph characters and rDNA sequences. Stud. Mycol. 45:63-82. [Google Scholar]
- 53.Sharma, R., C. K. T. Farmer, W. R. Grandsen, and C. S. Ogg. 1998. Peritonitis in continuous ambulatory peritoneal dialysis due to Cylindrocarpon lichenicola infection. Nephrol. Dial. Transplant. 13:2662-2664. [DOI] [PubMed] [Google Scholar]
- 54.Swofford, D. L. 2000. PAUP∗. Phylogenetic analysis using parsimony (∗and other methods), version 4. Sinauer Associates, Sunderland, Mass.
- 55.Thianprasit, M., and A. Sivayathorn. 1983. Black dot mycetoma. Mykosen 27:219-226. [DOI] [PubMed] [Google Scholar]
- 56.Van Etta, L. L., L. R. Peterson, and D. N. Gerding. 1983. Acremonium falciforme (Cephalosporium falciforme) mycetoma in a renal transplant patient. Arch. Dermatol. 119:707-708. [DOI] [PubMed] [Google Scholar]
- 57.Vilgalys, R., and M. Hester. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172:4238-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols, a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 59.Zapater, R. C. 1986. “Opportunistic fungus infections”—Fusarium infections—(keratomycosis by Fusarium). Jpn. J. Med. Mycol. 27:68-69. [Google Scholar]
- 60.Zoutman, D. E., and L. Sigler. 1991. Mycetoma of the foot caused by Cylindrocarpon destructans. J. Clin. Microbiol. 29:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]