Abstract
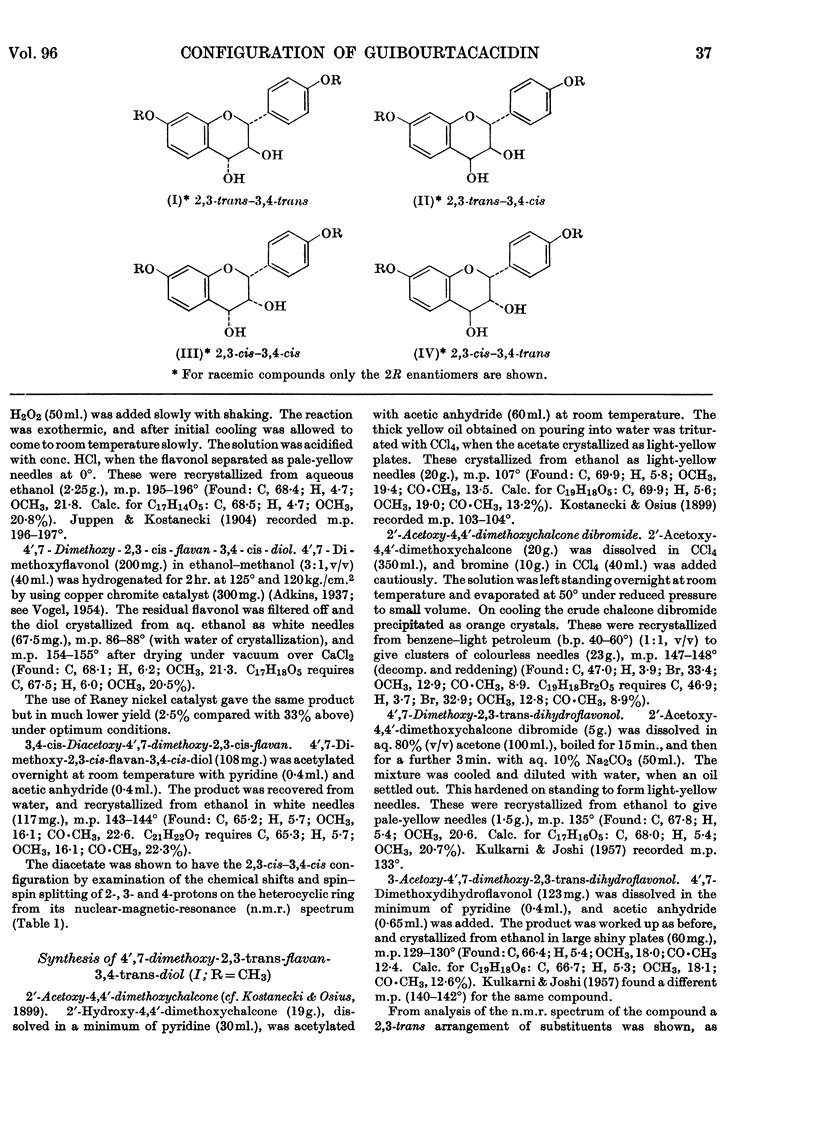
1. Diacetates of the four possible racemates of 4′,7-dimethoxyflavan-3,4-diol have been synthesized. 2. Comparison of their nuclear-magnetic-resonance spectra and the ionophoretic mobilities of the diols in borate buffer with those of the corresponding derivatives of guibourtacacidin shows that the natural 4′,7-dihydroxyflavan-3,4-diol has a 2,3-cis–3,4-trans configuration, but is accompanied by 2,3-trans–3,4-trans and 2,3-trans–3,4-cis isomers. These occur in the approximate proportions 5:1:1. 3. The occurrence of guibourtacacidins in Guibourtia coleosperma appears to be of taxonomic significance. Their association with a large excess of related tannins in the heartwood suggests that flavan-3,4-diols with these configurations are suitable precursors in tannin biosynthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DREWES S. E., ROUX D. G. CONDENSED TANNINS. OPTICALLY ACTIVE DIASTEREOISOMERS OF (+)-MOLLISACACIDIN BY EPIMERIZATION. Biochem J. 1965 Feb;94:482–487. doi: 10.1042/bj0940482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes S. E., Roux D. G. Condensed tannins. 19. Configuration of diol groups in flavan-3,4-diols by paper chromatography and paper ionophoresis. Biochem J. 1964 Sep;92(3):555–559. doi: 10.1042/bj0920555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux D. G., De Bruyn G. C. Condensed tannins. 17. Isolation of 4',7-dihydroxyflavan-3,4-diol from Guibourtia coleosperma. Biochem J. 1963 May;87(2):439–444. doi: 10.1042/bj0870439. [DOI] [PMC free article] [PubMed] [Google Scholar]


