Abstract
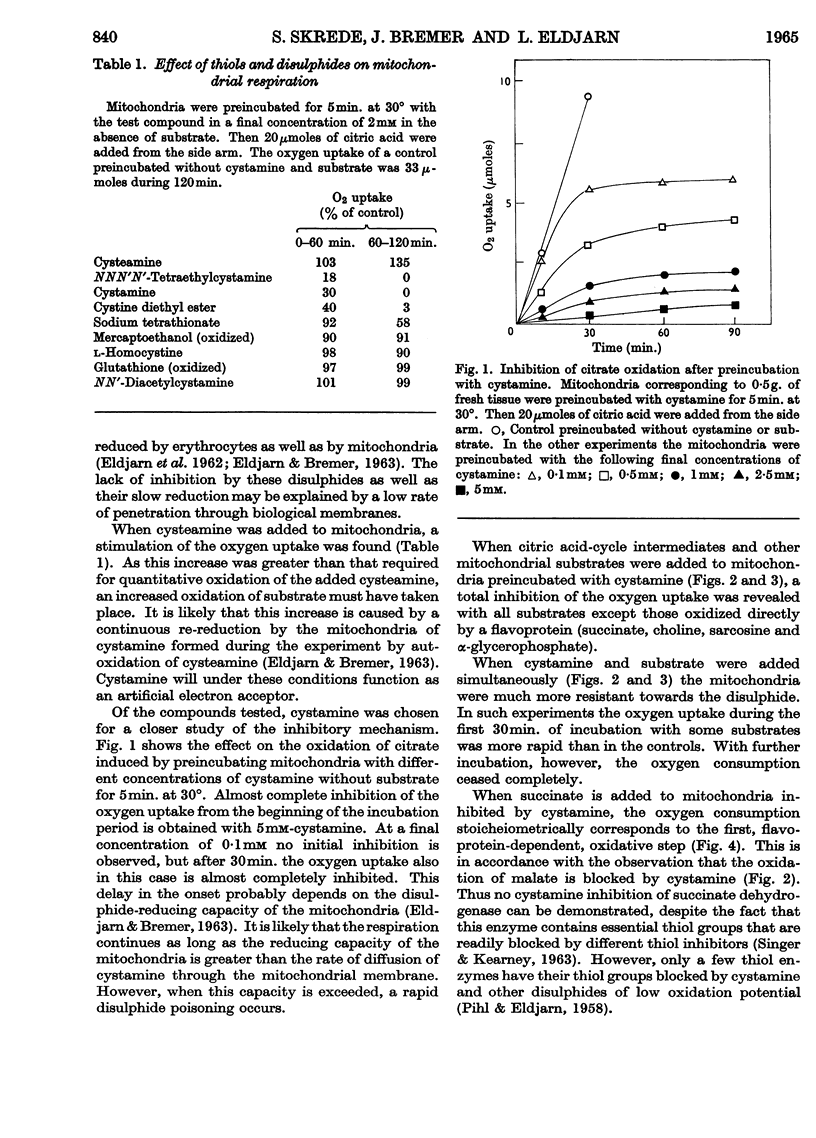
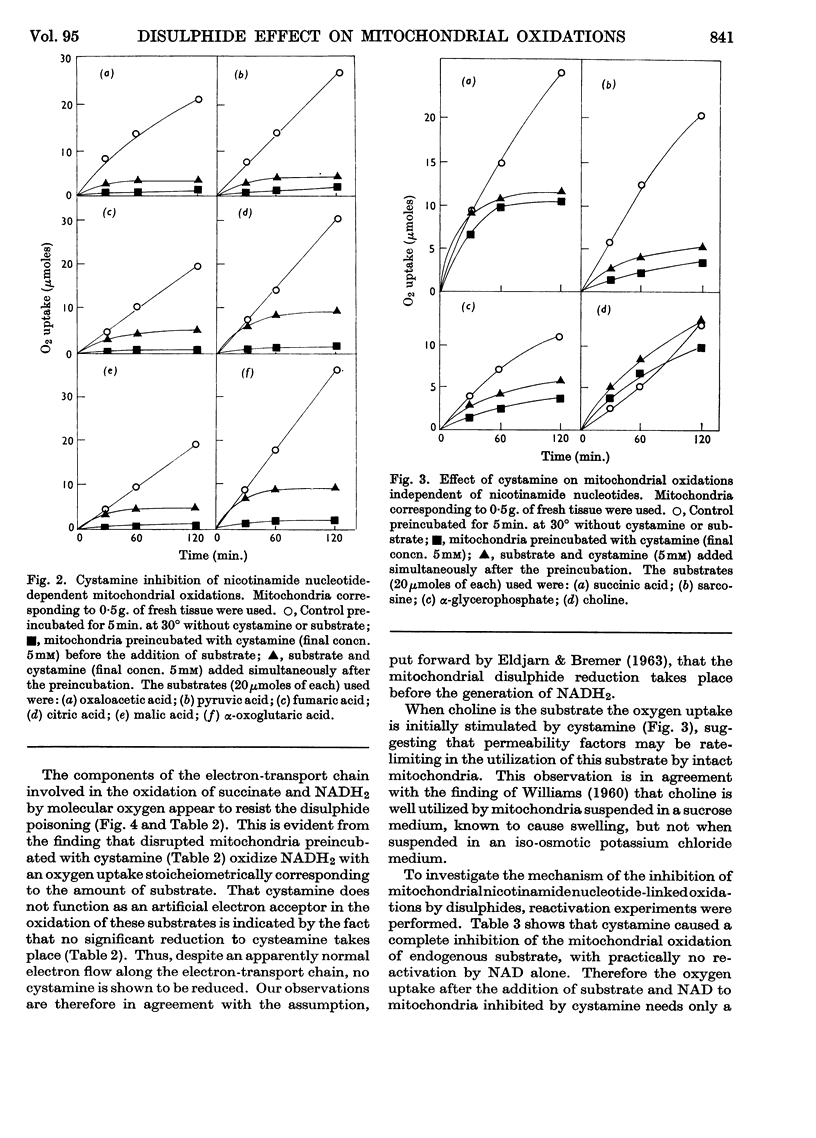
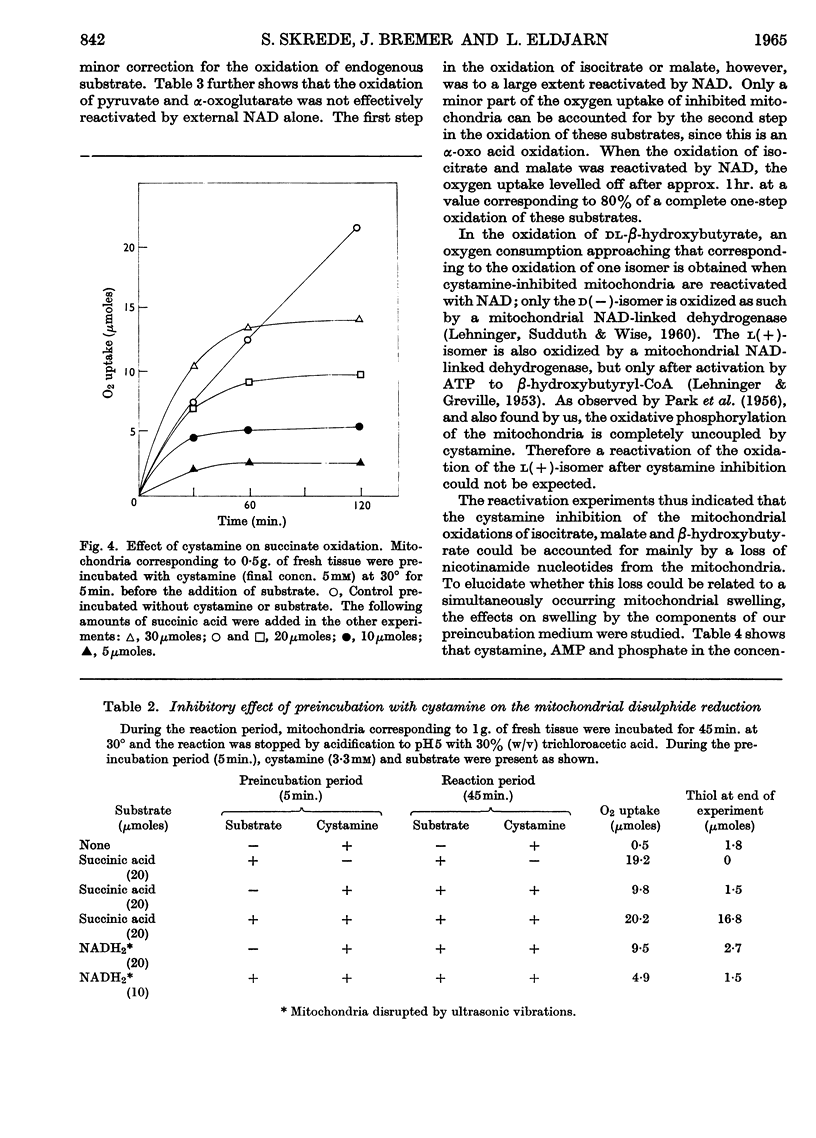
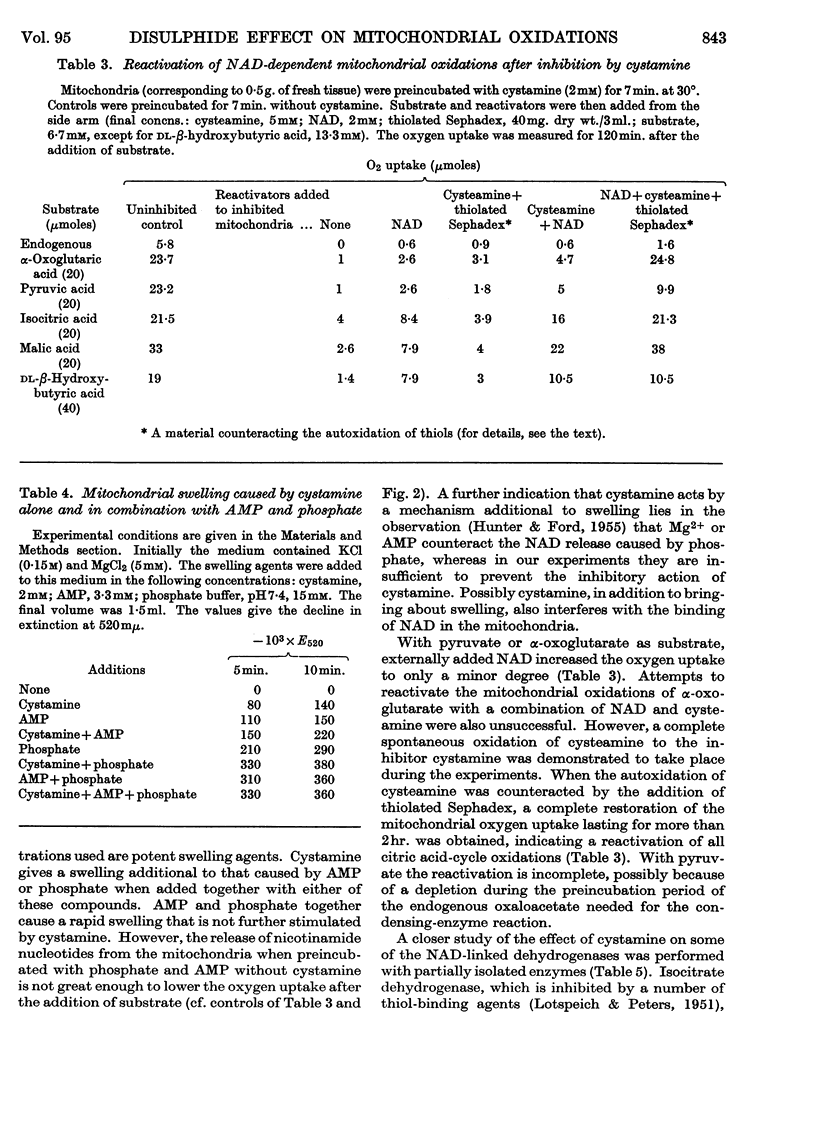
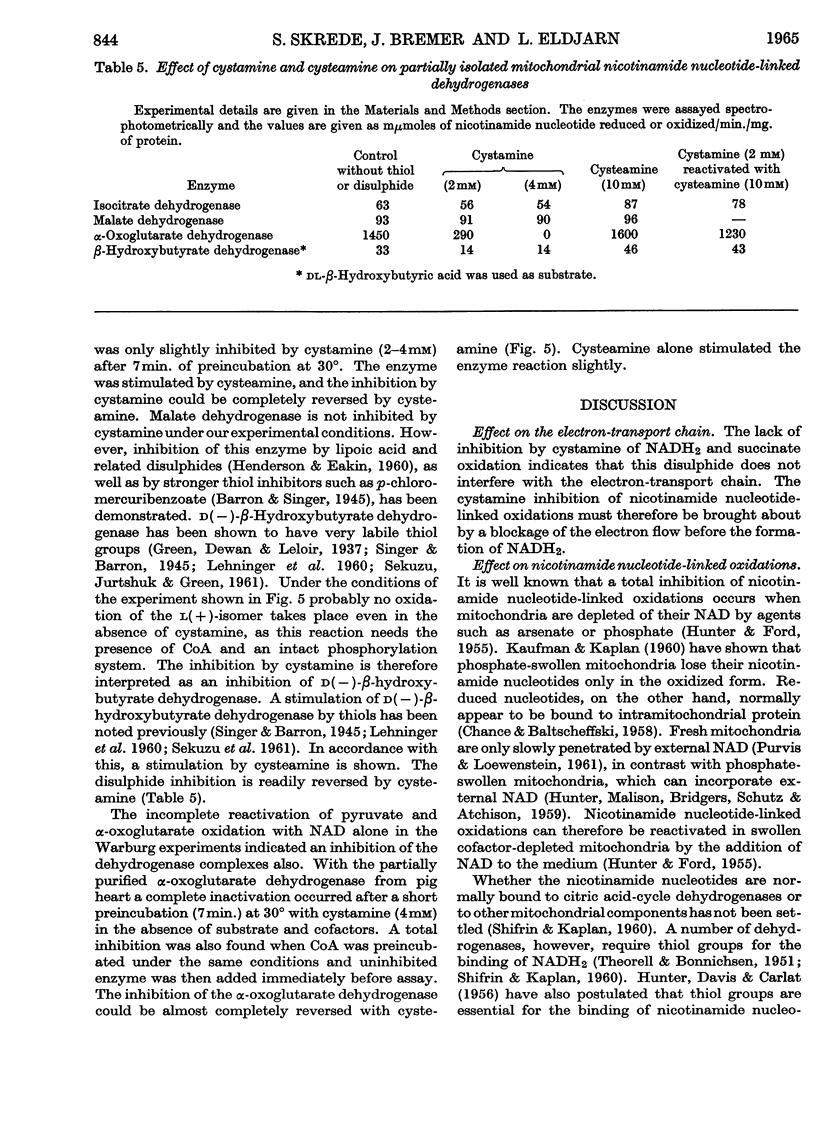
1. Nicotinamide nucleotide-linked mitochondrial oxidations were inhibited by the disulphides NNN′N′-tetraethylcystamine, cystamine and cystine diethyl ester, whereas l-homocystine, oxidized mercaptoethanol, oxidized glutathione, NN′-diacetylcystamine and tetrathionate were only slightly inhibitory. Mitochondrial oxidations were not blocked by the thiol cysteamine. 2. NAD-independent oxidations were not inhibited by cystamine. The oxidation of choline was initially stimulated. 3. The inactivation of isocitrate, malate and β-hydroxybutyrate oxidation of intact mitochondria could be partially reversed by external NAD. For the reactivation of α-oxoglutarate oxidation a thiol was also required. 4. A leakage of nicotinamide nucleotides from the mitochondria is suggested as the main cause of the inhibition. In addition, a strong inhibition of α-oxoglutarate dehydrogenase by cystamine was observed. A mixed disulphide formation with CoA and possibly also lipoic acid and lipoyl dehydrogenase is suggested to explain this inhibition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R. E., LARDY H. A., BENESCH R. The sulfhydryl groups of crystalline proteins. I. Some albumins, enzymes, and hemoglobins. J Biol Chem. 1955 Oct;216(2):663–676. [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958 Sep;233(3):736–739. [PubMed] [Google Scholar]
