Abstract
A quantitative PCR test, the Cobas Amplicor CMV Monitor, was used for the monitoring of viral load in the peripheral blood of 27 individual liver transplant patients and correlated with cytomegalovirus (CMV) pp65 antigenemia. Altogether, 243 specimens were analyzed. During the first 3 months, 20 patients showed PCR positivity which correlated with pp65 antigenemia. Of those, 13 patients developed symptomatic CMV infection 27 to 52 days after transplantation, with a significantly higher peak viral load in PCR and in pp65 assay compared with the seven asymptomatic infections (median 10,200 versus 2,240 copies/ml, P < 0.05, and median 100 versus 30 pp65-positive cells/50,000 leukocytes, P < 0.01). Five were primary infections of D+/R− cases (donor CMV seropositive and recipient seronegative) and demonstrated, except in one case, a high peak viral load (>10,000 copies/ml; range, 10,200 to 21,600 copies, and ≥50 positive cells, range, 50 to 800 cells). The peak viral loads of the six D+/R+ patients with symptomatic infection varied widely (range, 2,290 to 126,000 copies and 50 to 300 positive cells). Two D−/R+ patients developed symptomatic infection with a lower viral load (range, 1,120 to 6,510 copies and 25 to 100 positive cells). All symptomatic infections were successfully treated with ganciclovir. The asymptomatic infections all in D+/R+ patients with low copy numbers (<5,500 copies) were monitored until CMV disappeared. One of the seven PCR-negative patients had one sample with low antigenemia, but the subsequent specimens were all negative. The time-related correlation of the two methods was also good. In summary, quantitative PCR could equally well be used as the CMV pp65 assay for the monitoring of viral load in individual transplant patients.
Cytomegalovirus (CMV) infection is a common complication after liver transplantation. A variety of clinical manifestations of CMV, such as fever, leukopenia, thrombocytopenia, colitis, pneumonia, and hepatitis, have been described (16). Immunosuppressed organ transplant patients are usually frequently monitored for CMV and treated with antiviral agents. The antiviral treatment is based on the clinical symptoms and/or rapid laboratory diagnosis. Also, preemptive therapy, which is commonly used to prevent the development of CMV disease after transplantation, is based on monitoring the viral load of the patients (5, 6, 15, 20).
For more than 10 years, the CMV-pp65 antigenemia assay has been the most commonly used method for monitoring the appearance of CMV infection in transplant patients (2, 8, 22, 25). The antigenemia test is quantitative and can also be used to assess viral load and to monitor the response to antiviral treatment. Although there have been attempts to standardize the assay (8, 23), the great variety of in-house and commercial modifications of the method make it difficult to compare the results and run clinical trials based on this technique. Quantitative PCR techniques, which are easy to standardize, are less laborious for laboratory personnel, and can be automated, are slowly replacing the pp65 test for the follow-up of transplant patients. Most virological laboratories also run other PCR diagnostic methods, and the CMV PCR can easily be integrated in such laboratories.
Recently, commercial quantitative PCR methods have became available and have proven to be useful in the determination of viral load (1-4, 7, 9-12, 14, 17, 18-20, 22, 24). In the pp65 assay, the number of CMV-positive cells in the peripheral blood reflects the viral load, and high numbers of positive cells correlate with symptomatic infection. Although quantitative PCR methods are widely used in CMV diagnostics, the clinical correlation of the findings is not as clear as that with the pp65 assay. High viral loads, measured by quantitative DNA-PCR methods, have been shown to correlate with the appearance of symptomatic CMV infection in a similar way (3, 4, 7, 12, 17, 21, 24). As the tendency nowadays is towards replacement of the pp65 assay with PCR methods, the clinical use of these methods should be evaluated in parallel, not only in the diagnosis of CMV infection but also in the monitoring of individual transplant patients during an active infection.
We and others have previously demonstrated that there is a good correlation between the CMV pp65 antigenemia assay and a commercial quantitative DNA-PCR test, the Cobas Amplicor CMV Monitor, in the diagnosis of CMV infection of organ transplant patients (4, 17, 20, 24). In our previous study, the sensitivity of the plasma PCR test was 86% and the specificity was 94% in the diagnosis of CMV infection (17). Although the usefulness of the PCR method in the diagnosis of CMV infection has been proven by several groups, the follow-up of individual patients during the infection and antiviral treatment has not been studied previously.
In this study, we report the follow-up of clinically significant, ganciclovir-treated and asymptomatic, nontreated CMV infections in individual liver transplant patients in whom the viral load was monitored by both the quantitative plasma PCR test and the pp65 antigenemia assay.
