Abstract
Sporothrix schenckii isolates of fixed and lymphocutaneous clinical forms from Mexico (MX), Guatemala (GT), and Colombia (CO) as well as environmental isolates from MX were studied by analyzing their phenotypic characteristics (conidial length, thermotolerance by percent growth inhibition [GI] at 35 and 37°C, median lethal dose [LD50]) and genotypic characteristics (by random amplified polymorphic DNA [RAPD] analysis-PCR). A significant difference (P < 0.01) in the mean conidial length of S. schenckii clinical isolates from CO (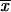 = 4.03 ± 1.04 μm) compared with those of clinical isolates from MX (
= 4.03 ± 1.04 μm) compared with those of clinical isolates from MX (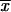 = 2.06 ± 0.53 μm) and GT (
= 2.06 ± 0.53 μm) and GT (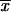 = 2.68 ± 0.83 μm) was observed. The lowest thermotolerance, as determined by measurement of percent GI, was exhibited by isolates from CO at 35°C (
= 2.68 ± 0.83 μm) was observed. The lowest thermotolerance, as determined by measurement of percent GI, was exhibited by isolates from CO at 35°C (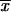 = 50.1% ± 15.9%) and 37°C (
= 50.1% ± 15.9%) and 37°C (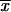 = 72.7% ± 10.9%). In general, the highest virulence, as determined by measurement of the LD50 for mice, was observed for the MX environmental isolates. RAPD analysis-PCR with 10-mer primers OPBG-01, OPBG-14, and OPBG-19 generated 52 reproducible bands. The 44 Sporothrix isolates fell into four major groups by hierarchical cluster analysis. The first group (group I), formed by 25 (of 27) isolates from MX, had two subgroups: subgroup Ia with 10 environmental isolates and subgroup Ib with 14 clinical isolates. The second group (group II) had two subgroups: subgroup IIa, formed by isolates from CO, and subgroup IIb, formed by isolates from GT. Groups III and IV each had only one clinical isolate from MX. A principal-component analysis of the same data yielded three distinct groups, depending on the geographical origins of the isolates, including the isolates in groups III and IV from MX, which were grouped with the isolates from MX by principal-component analysis. This study revealed that isolates from CO had low thermotolerances at 35 and 37°C and could be associated with superficial skin lesions in patients with fixed clinical forms of sporotrichosis, the most frequent form of the disease in CO. Distinct patterns dependent on geographical origins were also revealed by RAPD analysis-PCR, but these had no relation to the clinical form of the disease.
= 72.7% ± 10.9%). In general, the highest virulence, as determined by measurement of the LD50 for mice, was observed for the MX environmental isolates. RAPD analysis-PCR with 10-mer primers OPBG-01, OPBG-14, and OPBG-19 generated 52 reproducible bands. The 44 Sporothrix isolates fell into four major groups by hierarchical cluster analysis. The first group (group I), formed by 25 (of 27) isolates from MX, had two subgroups: subgroup Ia with 10 environmental isolates and subgroup Ib with 14 clinical isolates. The second group (group II) had two subgroups: subgroup IIa, formed by isolates from CO, and subgroup IIb, formed by isolates from GT. Groups III and IV each had only one clinical isolate from MX. A principal-component analysis of the same data yielded three distinct groups, depending on the geographical origins of the isolates, including the isolates in groups III and IV from MX, which were grouped with the isolates from MX by principal-component analysis. This study revealed that isolates from CO had low thermotolerances at 35 and 37°C and could be associated with superficial skin lesions in patients with fixed clinical forms of sporotrichosis, the most frequent form of the disease in CO. Distinct patterns dependent on geographical origins were also revealed by RAPD analysis-PCR, but these had no relation to the clinical form of the disease.