- CHEVREMONT S., CHEVREMONT M. Action de la beta-mercaptoethylamine sur la croissance et la mitose en culture de tissus. C R Seances Soc Biol Fil. 1953 Jan;147(1-2):164–166. [PubMed] [Google Scholar]
- CICCARONE P., MILANI R. EFFECTS OF CYSTAMINE ON THE METABOLISM OF YOSHIDA HEPATOMA ASCITES CELLS IN VITRO. Biochem Pharmacol. 1964 Feb;13:183–190. doi: 10.1016/0006-2952(64)90135-2. [DOI] [PubMed] [Google Scholar]
- ELDJARN L., BREMER J., BORRESEN H. C. The reduction of disulphides by human erythrocytes. Biochem J. 1962 Jan;82:192–197. doi: 10.1042/bj0820192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDJARN L., PIHL A. On the mode of action of x-ray protective agents. II. Interaction between biologically important thiols and disulfides. J Biol Chem. 1957 Mar;225(1):499–510. [PubMed] [Google Scholar]
- ELDJARN L., PIHL A., SVERDRUP A. The synthesis of S35-labeled hypotaurine and its metabolism in rats and mice. J Biol Chem. 1956 Nov;223(1):353–358. [PubMed] [Google Scholar]
- Green D. E., Dewan J. G., Leloir L. F. The beta-hydroxybutyric dehydrogenase of animal tissues. Biochem J. 1937 Jun;31(6):934–949. doi: 10.1042/bj0310934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON R. F., EAKIN R. E. Inhibition of malic dehydrogenase by cyclic disulfides. Biochem Biophys Res Commun. 