Abstract
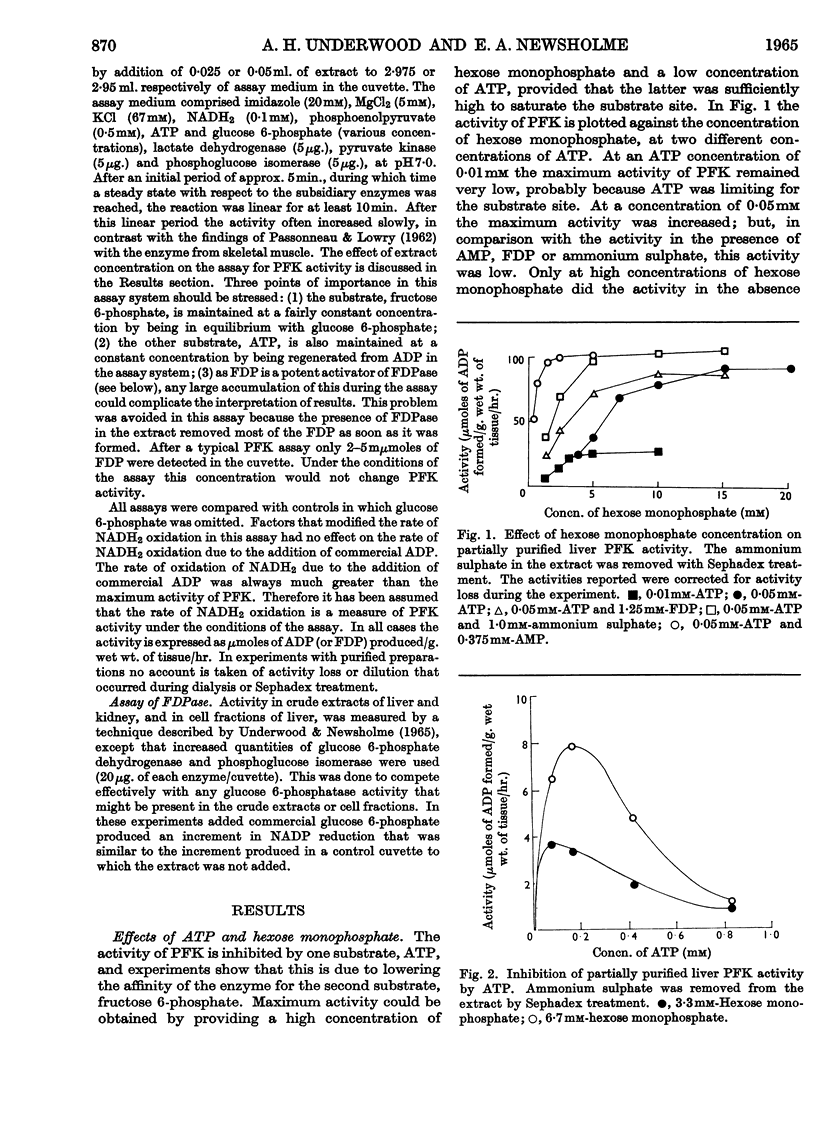
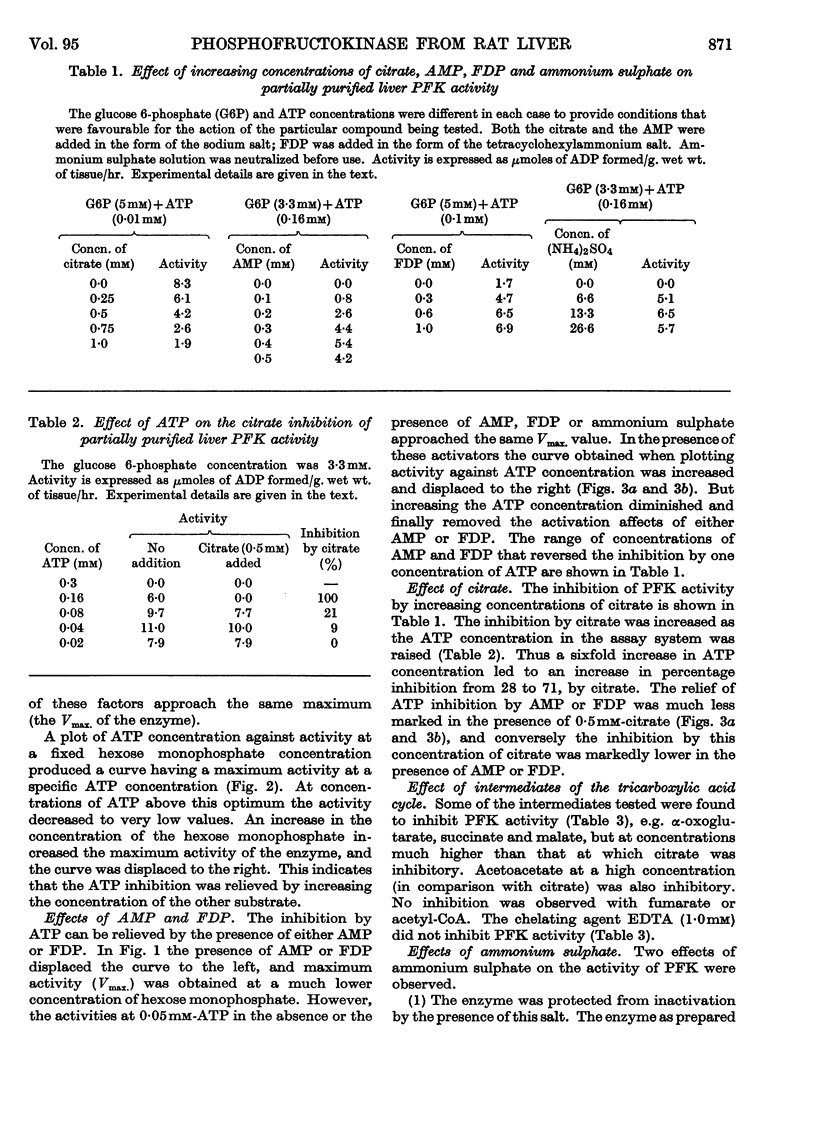
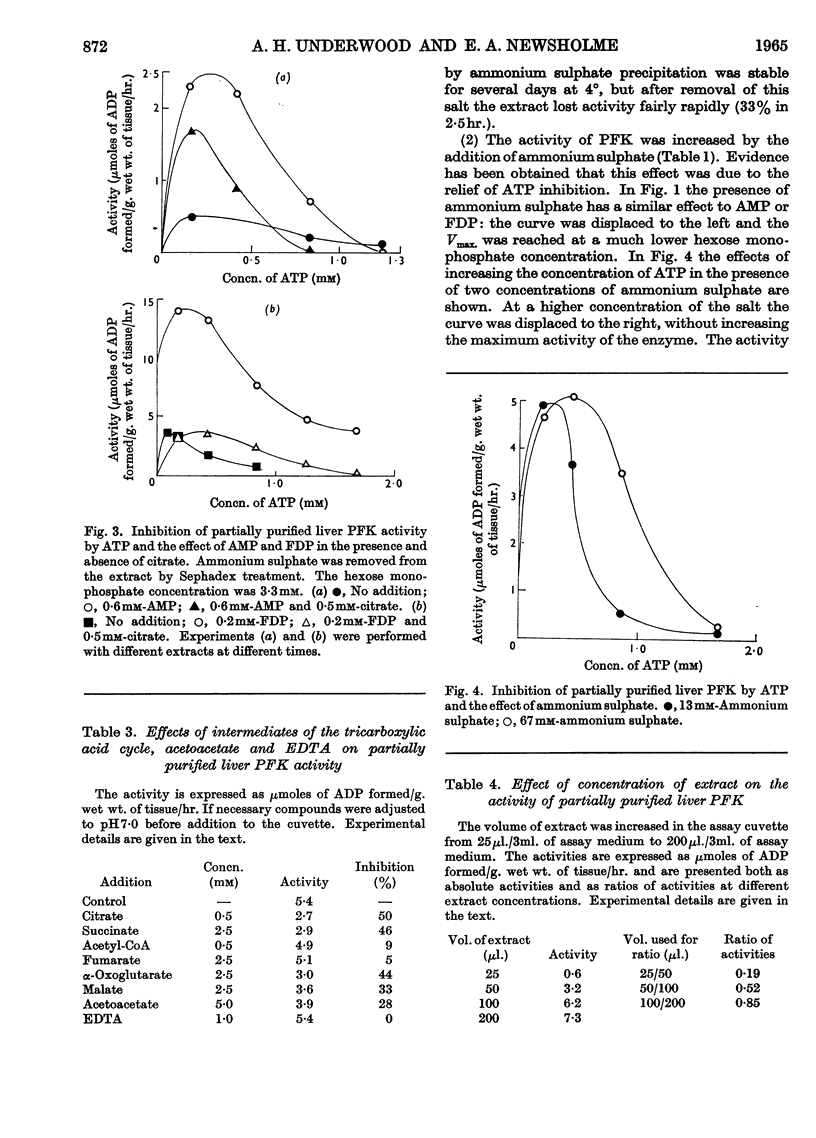
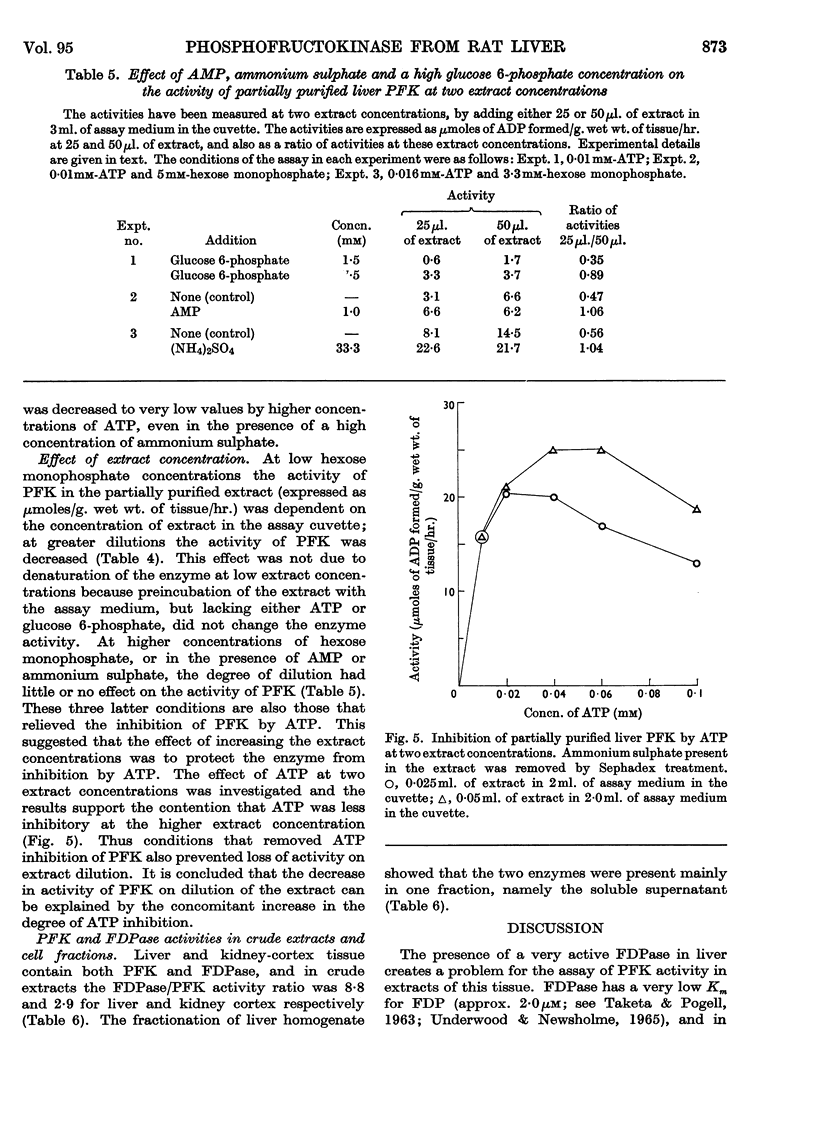
1. Phosphofructokinase from rat liver has been partially purified by ammonium sulphate precipitation so as to remove enzymes that interfere in one assay for phosphofructokinase. The properties of this enzyme were found to be similar to those of the same enzyme from other tissues (e.g. cardiac muscle, skeletal muscle and brain) that were previously investigated by other workers. 2. Low concentrations of ATP inhibited phosphofructokinase activity by decreasing the affinity of the enzyme for the other substrate, fructose 6-phosphate. Citrate, and other intermediates of the tricarboxylic acid cycle, also inhibited the activity of phosphofructokinase. 