Abstract
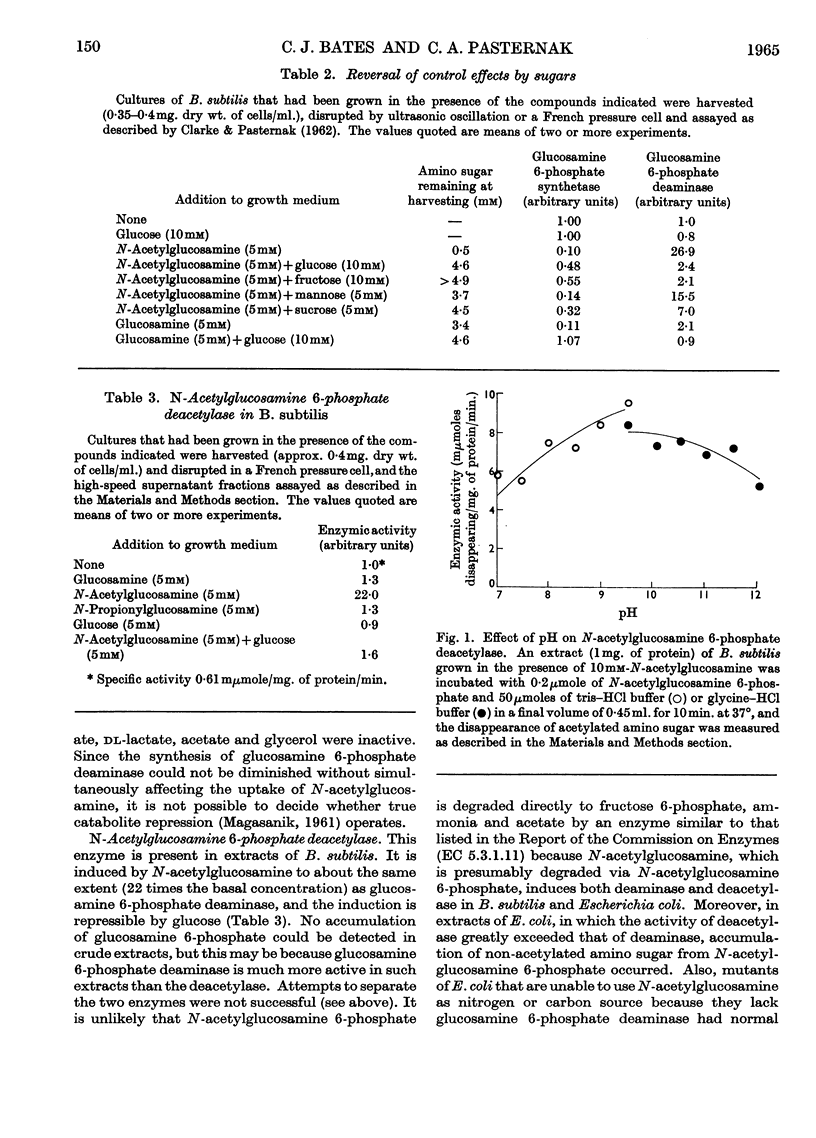
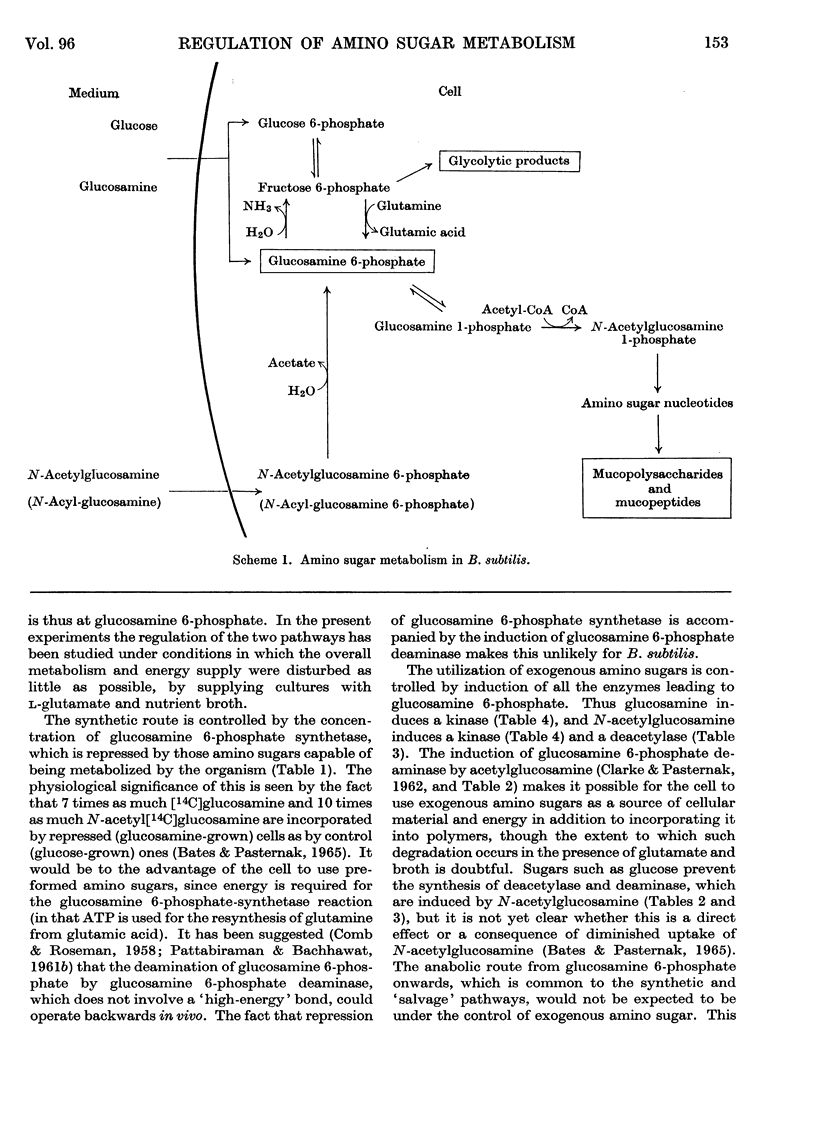
1. Glucosamine 6-phosphate deaminase [2-amino-2-deoxy-d-glucose 6-phosphate ketol-isomerase (deaminating), EC 5.3.1.10] of Bacillus subtilis has been partially purified. Its Km is 3·0mm. 2. Extracts of B. subtilis contain N-acetylglucosamine 6-phosphate deacetylase (Km 1·4mm), glucosamine 1-phosphate acetylase and amino sugar kinases (EC 2.7.1.8 and 2.7.1.9). 3. Glucosamine 6-phosphate synthetase (l-glutamine–d-fructose 6-phosphate aminotransferase, EC 2.6.1.16) is repressed by growth of B. subtilis in the presence of glucosamine, N-acetylglucosamine, N-propionylglucosamine or N-formylglucosamine. Glucosamine 6-phosphate deaminase and N-acetylglucosamine 6-phosphate deacetylase are induced by N-acetylglucosamine. Amino sugar kinases are induced by glucose, glucosamine and N-acetylglucosamine. The synthesis of glucosamine 1-phosphate acetylase is unaffected by amino sugars. 4. Glucose in the growth medium prevents the induction of glucosamine 6-phosphate deaminase and of N-acetylglucosamine 6-phosphate deacetylase caused by N-acetylglucosamine; glucose also alleviates the repression of glucosamine 6-phosphate synthetase caused by amino sugars. 5. Glucosamine 6-phosphate deaminase increases in bacteria incubated beyond the exponential phase of growth. This increase is prevented by glucose.