1960 Aug;3:169–172. doi: 10.1016/0006-291x(60)90216-3. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, DAVIS J., CARLAT L. The stability of oxidative and phosphorylative systems in mitochondria under anaerobic conditions. Biochim Biophys Acta. 1956 Apr;20(1):237–242. doi: 10.1016/0006-3002(56)90282-7. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, FORD L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J Biol Chem. 1955 Sep;216(1):357–369. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, MALISON R., BRIDGERS W. F., SCHUTZ B., ATCHISON A. Reincorporation of diphosphopyridine nucleotide into mitochondrial enzyme systems. J Biol Chem. 1959 Mar;234(3):693–699. [PubMed] [Google Scholar]
- KAUFMAN B. T., KAPLAN N. O. Mechanism of depletion of mitochondrial pyridine nucleotides. Biochim Biophys Acta. 1960 Apr 8;39:332–342. doi: 10.1016/0006-3002(60)90171-2. [DOI] [PubMed] [Google Scholar]
- KIELER J. The five-membered disulphide ring system. II. Cytostatic effects. Biochem Pharmacol. 1962 Jun;11:453–466. doi: 10.1016/0006-2952(62)90228-9. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., GREVILLE G. D. The enzymic oxidation of alpha- and 2-beta-hydroxybutyrate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):188–202. doi: 10.1016/0006-3002(53)90138-3. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- LELIEVRE P. [Action of cystamine on oxygen consumption and coupled phosphorylation of rat organ mitochondria]. C R Seances Soc Biol Fil. 1963 Jul 10;157:693–696. [PubMed] [Google Scholar]