MATERIALS AND METHODS
Patients.
Twenty-seven adult liver allograft recipients transplanted between 1999 and 2000 were frequently monitored for CMV with the pp65 antigenemia test and quantitative plasma PCR during the first 3 months after transplantation. Most patients (20 of 27, 74%) were CMV seropositive before transplantation. According to the CMV serostatus of the organ donor and the recipient, the patients were of the following subgroups: D+/R+ (n = 15), D+/R− (n = 6), D−/R+ (n = 5), and D−/R− (n = 1). As basic immunosuppressive therapy, the patients received triple drug therapy with various combinations of steroids, azathioprine, and cyclosporin or tacrolimus. Rejections were treated with high doses of steroids. numberroutine antiviral prophylaxis was given besides ganciclovir prophylaxis (5 mg/kg twice daily for 5 to 10 days intravenously) when acute rejection occurred. Symptomatic CMV infections were treated with intravenous ganciclovir (5 mg/kg twice daily) for at least 2 weeks. Diagnosis of symptomatic CMV infection was based on positive pp65 antigenemia assay results and clinical signs of CMV infection, typically fever which could not be explained otherwise. In addition to the pp65 antigenemia test and quantitative PCR, rapid shell vial cultures were performed from liver biopsies or bronchoalveolar lavage specimens when these were taken for the organ-specific diagnosis of CMV infection.
Blood specimens.
The clinical specimen material consisted of 243 consecutive blood samples from adult liver transplant patients. Peripheral blood specimens were obtained weekly during the patients' hospitalization and thereafter once every 1 to 2 weeks, up to 3 months after transplantation and in any case of suspected viral infection. In the case of symptomatic CMV infection and ganciclovir treatment, the patients were monitored to follow their response to the antiviral treatment. In the case of asymptomatic infections, the patients were monitored for CMV until the pp65 antigenemia disappeared. Blood specimens were used for both PCR and the pp65 assay. The analyses were performed in parallel from samples obtained concurrently.
CMV PCR test.
The EDTA-treated blood samples were centrifuged within 24 h after collection, and the plasma was used for the PCR analysis. The commercial Cobas Amplicor CMV Monitor Test (Roche) was used for the quantification of CMV DNA in the samples. The test was run according to the manufacturer's instructions. Briefly, DNA was isolated from plasma samples by lysis of virus particles, followed by alcohol precipitation. Adding a known number of quantitation standard DNA molecules to each sample compensated for the effects of inhibition. The quantitation standard was plasmid DNA containing the same primer binding sites as the CMV target and a unique probe-binding region. The biotinylated primers were chosen from the DNA polymerase gene, and the length of the amplified product was 365 bp. PCR products were serially diluted to achieve a larger dynamic range for quantification in the hybridization step. Specific probes were used for CMV and quantitation standard products. CMV DNA levels were calculated in the test sample by comparing the CMV signal to the quantitation standard signal for each sample. The linear area of the method was 400 to 100,000 copies/ml of plasma.
CMV pp65 antigenemia test.
The standard CMV pp65 antigenemia assay was performed in parallel (13, 22, 24). The leukocytes from peripheral blood specimens were isolated and cytocentrifuged onto microscope slides. The slides were dried at room temperature and fixed in cold acetone. Immunoperoxidase staining and a monoclonal antibody against CMV pp65 antigen (Biotest, Frankfurt, Germany) were used to demonstrate the viral proteins in the leukocytes. The positive results were quantified by counting the number of pp65-expressing cells per 50,000 leukocytes on the slide, as described for the original CMV pp65 antigenemia assay (22, 25). The viral loads of individual patients are demonstrated as PCR results (copies per milliliter) and compared with the results of the quantitative pp65 antigenemia test (number of positive cells).
Statistical analysis.
For statistical analysis, the Mann-Whitney U test was used. The results were expressed as medians, and P values of <0.05 were considered significant.
RESULTS
Monitoring of individual patients.