Sporotrichosis is a subcutaneous mycosis especially frequent in Latin American countries with temperate and tropical climates. Due to the presence of Sporothrix schenckii fungal elements in vegetative matter, this disease is associated with different occupations such as gardening, forestry, and fieldwork (3, 6, 9, 11, 16, 27). In Mexico (MX), Guatemala (GT), and Colombia (CO), it is a relevant mycosis mainly in farmers who work with a variety of vegetation in the field and individuals who manipulate fungus-contaminated material (10, 17, 21, 37). The fungus has been isolated from soil, different plants, and sphagnum moss (9, 11, 16, 19, 20), and when it is introduced into the host via trauma, the mycelial-saprophytic form changes to the yeast-parasitic form. Previous reports have demonstrated other forms of inoculation of infecting mycelia, such as mosquito bites and, probably, cat scratches, as recently demonstrated by molecular techniques in Brazil (R. S. Reis, T. M. P. Schubach, A. J. Guimarães, P. C. F. Monteiro, and R. M. Zancopé-Oliveira, Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol., abstr. 498, p. 133, 2000). Although the prevalence of this mycosis among AIDS patients is low, it is extremely serious in human immunodeficiency virus-seropositive patients and becomes disseminated, with a poor prognosis (30).
Researchers from Japan and the United States have performed epidemiological studies with S. schenckii isolates from Asia, the United States, and Australia based on restriction fragment length polymorphisms. They have shown 24 different genotypes divided into two groups: group A includes isolates from the United States, and group B includes isolates from Asia and Australia (12, 13; H. Ishizaki, H., M. Aoki, J. Lin, S. Wu, and J. A. Kim, Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol., abstr. 56, p. 120, 2000). Kawasaki et al. (M. Kawasaki, R. Arenas, C. Zaitz, J. T. Yamashita, and C. Rubio, Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol., abstr. 54, p. 120, 2000) also studied by restriction fragment length polymorphism analysis isolates from Brazil, Mexico, and Spain, and again, two groups were formed: isolates from Brazil and Mexico were in group A, and those from Spain were in group B. Despite the widespread use of the random amplified polymorphic DNA (RAPD) analysis method for genotypic characterization of other pathogenic fungi (5, 18, 23, 25, 34), identical DNA patterns for clinical isolates and isolates from infecting cats were only recently reported for S. schenckii (Reis et al., Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol.).
The RAPD analysis method detects subtle genotypic changes among close groups of isolates, as has been observed for other fungal pathogens (5, 18, 23, 25, 34). In MX, GT, and CO, the molecular epidemiology of this mycosis is unknown. In contrast, the clinical epidemiology of sporotrichosis shows that the lymphocutaneous form of the disease is prevalent in MX and GT (6, 21, 22), whereas the fixed cutaneous form prevails in CO (36, 37). Hence, this study was aimed at determining the genotypic (by RAPD analysis) and phenotypic relatedness among S. schenckii isolates from MX, GT, and CO.
MATERIALS AND METHODS
Fungi.
A total of 44 clinical and environmental isolates of S. schenckii were studied. All clinical isolates were from patients with fixed or lymphocutaneous forms of sporotrichosis from MX, GT, and CO. Environmental isolates from MX were obtained from soil, coffee, and carnation and rose plants by the plate dilution technique (Table 1). Isolates were preserved in sterile water or mineral oil and cryopreserved in liquid nitrogen at −196°C and were deposited in the fungal collection of the Laboratory of Basic Mycology of the Department of Microbiology and Parasitology, Faculty of Medicine, Universidad Nacional Autónoma de México. Isolates were cultured on 1.5% agar (Bioxon) slants with 1% yeast extract (Yestal, Mexico City, Mexico), 2% peptone (Bioxon, Mexico City, Mexico), and 2% glucose (YPG) or on potato dextrose agar (PDA; Bioxon) slants for further assays.
TABLE 1.
