Abstract
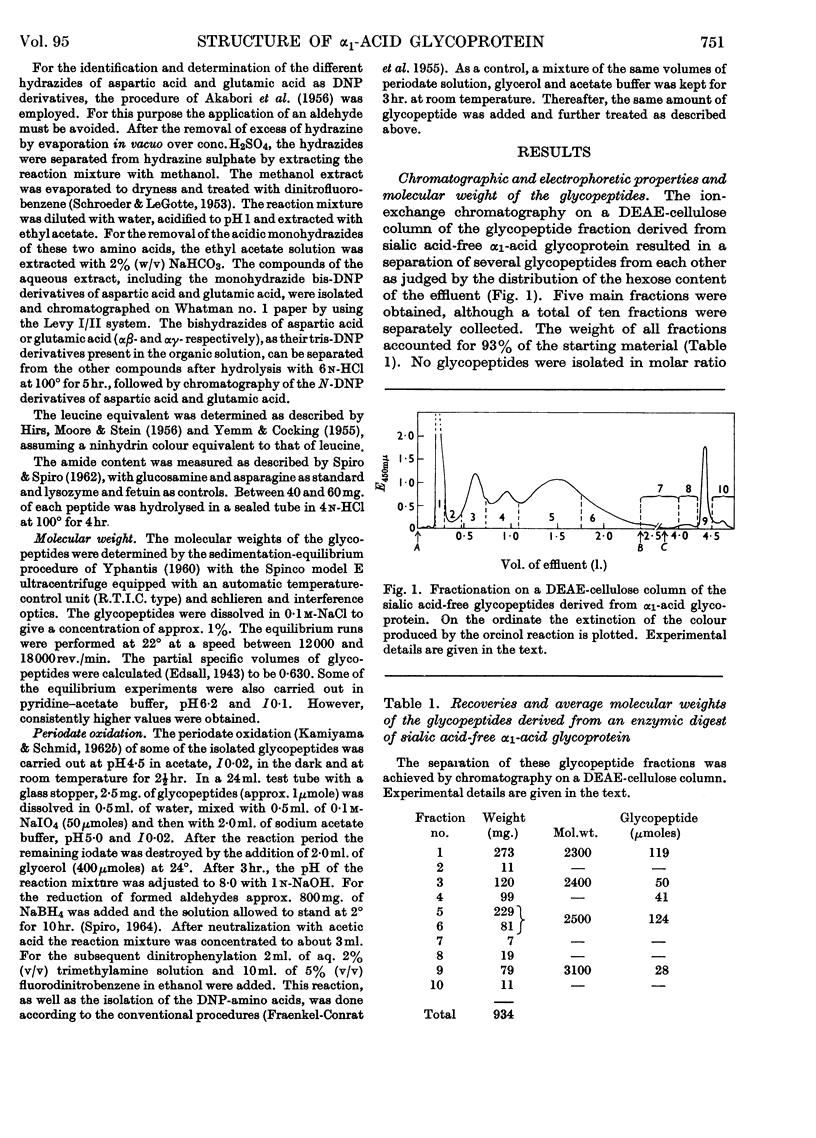
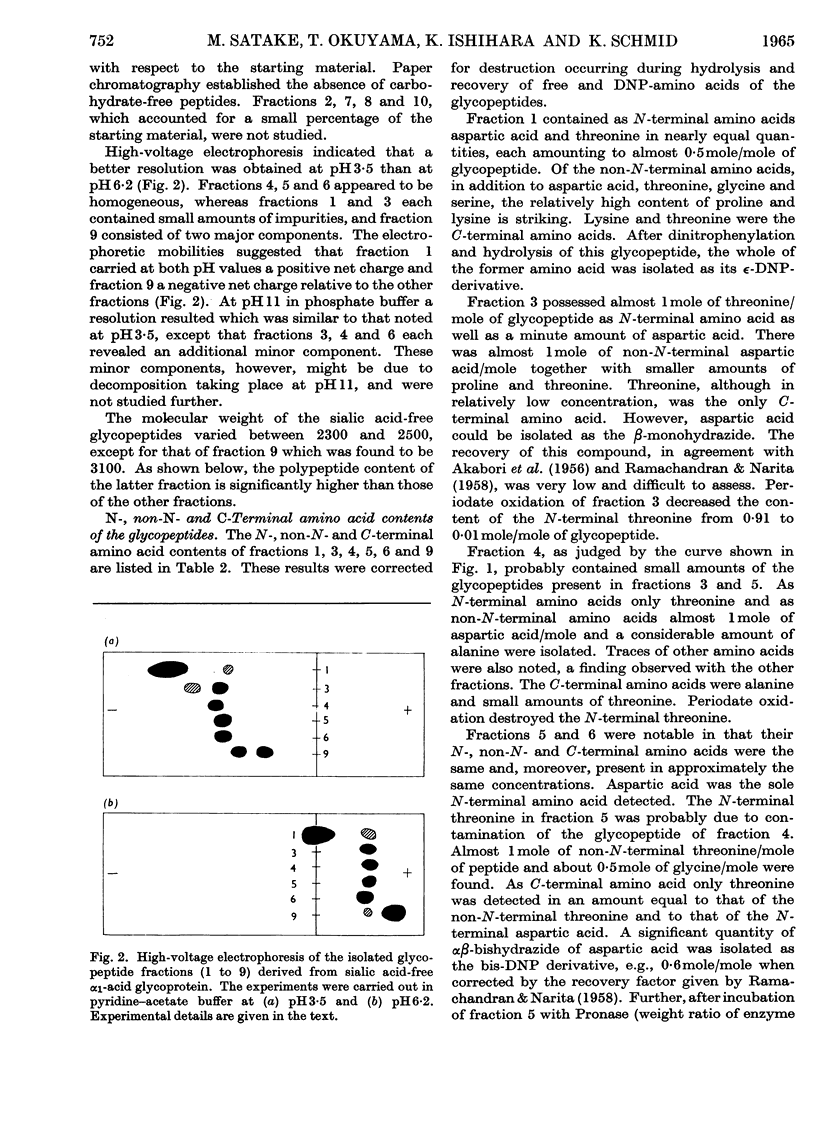
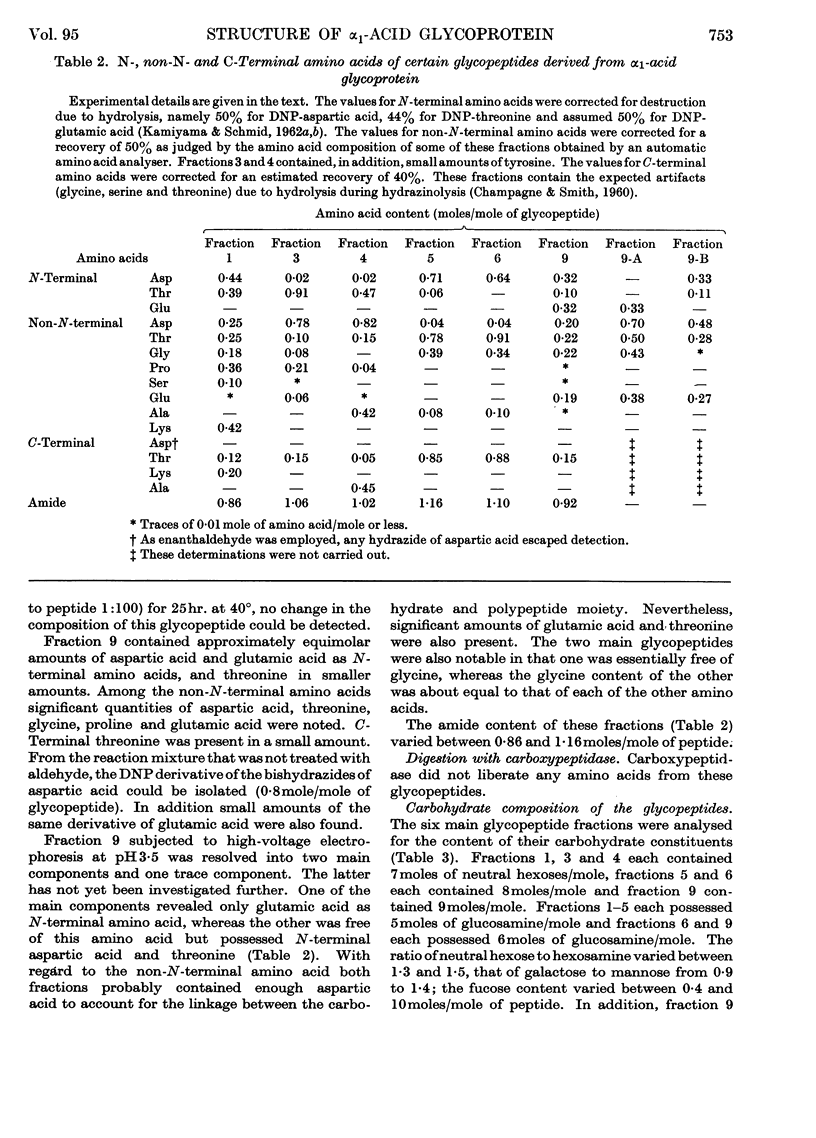
1. The glycopeptides derived from a proteolytic digest of sialic acid-free α1-acid glycoprotein were separated on a DEAE-cellulose column into five main fractions. 2. The average molecular weight of these glycopeptides was 2400, except for one fraction whose molecular weight was 3100. The average molecular weight of the sialic acid-free carbohydrate units was found to be 2200. From these data and the carbohydrate content of the native protein and the assumed molecular weight of 44000, it was concluded that α1-acid glycoprotein probably possesses five carbohydrate units. The sialic acid-containing carbohydrate units of this glycoprotein have an average molecular weight of 3000, except for one unit the molecular weight of which is significantly higher. 3. The N-, non-N- and C-terminal amino acids of the main glycopeptides were determined. Aspartic acid and threonine occur in most peptides. Alanine, glycine, proline, serine and lysine were present in varying amounts. Traces of other amino acids were also found. 4. The amino acid sequence of three main glycopeptides was established and indicated that these glycopeptides are located at different positions of the polypeptide chain of the glycoprotein. These sequences are: Asp(NH2)-Pro-Lys; Thr-Asp(NH2)-Ala; Asp(NH2)-Gly-Thr. 5. From the results of a series of chemical reactions (periodate oxidation, hydrazinolysis, dinitrophenylation, mild acid hydrolysis) it was shown