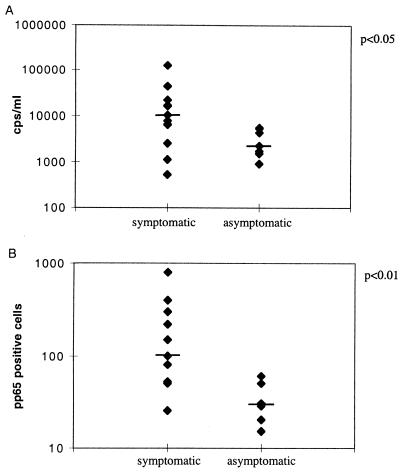
By frequent monitoring, CMV DNA and pp65 were detected in the blood specimens of 20 patients (numbered 1 to 20). Of these patients, 13 developed a clinically significant, symptomatic CMV infection 27 to 52 days after transplantation, with a significantly higher peak viral load in PCR and in the pp65 assay than the seven asymptomatic infections (median 10,200 versus 2,240 cps/ml, P < 0.05, and median 100 versus 30 pp65-positive cells/50,000 leukocytes, P < 0.01), as demonstrated in Fig. 1. All symptomatic CMV infections were successfully treated with intravenous ganciclovir.
FIG. 1.
Median values of peak viral loads of symptomatic and asymptomatic CMV infections demonstrated by PCR and by the pp65 antigenemia assay (positive cells/50,000 leukocytes).
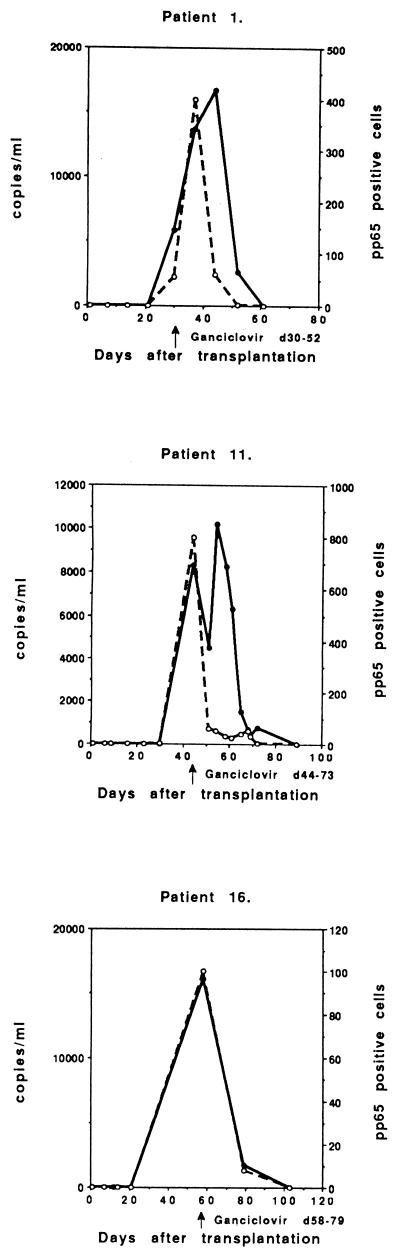
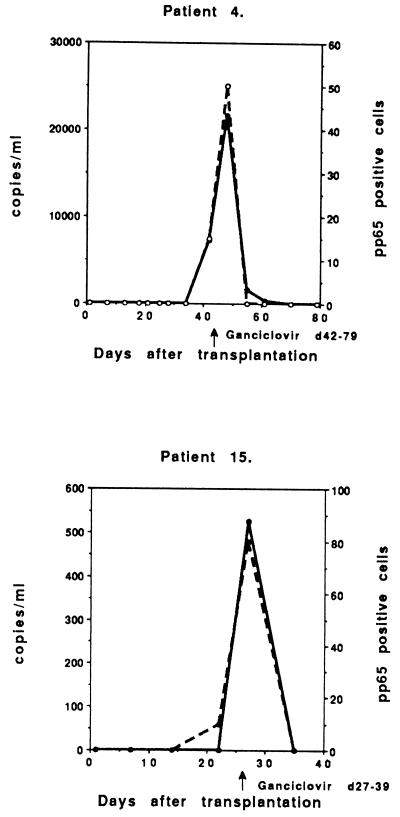
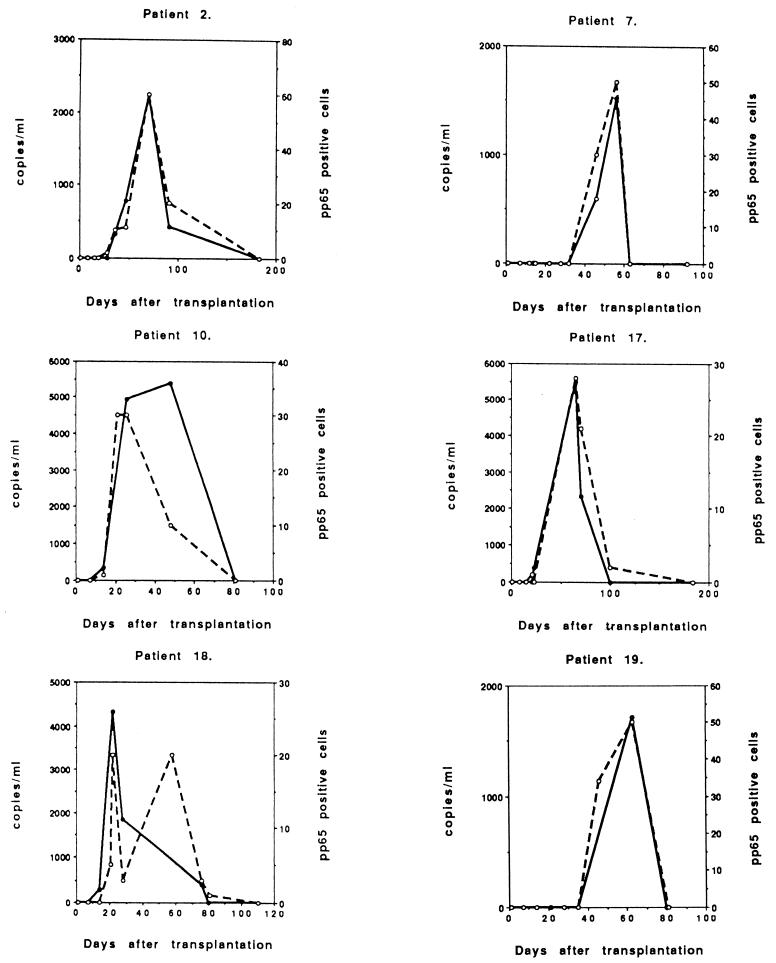
Five of the 13 symptomatic infections were primary infections (D+/R−), with the peak viral load occurring 27 to 52 days after transplantation (Fig. 2). All patients were successfully treated with intravenous ganciclovir. The peak CMV DNA level was usually high (in four cases >10,000 copies/ml; range, 10,200 to 21,600 copies), which correlated very well with the peak of CMV pp65 antigenemia (≥50 positive cells/50,000 leukocytes; range, 50 to 800 positive cells). One patient (number 15) demonstrated a low level of CMV DNA (526 copies/ml) at day 27, but had already had a significant pp65 antigenemia (80 positive cells/50,000) and was immediately treated with ganciclovir.