- LIPMANN F., MERIWETHER B. P., MUDD S. H., PARK C. R., PARK J. H. Glutathione and ethylenediaminetetraacetate antagonism of uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1956 Nov;22(2):403–404. doi: 10.1016/0006-3002(56)90174-3. [DOI] [PubMed] [Google Scholar]
- LOTSPEICH W. D., PETERS R. A. The action of sulphydryl inhibitors upon isocitric dehydrogenase with especial reference to the behaviour of some trivalent arsenicals. Biochem J. 1951 Oct;49(5):704–709. doi: 10.1042/bj0490704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., SUMNER J. B. D-Glyceraldehyde 3-phosphate dehydrogenase; a comparison with liver aldehyde dehydrogenase. Arch Biochem Biophys. 1952 Jul;39(1):119–128. doi: 10.1016/0003-9861(52)90266-x. [DOI] [PubMed] [Google Scholar]
- Nesbakken R., Eldjarn L. The inhibition of hexokinase by disulphides. Biochem J. 1963 Jun;87(3):526–532. doi: 10.1042/bj0870526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIHL A., ELDJARN L., BREMER J. On the mode of action of x-ray protective agents. III. The enzymatic reduction of disulfides. J Biol Chem. 1957 Jul;227(1):339–345. [PubMed] [Google Scholar]