that the hydroxyl group of the N-terminal threonine and the ∈-amino group of lysine are free and that the β-carboxyl group of aspartic acid is present as amide. It was concluded that this amide group is involved in the carbohydrate–polypeptide linkages of at least four carbohydrate units of α1-acid glycoprotein. 6. The carbohydrate composition of the sialic acid-free glycopeptides was determined in terms of moles of neutral hexoses, glucosamine and fucose/mole. 7. Fucose, at least to the larger part, is not linked to sialic acid, and its (glycosidic) linkage is significantly more stable toward acid hydrolysis than the bond of the sialyl residues. 8. Heterogeneity of the carbohydrate units of α1-acid glycoprotein was found with regard to size and to content of fucose and sialic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURRILLON R., GOT R., MEYER D. ETUDES SUR LA STRUCTURE D'UNE ALPHA-1-GLYCOPROT'EINE. II. S'EQUENCE DES AMINOACIDES AU VOISINAGE DE LA LIAISON GLUCIDE-PEPTIDE. Biochim Biophys Acta. 1964 Jul 7;83:178–188. [PubMed] [Google Scholar]
- BRADBURY J. H. The kinetics of hydrazinolysis of simple peptides in anhydrous hydrazine. Biochem J. 1958 Mar;68(3):475–482. doi: 10.1042/bj0680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavanandan V. P., Buddecke E., Carubelli R., Gottschalk A. The complete enzymic degradation of glycopeptides containing O-seryl and O-threonyl linked carbohydrate. Biochem Biophys Res Commun. 1964 Jul 1;16(4):353–357. doi: 10.1016/0006-291x(64)90039-7. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- DIXON G. H., KAUFFMAN D. L., NEURATH H. Amino acid sequence in the region of diisopropylphosphoryl binding in diisopropylphosphoryl-trypsin. J Biol Chem. 1958 Dec;233(6):1373–1381. [PubMed] [Google Scholar]