FIG. 2.
Follow-up of copy numbers from PCR (solid circles and continuous line) and positive cells from the pp65 test (open circles and broken line) of symptomatic primary CMV infections of individual D+/R− patients treated with ganciclovir (n = 5).
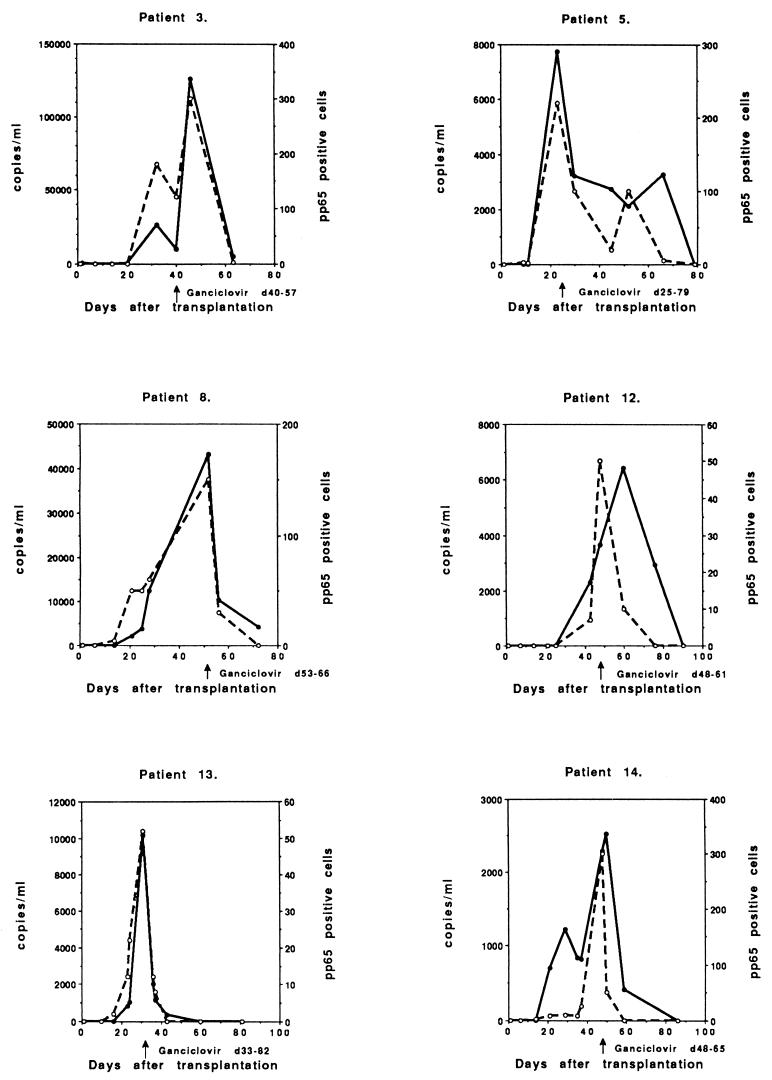
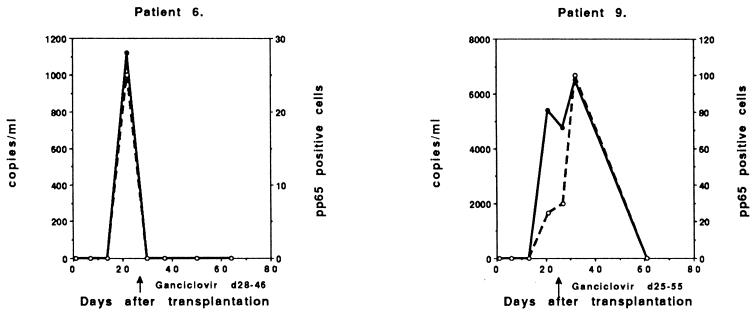
Six patients with symptomatic, ganciclovir-treated CMV infections had reactivations or reinfections (D+/R+), with peak viral load occurring 23 to 52 days after transplantation (Fig. 3). These patients also demonstrated a high peak of CMV DNA (>10,000 copies/ml; range, 10,200 to 126,000 copies) and CMV pp65 (>50 positive cells/50,000 leukocytes; range, 52 to 300 positive cells) in three cases. Three patients had a lower peak of CMV DNA (<10,000 copies/ml; range, 2,290 to 7,730 copies), but the peak antigenemia was approximately the same level as that of the other D+/R+ patients (>50 positive cells/50,000 leukocytes; range, 50 to 300 positive cells).
FIG. 3.
Follow-up of symptomatic CMV infections in individual ganciclovir-treated D+/R+ patients (n = 6).
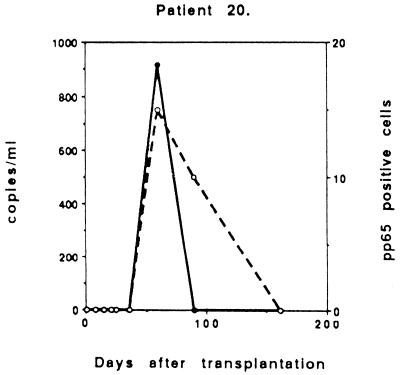