S. schenckii isolates studied and their phenotypic characteristics
| Isolate origin, source, and isolate no.k | Conidial sizea (width × length) (μm) | Thermo- tolerance (% GIb) at:
|
LD50c (no. of conidia [107]/ml) | |
|---|---|---|---|---|
| 35°C | 37°C | |||
| MX, clinical | ||||
| EH-143,d Fe | 1.85 × 2.5 | 35.9 | 75.9 | 7.25 |
| EH-176, Ff | 2.4 × 2.9 | 23.1 | 65.2 | |
| EH-184, Lf | 2.5 × 2.6 | 47.1 | 68.7 | 135 |
| EH-189, Lg | 2.5 × 2.5 | 37.4 | 47.6 | 50 |
| EH-197, Fe | 1.5 × 1.6 | 29.4 | 54.6 | 237 |
| EH-198, Le | 2.1 × 2.1 | 33.9 | 56.5 | |
| EH-199, Le | 1.5 × 1.5 | 30.7 | 49.1 | |
| EH-200, Le | 1.4 × 1.5 | 24.0 | 57.5 | |
| EH-217, Fh | 1.7 × 1.7 | 50.4 | 63.1 | 7.0 |
| EH-218, Lh | 2.7 × 2.7 | 55.1 | 65.2 | |
| EH-220, Lh | 2.0 × 2.1 | 36.5 | 55.3 | |
| EH-221, Fh | 2.1 × 2.1 | 44.3 | 62.6 | |
| EH-241, Ff | 1.6 × 1.6 | 55.4 | 69.3 | |
| EH-247, Fh | 1.2 × 1.3 | 31.0 | 74.1 | 8.5 |
| EH-248, Lh | 1.2 × 1.3 | 46.0 | 66.4 | |
| EH-249, Lh | 1.2 × 1.3 | 49.0 | 67.6 | |
| EH-250, Fh | 2.5 × 2.5 | 42.2 | 66.7 | |
| MX, environmentale | ||||
| EH-193 | 1.8 × 1.8 | 30.9 | 45.9 | 6.7 |
| EH-194 | 1.5 × 1.5 | 24.8 | 24.7 | 1.4 |
| EH-195 | 1.3 × 1.4 | 33.2 | 54.3 | 2.5 |
| EH-251 | 2.7 × 2.8 | 47.2 | 70.1 | 12.0 |
| EH-252 | 1.6 × 1.6 | 40.9 | 61.2 | |
| EH-253 | 2.4 × 2.5 | 53.9 | 70.6 | |
| EH-254 | 1.3 × 1.3 | 24.5 | 61.2 | 29.5 |
| EH-255 | 1.2 × 1.2 | 28.9 | 63.4 | |
| EH-256 | 3.0 × 3.1 | 23.7 | 62.5 | |
| EH-257 | 3.0 × 3.2 | 23.6 | 66.6 | 5.0 |
| GT, clinicali | ||||
| EH-230, L | 3.0 × 3.0 | 16.4 | 69.1 | 2.4 |
| EH-232, F | 1.3 × 1.3 | 39.2 | 70.2 | |
| EH-233, L | 3.0 × 3.5 | 33.6 | 66.2 | 28.5 |
| EH-243, F | 2.5 × 3.0 | 18.2 | 70.7 | |
| EH-244, F | 3.0 × 3.1 | 24.7 | 77.0 | |
| EH-245, L | 3.5 × 3.8 | 29.3 | 69.8 | 24.5 |
| EH-246, L | 1.6 × 1.6 | 30.7 | 66.7 | 4.8 |
| CO, clinicalj | ||||
| 022, F | 1.6 × 4.1 | 51.8 | 63.7 | |
| 6067, F | 1.6 × 3.3 | 68.0 | 74.0 | 3.5 |
| 6514, L | 1.6 × 4.0 | 48.2 | 80.3 | 290 |
| 6780, F | 1.6 × 3.3 | 31.9 | 68.9 | 12.0 |
| 9362, L | 1.9 × 4.8 | 68.4 | 81.8 | |
| 10937; F | 1.9 × 4.1 | 30.8 | 70.9 | |
| 12926, F | 1.6 × 3.3 | 28.0 | 79.5 | |
| 15895, F | 1.9 × 3.8 | 71.0 | 74.5 | 170 |
| 9805079; F | 1.6 × 3.8 | 56.0 | 71.9 | |
| 9806027, L | 2.2 × 5.4 | 51.9 | 79.1 | 9.5 |
A minimum of 10 conidia from each isolate were grown on PDA plates for 15 days at 28°C, and measurements were made with a calibrated ocular.
