Abstract
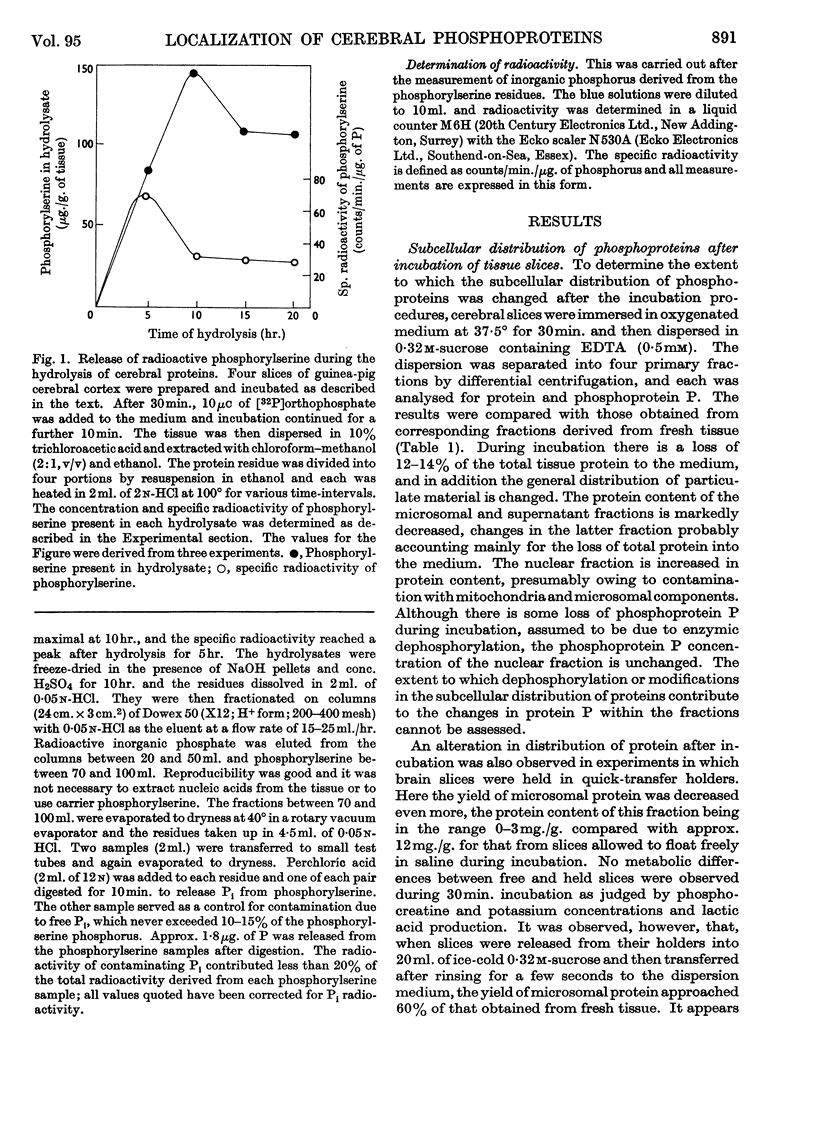
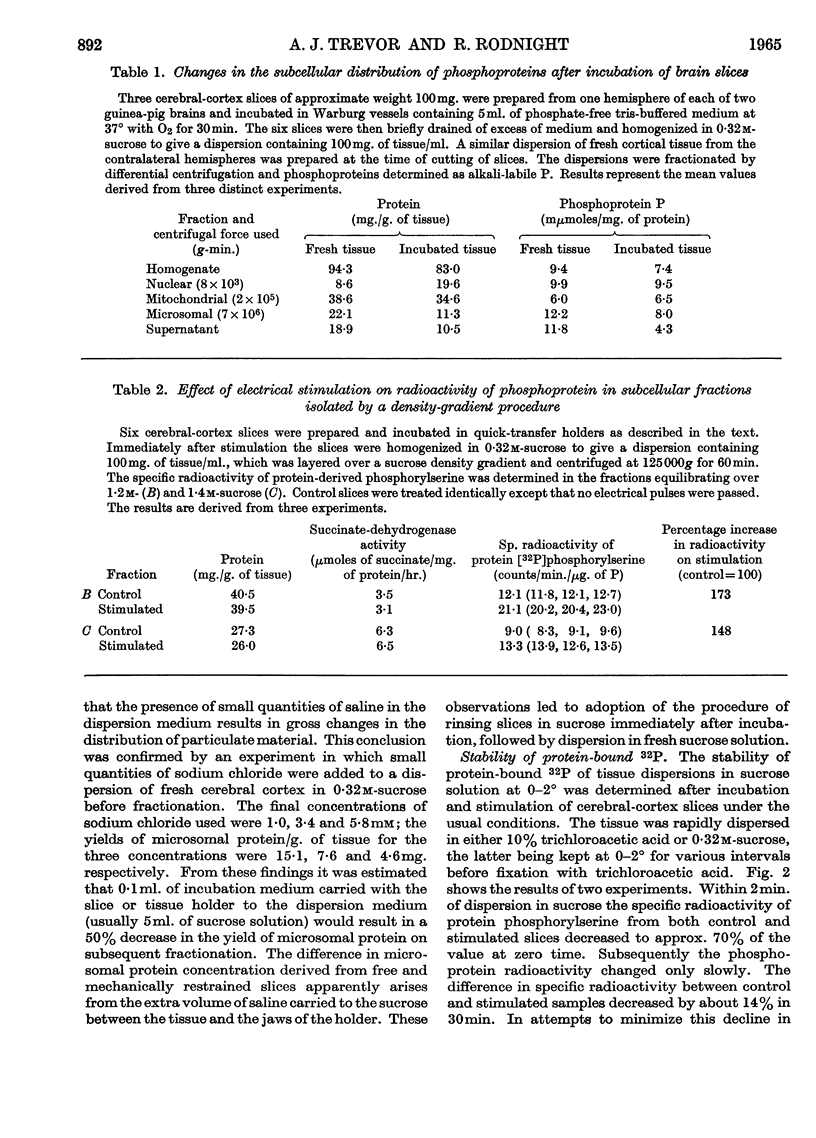
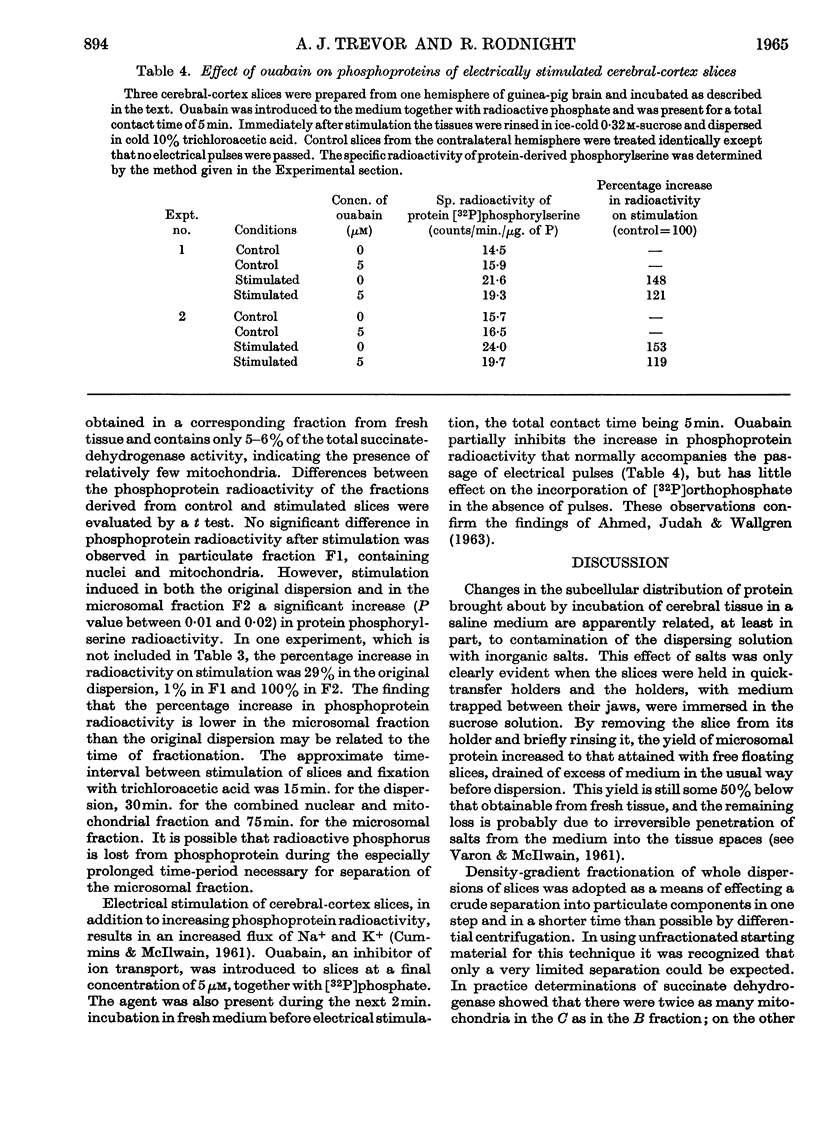
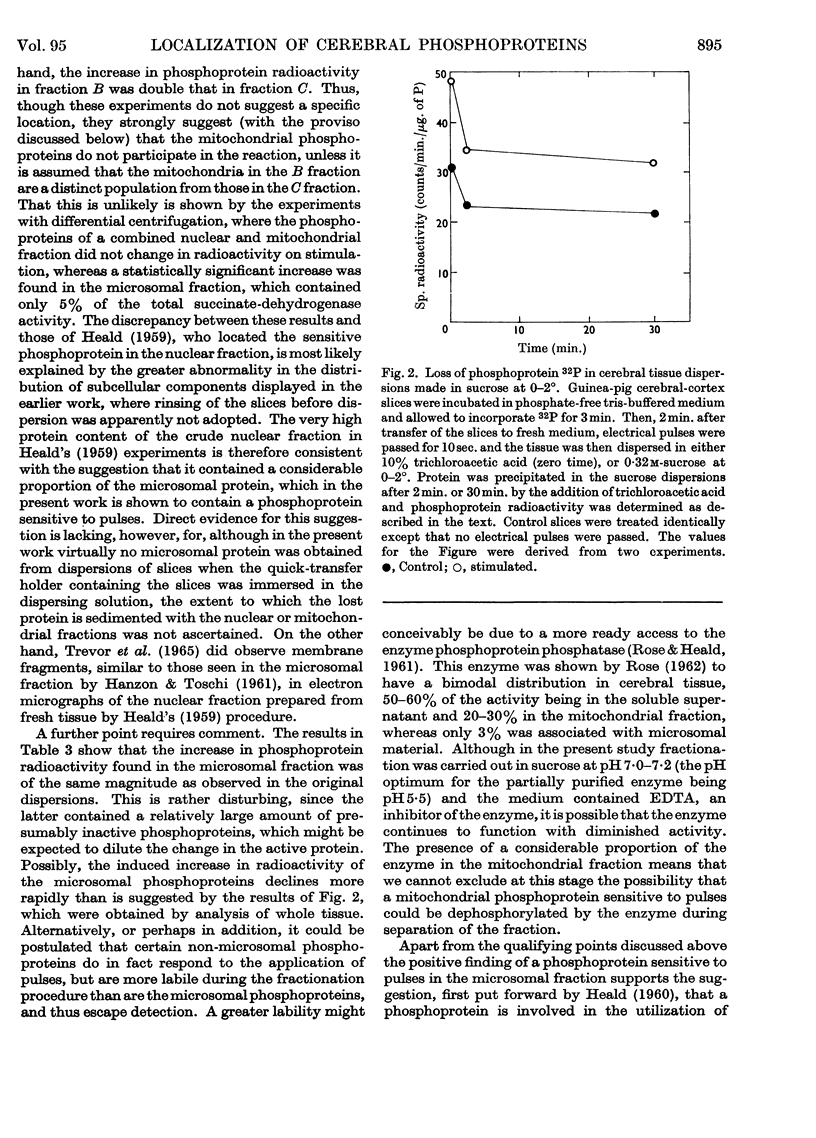
1. On incubating cerebral-cortex slices at 37° in an oxygenated medium marked changes resulted in the subcellular distribution of proteins and phosphoproteins in the tissue. The protein content of the nuclear fraction more than doubled, whereas the yields of microsomal and supernatant proteins were both markedly decreased. The amount of phosphoprotein/mg. of protein decreased in the microsomal and supernatant fractions, but showed little change in the nuclear and mitochondrial fractions. The loss of microsomal protein could be partly prevented by rinsing the slices briefly in cold sucrose solution before dispersion; the altered subcellular distribution was apparently related to contamination of the dispersing solution with traces of salts from the medium. 2. The subcellular location of the phosphoprotein sensitive to the effects of electrical pulses applied to cerebral slices in vitro has been reinvestigated by two different procedures. Comparison between unstimulated and stimulated slices after incubation in the presence of [32P]orthophosphate showed that