Two patients probably had symptomatic reactivation (D−/R+), though reinfection could not be excluded, with the peak viral load occurring 22 and 32 days after transplantation (Fig. 4). The viral load was low (1,120 copies and 25 pp65-positive cells) in one case (number 6), but the patient had undergone retransplantation and was immediately treated when CMV was detected together with fever. The other patients demonstrated a moderate peak viral load (6,510 copies and 100 positive cells).
FIG. 4.
Two patients with symptomatic, ganciclovir-treated CMV infection in the D−/R+ group.
In addition to the symptomatic and treated patients, seven patients developed asymptomatic CMV infections, with a peak 22 to 70 days posttransplantation, and were not treated with antivirals (Fig. 5). All asymptomatic infections were reactivations or reinfections (D+/R+). The peak of CMV DNA was significantly lower than that of the symptomatic infections (Table 1) and exceeded <5,500 copies/ml (range, 915 to 5,490 copies). The pp65 antigenemia levels were also significantly lower than those of the symptomatic patients (range, 15 to 60 positive cells). These patients were also monitored until no evidence of CMV was detected in the peripheral blood. They did not develop symptomatic CMV infection.
FIG. 5.
Follow-up of copy numbers and pp65-positive cells of individual asymptomatic nontreated CMV infections in individual D+/R+ patients (n = 7).
TABLE 1.