3. This inhibition was relieved by either AMP or fructose 1,6-diphosphate; however, higher concentrations of ATP decreased and finally removed the effect of these activators. 4. Ammonium sulphate protected the enzyme from inactivation, and increased the activity by relieving the inhibition due to ATP. The latter effect was similar to that of AMP. 5. Phosphofructokinase was found in the same cellular compartment as fructose 1,6-diphosphatase, namely the soluble cytoplasm. 6. The properties of phosphofructokinase and fructose 1,6-diphosphatase are compared and a theory is proposed that affords dual control of both enzymes in the liver. The relation of this to the control of glycolysis and gluconeogenesis is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAHILL G. F., Jr, ASHMORE J., RENOLD A. E., HASTINGS A. B. Blood glucose and the liver. Am J Med. 1959 Feb;26(2):264–282. doi: 10.1016/0002-9343(59)90316-x. [DOI] [PubMed] [Google Scholar]
- GETZ G. S., BARTLEY W. The intracellular distribution of fatty acids in rat liver. The fatty acids of intracellular compartments. Biochem J. 1961 Feb;78:307–312. doi: 10.1042/bj0780307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Salas M. L., Salas M., Sols A. Two interconvertible forms of yeast phosphofructokinase with different sensitivity to endproduct inhibition. Biochem Biophys Res Commun. 1964 Mar 26;15(3):243–249. doi: 10.1016/0006-291x(64)90154-8. [DOI] [PubMed] [Google Scholar]
- Wu R. Control of glycolysis by phosphofructokinase in slices of rat liver, Novikoff hepatoma, and adenocarcinomas. Biochem Biophys Res Commun. 1964;14:79–85. doi: 10.1016/0006-291x(63)90215-8. [DOI] [PubMed] [Google Scholar]