phosphoprotein radioactivity increased on stimulation to a greater extent in a membrane-rich fraction than in a mitochondria-rich fraction, these being obtained by immediate density-gradient fractionation of the tissue dispersion. With fractions isolated by differential centrifuging the percentage increase in a combined mitochondrial and nuclear fraction was 5% as compared with 24% (P<0·02) in the microsomal fraction and 30% in the original dispersion before fractionation. The sensitive phosphoprotein therefore appears to be located in structures sedimenting with the microsomal fraction, rather than with the nuclear fraction as previously claimed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., JUDAH J. D., WALLGREN H. Phosphoproteins and ion transport of cerebral cortex slices. Biochim Biophys Acta. 1963 Feb 5;69:428–430. doi: 10.1016/0006-3002(63)91285-x. [DOI] [PubMed] [Google Scholar]
- AYRES P. J., McILWAIN H. Techniques in tissue metabolism. II. Application of electrical impulses to separated tissues in aqueous media. Biochem J. 1953 Nov;55(4):607–617. doi: 10.1042/bj0550607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. T., McIlwain H. Electrical pulses and the potassium and other ions of isolated cerebral tissues. Biochem J. 1961 May;79(2):330–341. doi: 10.1042/bj0790330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. Analysis of radioactive phosphates in extracts of cerebral tissues. Biochem J. 1956 Jun;63(2):235–242. doi: 10.1042/bj0630235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. Phosphorylserine and cerebral phosphoprotein. Biochem J. 1958 Apr;68(4):580–584. doi: 10.1042/bj0680580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. Studies on the phosphoproteins of brain: the intracellular localization in brain of a phosphoprotein involved in the metabolic response of cortical slices to electrical stimulation. Biochem J. 1959 Sep;73:132–141. doi: 10.1042/bj0730132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. The incorporation of phosphate into cerebral phosphoportein promoted by electrical impulses. Biochem J. 1957 Aug;66(4):659–663. doi: 10.1042/bj0660659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- ROSE S. P., HEALD P. J. A phosphoprotein phosphatase from ox brain. Biochem J. 1961 Nov;81:339–347. doi: 10.1042/bj0810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE S. P. The localization of cerebral phosphoprotein phosphatase. Biochem J. 1962 Jun;83:614–622. doi: 10.1042/bj0830614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSTEIN A. Functional implications of interactions of extracellular ions with ligands of the cell membrane. Circulation. 