Peak viral loads demonstrated by CMV PCR and the pp65 antigenemia assay in different patient groups
| Status | No. of patients | PCR peak range, copies/ml (median) | pp65 assay peak range, positive cells/50,000 leukocytes (median) |
|---|---|---|---|
| D+/R− (symptomatic) | 5 | 526–21,600 (16,100) | 50–800 (100) |
| D+/R+ (symptomatic) | 6 | 2,520–126,000 (8,965) | 50–300 (185) |
| D−/R+ (symptomatic) | 2 | 1,120–6,510 (3,815) | 25–100 (62) |
| D+/R+ (asymptomatic) | 7 | 915–5,490 (2,240) | 15–60 (29) |
Table 1 demonstrates the results of the two quantitative methods, PCR and the pp65 assay, at the peak viral load for each patient group.
Seven patients remained PCR negative for CMV throughout the follow-up period. One of these patients demonstrated a very low pp65 antigenemia (3 positive cells) 76 days after transplantation. The subsequent specimens were, however, all negative. There were PCR-negative patients from each of the D/R subgroups: D+/R− (n = 1), who had received ganciclovir prophylaxis during rejection treatment, D+/R+ (n = 2), D−/R+ (n = 3), and D−/R− (n = 1).
General correlation between CMV PCR and CMV pp65 results.
In general, the appearance of CMV DNA in the plasma and pp65-positive cells in the blood correlated well. Eight of 20 patients developed antigenemia before the DNA appeared. In these cases, the levels of antigenemia were very low (1 to 10 positive cells/50,000 leukocytes). On the other hand, in two patients low levels of CMV DNA (282 to 478 cps/ml of plasma) appeared before the antigenemia. In three cases, pp65-positive cells were seen in the blood after CMV DNA had disappeared from the plasma. The antigenemia levels were, however, low (1 to 10 positive cells/50,000 leukocytes). In four cases, low levels of CMV DNA (346 to 2,940 copies/ml) were seen after the antigen had already disappeared. All four of these cases represented patients given antiviral treatment.
DISCUSSION
Modern strategies for the prevention and therapy of CMV infection and disease in organ transplantation include prophylaxis, preemptive therapy, and treatment of symptomatic infections (15, 20). The overall recommendations are generally accepted: prophylaxis should be given to high-risk patients, such as seronegative recipients receiving an organ from a seropositive donor and those given anti-T-cell antibodies as induction therapy, preemptive therapy based on the frequent monitoring of the patient either by PCR, pp65 antigenemia, or shell vial cultures, and treatment of the disease based on clinical signs and on adequate virological methods. In practice, however, numerous variations of the strategies exist in different transplant centers depending on the availability of the laboratory service and also on the economic and organizational factors.
In our population, CMV seroprevalence was high, 70 to 80%, and transplant recipients were usually seropositive (74% in this study). Thus, CMV infections are normally reactivations and often asymptomatic, as was the case in 35% of all CMV infections in this study. Severe life-threatening CMV disease is also quite rare in this patient population. At our center, routine antiviral prophylaxis is not given, but transplant recipients, especially the few seronegative patients, are carefully monitored. The patients receive intravenous ganciclovir prophylaxis only during rejection treatment with high doses of immunosuppressive drugs. Based on frequent monitoring, symptomatic CMV infections are immediately treated with intravenous ganciclovir for at least 2 weeks. The frequent monitoring is based on rapid methods, such as pp65 antigenemia and nowadays also quantitative PCR, and laboratory results can be provided daily.
Our previous (17) and present experiences with this plasma PCR method are in accordance with recent reports from other groups (4, 12). For predicting CMV disease, the quantitative plasma PCR has been found useful for transplant patients. In a previous study, the mean peak viral load (73,715 copies/ml; range, 9,230 to 195,000) of the symptomatic infections was significantly higher than that of asymptomatic CMV infections (3,615 copies/ml; range, 400 to 15,900) (12). In that study, the optimal cutoff level for predicting CMV disease was found for a viral load in the range from 2,000 to 5,000 copies/ml. In another study, the cutoff level of low sensitivity of 40,000 copies/ml could be used for differential diagnosis of CMV disease, but a lower cutoff level of 1,000 copies/ml improved the sensitivity (24). These viral load levels are quite similar to our findings (17).
In the present study, high peak viral loads were seen especially in the D+/R− patient group with primary CMV infections (>10,000 copies/ml), although the patients were immediately treated with ganciclovir after the first positive CMV finding. In the symptomatic infections of the D+/R+ patients, the peak viral load varied greatly, as it did in the D−/R+ patients. The overall viral peak loads of the symptomatic infections were significantly higher than those of the asymptomatic infections (median 10,200 versus 2,240 copies/ml). The peak viral loads of the asymptomatic nontreated D+/R+ patients did not exceed 5,500 copies/ml, and only two patients had viral loads of over 5,000 copies/ml. Thus, it might be that the optimal cutoff level for the seropositive liver transplant patient population would be 2,000 to 5,000 copies/ml.