- EYLAR E. H. The carbohydrate-protein linkage in the alpha1-glycoprotein of human plasma. Biochem Biophys Res Commun. 1962 Jul 3;8:195–199. doi: 10.1016/0006-291x(62)90262-0. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. A. THE SENSITIVITY OF THE NEURAMINOSIDIC LINKAGE IN MUCOSUBSTANCES TOWARDS ACID AND TOWARDS NEURAMINIDASE. Biochem J. 1963 Nov;89:380–391. doi: 10.1042/bj0890380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOT R., FONT J., BOURRILLON R. TROIS HETEROSACCHARIDES DU COLOSTRUM HUMAIN. Biochim Biophys Acta. 1963 Oct 29;78:367–369. doi: 10.1016/0006-3002(63)91647-0. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by fingerprinting. Biochim Biophys Acta. 1958 Jun;28(3):539–545. doi: 10.1016/0006-3002(58)90516-x. [DOI] [PubMed] [Google Scholar]
- KAMIYAMA S., SCHMID K. Studies on the structure of alpha1-acid glycoprotein. II. Preparation and characterization of a glycopeptide fraction. Biochim Biophys Acta. 1962 Mar 26;58:80–87. doi: 10.1016/0006-3002(62)90819-3. [DOI] [PubMed] [Google Scholar]
- MASAMUNE H., HAKOMORI S., NUMABE H., AKAMA Z., KAMIYAMA S. Synthesis of artificial group A and B substances. Tohoku J Exp Med. 1955 Feb 25;61(2-3):283–301. doi: 10.1620/tjem.61.283. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., SPIK G., CHOSSON A., SEGARD E., SCHEPPLER N. LES PROC'ED'ES D''ETUDE DE LA STRUCTURE DES GLYCOPROT'EIDES. J Pharm Belg. 1963 Nov-Dec;18:529–546. [PubMed] [Google Scholar]