1962 Nov;26:1189–1200. doi: 10.1161/01.cir.26.5.1189. [DOI] [PubMed] [Google Scholar]
- Rodnight R., Lavin B. E. Phosvitin kinase from brain: activation by ions and subcellular distribution. Biochem J. 1964 Oct;93(1):84–91. doi: 10.1042/bj0930084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFER N. K., MAY S. C., Jr, SUMMERSON W. H. Serine phosphoric acid from diisopropylphosphoryl chymotrypsin. J Biol Chem. 1953 May;202(1):67–76. [PubMed] [Google Scholar]
- TREVOR A. J., RODNIGHT R., SCHWARTZ A. THE SUBCELLULAR DISTRIBUTION OF CEREBRAL PHOSPHOPROTEINS. Biochem J. 1965 Jun;95:883–888. doi: 10.1042/bj0950883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARON S., McILWAIN H. Fluid content and compartments in isolated cerebral tissues. J Neurochem. 1961 Dec;8:262–275. doi: 10.1111/j.1471-4159.1961.tb13552.x. [DOI] [PubMed] [Google Scholar]
- WOLFE L. S., McILWAIN H. Migration of histones from the nuclei of isolated cerebral tissues kept in cold media. Biochem J. 1961 Jan;78:33–40. doi: 10.1042/bj0780033. [DOI] [PMC free article] [PubMed] [Google Scholar]