In the monitoring of individual patients, the usefulness of the quantitative PCR method has not been described previously. In our study, this quantitative PCR method was also suitable for monitoring the response to antiviral treatment. All infections were successfully treated with ganciclovir, and the viral load declined. Compared with the duration of pp65 antigenemia, the PCR follow-up curves of individual patients were almost uniform. During the antiviral treatment, PCR positivity lasted a little longer that pp65 antigenemia in 4 of 13 cases. However, there were no significant discrepancies between the two methods in the response to antiviral treatment. The nontreated asymptomatic patients, who were monitored until the pp65 antigenemia and CMV DNA-emia disappeared, also demonstrated concordant follow-up curves. Thus, the organ transplant patient population with mainly asymptomatic or mild CMV reactivations can also be monitored in similar ways by quantitative PCR and by pp65 antigenemia.
In summary, the quantitative PCR test produced follow-up curves of viral loads of individual liver transplant patients which were almost uniform and concordant with those of pp65 antigenemia during active CMV infection. High copy numbers, as well as high numbers of pp65-positive cells, were usually seen in symptomatic infections, although individual differences were recorded. Asymptomatic nontreated CMV infections demonstrated low viral loads in PCR and became negative, as they also did according to pp65 antigenemia during the follow-up. Thus, this quantitative PCR method can be used as well as the pp65 antigenemia assay for the monitoring of viral loads during active CMV infections of individual transplant patients.
Acknowledgments
This study was supported by grants from the Sigrid Juselius Foundation and the Helsinki University Hospital Research Funds (EVO).
We thank Marjatta Palovaara and Teija Tekkala for technical assistance and Stephen Venn for correcting the English text.
REFERENCES
- 1.Aitken, C., W. Barrett-Muir, C. Millar, K. Templeton, J. Thomas, F. Sheridan, D. Jeffries, M. Yaqoob, and J. Breuer. 1999. Use of molecular assays in diagnosis and monitoring of cytomegalovirus disease following renal transplantation. J. Clin. Microbiol. 37:2804-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeckh, M., and G. Boivin. 1998. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol Rev 11:5333-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin, G., R. Belanger, R. Delage, C. Beliveau, C. Demers, N. Goyette, and J. Roy. 1999. Quantitative analysis of cytomegalovirus (CMV) viremia with the pp65 antigenemia assay and the COBAS AMPLICOR CMV MONITOR PCR test after marrow allogenic transplantation. J. Clin. Microbiol. 38:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caliendo, A., K. St George, S. Kao, J. Allega, B-H. Tan, R. LaFontaine, L. Bui, and C. R. Rinaldo. 2000. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J. Clin. Microbiol. 38:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emery, V. C. 2001. Prophylaxis for CMV should not now replace preemptive therapy in solid organ transplantation. Rev. Med. Virol. 11:83-86. [DOI] [PubMed] [Google Scholar]
- 6.Emery, V. C., C. A. Sabin, A. V. Cope, D. Cor, A. F. Hasan-Walker, and P. D. Griffiths.2000. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032-2036. [DOI] [PubMed] [Google Scholar]
- 7.Flexman, J., I. Kay, R. Fonte, R. Herrmann, E. Gabbay, and S. Palladino. 2001. Differences between quantitative antigenemia assay and the Cobas Amplicor Monitor quantitative PCR assay for detecting CMV viremia in bone marrow and solid organ transplant patients. J. Med. Virol. 64:275-282. [DOI] [PubMed] [Google Scholar]
- 8.Gerna, G., E. Percivalle, M. Torcellini, and M. G. Revello. 1998. Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. J. Clin. Microbiol. 36:3585-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guiver, M., A. J. Fox, K. Mutton, N. Mogulkoc, and J. Egan. 2001. Evaluation of CMV viral load with Taqman CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplantation 71:1609-1615. [DOI] [PubMed] [Google Scholar]