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES C. J., PASTERNAK C. A. THE INCORPORATION OF LABELLED AMINO SUGARS BY BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:155–158. doi: 10.1042/bj0960155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G., JACOB F., MONOD J. [Constituent synthesis of galactokinase following the development of lambda bacteriophages in Escherichia coli K 12]. C R Hebd Seances Acad Sci. 1960 Mar 28;250:2471–2473. [PubMed] [Google Scholar]
- CLARKE J. S., PASTERNAK C. A. The regulation of amino sugar metabolism in Bacillus subtilis. Biochem J. 1962 Jul;84:185–191. doi: 10.1042/bj0840185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. Glucosamine metabolism. IV. Glucosamine-6-phosphate deaminase. J Biol Chem. 1958 Jun;232(2):807–827. [PubMed] [Google Scholar]
- HOSODA J., NOMURA M. Nature of the primary action of the autolysin of Bacillus subtilis. J Bacteriol. 1956 Nov;72(5):573–581. doi: 10.1128/jb.72.5.573-581.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNFELD S., GLASER L. The synthesis of thymidine-linked sugars. v. thymidine diphosphate-amino sugars. J Biol Chem. 1962 Oct;237:3052–3059. [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- PATTABIRAMAN T. N., BACHHAWAT B. K. Purification of glucosamine 6-phosphate deaminase from human brain. Biochim Biophys Acta. 1961 Dec 9;54:273–283. doi: 10.1016/0006-3002(61)90366-3. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Formation of a lytic enzyme by a strain of Bacillus subtilis. Biochim Biophys Acta. 1959 May;33(1):78–92. doi: 10.1016/0006-3002(59)90500-1. [DOI] [PubMed] [Google Scholar]
- ROSEMAN S. Metabolism of connective tissue. Annu Rev Biochem. 1959;28:545–578. doi: 10.1146/annurev.bi.28.070159.002553. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]