- PIHL A., LANGE R. The interaction of oxidized glutathione, cystamine monosulfoxide, and tetrathionate with the-SH groups of rabbit muscle D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1962 Apr;237:1356–1362. [PubMed] [Google Scholar]
- PURVIS J. L., LOWENSTEIN J. M. The relation between intra- and extramitochondrial pyridine nucleotides. J Biol Chem. 1961 Oct;236:2794–2803. [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W., BOCK R. M. Studies on alpha-ketoglutaric oxidase. II. Purification and properties. J Biol Chem. 1952 May;197(2):851–862. [PubMed] [Google Scholar]
- SANNER T., PIHL A. Studies on the active--SH group of papain and on the mechanism of papain activation by thiols. J Biol Chem. 1963 Jan;238:165–171. [PubMed] [Google Scholar]
- SEARLS R. L., PETERS J. M., SANADI D. R. alpha-Ketoglutaric dehydrogenase. X. On the mechanism of dihydrolipoyl dehydrogenase reaction. J Biol Chem. 1961 Aug;236:2317–2322. [PubMed] [Google Scholar]
- SEARLS R. L., SANADI D. R. alpha-Ketoglutaric dehydrogenase. 8. Isolation and some properties of a flavoprotein compnent. J Biol Chem. 1960 Aug;235:2485–2491. [PubMed] [Google Scholar]
- WALKER J. B., WALKER M. S. Inhibition of sulfhydryl enzymes by formamidine disulfide. Arch Biochem Biophys. 1960 Jan;86:80–84. doi: 10.1016/0003-9861(60)90372-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G. R. The limitation by ionic solutions of the access of substrate to mitochondrial choline oxidase. J Biol Chem. 1960 Apr;235:1192–1195. [PubMed] [Google Scholar]