- 10.Halwachs-Bauman, G., M. Wilders-Trusching, G. Enzinger, M. Eibl, W. Linkesh, H. J. Dornbush, B. I. Santner, E. Marth, and H. H. Kessler. 2001. Cytomegalovirus diagnosis in renal and bone marrow transplant recipients: the impact of molecular assays. J. Clin. Virol 20:49-57. [DOI] [PubMed] [Google Scholar]
- 11.Hioshi, M., S. Tagawa, T. Takubo, K. Tanaka, T. Nakao, Y. Higeno, K. Tamura, M. Shimaoka, A. Fujii, M. Higashihata, Y. Yasui, T. Kim, A. Hiraoka, and N. Tatsumi. 1997. Evaluation of the AMPLICOR CMV test for direct detection of cytomegalovirus in plasma specimens. J. Clin. Microbiol. 35:2692-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humar, A., D. Gregson, A. M. Caliendo, A. McGreer, G. Malkan, M. Krajden, P. Corey, P. Greig, S. Walmsley, G. Levy, and T. Mazzulli. 1999. Clinical utility of quantitative cytomegalovirus viral load determination for predicting cytomegalovirus disease in liver transplant recipients. Transplantation 68:1305-1311. [DOI] [PubMed] [Google Scholar]
- 13.Koskinen, P. K., M. S. Nieminen, S. P. Mattila, P. J. Häyry, and I. T. Lautenschlager. 1993. The correlation between symptomatic CMV infection and CMV antigenemia in heart allograft recipients. Transplantation 55:547-551. [DOI] [PubMed] [Google Scholar]
- 14.Mendez, J., M. Espy, T. F. Smith, J. Wilson, R. Wiesner, and C. Paya. 1998. Clinical significance of viral load in the diagnosis of cytomegalovirus disease after liver transplantation. Transplantation. 65:1477-1481. [DOI] [PubMed] [Google Scholar]
- 15.Paya, C. V. 2001. Prevention of cytomegalovirus disease in recipients of solid-organ transplants. Clin. Infect. Dis. 32:596-603. [DOI] [PubMed] [Google Scholar]
- 16.Patel, R., and C. V. Paya. 1998. Cytomegalovirus infection and disease in solid organ transplant recipients, p. 229-244. In R. A. Bowden, P. Ljungman, and C. V. Paya (ed.), Transplant infections. Lippincott-Raven Publishers, Philadelphia, Pa.
- 17.Piiparinen, H., K. Höckerstedt, C. Grönhagen-Riska, M. Lappalainen, J. Suni, and I. Lautenschlager. 2001. Comparison of plasma polymerase chain reaction and pp65 antigenemia assay in the quantification of cytomegalovirus in liver and kidney transplant patients. J. Clin. Virol. 22:111-116. [DOI] [PubMed] [Google Scholar]
- 18.Preiser, W., S. Brauninger, R. Scherdtfeger, U. Ayliffe, J. A. Carson, N. S. Brink, S. Frank, H. W. Doerr, and H. F. Rabenau. 2001. Evaluation of diagnostic methods for the detection of cytomegalovirus in recipients of allogenic stem cell transplants. J. Clin. Virol. 20:59-70. [DOI] [PubMed] [Google Scholar]
- 19.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2000. Detection of cytomegalovirus DNA in human specimens by Light Cycler PCR. J. Clin. Microbiol. 38:4006-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sia, I. G., and R. Patel. 2000. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin. Microbiol. Rev. 13:83-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sia, I. G., J. A. Wilson, M. J. Espy, C. V. Paya, and T. F. Smith. 2000. Evaluation of the COBAS AMPLICOR CMV MONITOR test for the detection of viral DNA in specimens taken from patients after liver transplantation. J. Clin. Microbiol. 38:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The, T. H., W. van der Bij, A. P. van der Berg, M. van der Giessen, J. Weits, H. G. Sprenger, and W. J. van Son. 1990. Cytomegalovirus antigenemia. Rev. Infect. Dis. 12(Suppl. 7):S734-S744. [PubMed] [Google Scholar]
- 23.The, T. H., A. P. Van der Berg, M. C. Harmsen, W. van der Bij, and W. J. van Son. 1995. The cytomegalovirus antigenemia assay: a plea for standardization. Scand. J. Infect. Dis. 99:S25-S29. [PubMed] [Google Scholar]
- 24.Tong, C. Y., L. E. Cuevas, H. Williams, and A. Bakran. 2000. Comparison of two commercial methods for measurement of cytomegalovirus load in blood samples after renal transplantation. J. Clin. Microbiol. 38:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Berg, A. P., W. van der Bij, W. J. van Son, J. Anema, M. van der Giessen, J. Schirm, A. M. Tegzess, and T. H. The. 1989. Cytomegalovirus-antigenemia as a useful marker of symptomatic cytomegalovirus disease after renal transplantation: a report of 130 consecutive patients. Transplantation 48:991-995. [DOI] [PubMed] [Google Scholar]