- Marks G. S., Marshall R. D., Neuberger A. Carbohydrates in protein. 6. Studies on the carbohydrate-peptide bond in hen's-egg albumin. Biochem J. 1963 May;87(2):274–281. doi: 10.1042/bj0870274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPKOFF H. PREPARATION OF A GLYCOPEPTIDE FROM OVINE INTERSTITIAL CELL-STIMULATING HORMONE. Biochim Biophys Acta. 1963 Oct 29;78:384–387. doi: 10.1016/0006-3002(63)91654-8. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN L. K., NARITA K. Reactions involving the amide and carboxyl groups of tobacco mosaic virus (TMV) protein. Biochim Biophys Acta. 1958 Dec;30(3):616–624. doi: 10.1016/0006-3002(58)90109-4. [DOI] [PubMed] [Google Scholar]
- SPIRO M. J., SPIRO R. G. Composition of the peptide portion of fetuin. J Biol Chem. 1962 May;237:1507–1510. [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEIMER H. E., MEHL J. W., WINZLER R. J. Studies on the mucoproteins of human plasma. V. Isolation and characterization of a homogeneous mucoprotein. J Biol Chem. 1950 Aug;185(2):561–568. [PubMed] [Google Scholar]
- WERNER I., ODIN L. On the presence of sialic acid in certain glycoproteins and in gangliosides. Acta Soc Med Ups. 1952;57(3-4):230–241. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]
- ZILLIKEN F., BRAUN G. A., GYORGY P. Gynaminic acid, a naturally occurring form of neuraminic acid in human milk. Arch Biochem Biophys. 1955 Feb;54(2):564–566. doi: 10.1016/0003-9861(55)90073-4. [DOI] [PubMed] [Google Scholar]