Percent GIs were calculated in triplicate for samples from three different assays by the formula provided in Materials and Methods.
The LD50 was calculated for selected isolates from each geographical origin. For a better comparison of results, all LD50s were expressed as the number of conidia × 107 (same exponent).
Reference isolate.
From A. Espinosa Texis, Universidad Autónoma de Puebla, Puebla, Mexico.
From R. Arenas, Hospital Gea González, González, González, Mexico City, Mexico.
From A. Bonifaz, Hospital General de México, Mexico City, Mexico.
From D. Martinez, Instituto Dermatológico de Guadalajara, Guadalajara, Mexico.
From H. Logemann, Universidad de San Carlos de Guatemala. Guatemala City, Guatemala.
From A. Mesa Arango, Universidad de Antioquia, Medellín, Colombia.
F, fixed form of sporotrichosis; L, lymphocutaneous form of sporotrichosis.
Isolate identification.
Colony morphology on PDA slants and microscopic observations of fungal fragments were recorded after 15 days of incubation at 28°C.
All isolates were tested for conversion to the yeast phase by the following procedure. Seven-day-old conidia from YPG agar slants at 28°C were collected, and a suspension of 106 conidia/ml was prepared. Several 96-well plates (Nunc, Roskilde, Denmark) were prepared to test for the conversion from the mycelial to the yeast form. Sterile medium (pH 7.2) was prepared as described by Rodríguez del Valle et al. (28) and added (100 μl) to each well along with 50 μl of the conidial suspension described above. Ten wells were used for each isolate. The plates were incubated at 37°C for 72 h and were searched for yeasts under a light microscope.
All isolates were grown in Erlenmeyer flasks with liquid medium (pH 5.0) prepared as described by Rodríguez del Valle et al. (28), incubated at 28°C with agitation for 20 days, filtrated, and concentrated 10-fold. Exoantigens (32) were tested by immunodiffusion assays with a reference S. schenckii antigen as a control and a rabbit anti-S. schenckii hyperimmune serum, previously standardized in our laboratory (2).
Phenotypic characterization. (i) Conidial measurements.
The widths and lengths of the conidia were measured with a calibrated ocular micrometer (Olympus America Inc., Melville, N.Y.). All measurements were estimated on the basis of the results obtained with at least 10 conidia from 15-day-old PDA slants at 28°C, as described by Dixon et al. (9).
(ii) Thermotolerance.
The sensitivities of the isolates to different temperatures were detected by a simple agar medium assay. Seven-day-old conidia were collected from PDA slants at 28°C, and a suspension of 106 conidia/ml was prepared. The conidial suspension (100 μl) was added to a central 2.5-mm-diameter well made in petri dishes with PDA culture medium. Three to five replicate plates were used for each temperature, and the plates were incubated at 28, 35, and 37°C. All experiments were repeated separately at least three times. The colony diameters (in millimeters) were measured at day 15 and were always measured at the same premarked line. The percent growth inhibition (GI) was calculated at 35 and 37°C by the following formula: [(colony diameter at 28°C − colony diameter at 35 or 37°C)/colony diameter at 28°C] × 100. The conidial lengths and percent GIs were analyzed by analysis of variance (α = 0.01 and 0.05) to detect significance.
(iii) S. schenckii virulence assessed by median lethal dose (LD50) determination.
Fungal isolates selected from each country and source (6 of 17 clinical isolates from MX, 6 of 10 environmental isolates from MX, 4 of 7 isolates from GT, and 5 of 10 isolates from CO, giving a total of 21 isolates) were grown in Erlenmeyer flasks with 100 ml of YPG broth and were incubated at 28°C for 20 days. Then, each fungal culture was collected and centrifuged at 1,400 × g for 10 min. The pellet was resuspended in sterile saline, washed thrice, and filtered through Whatman no. 1 filter paper to eliminate mycelia and separate the conidium-enriched suspension. This suspension was adjusted to approximately 109 conidia/ml for each isolate studied, and four different dilutions were obtained: 10−1, 10−2, 10−3, and 10−4. The conidia were counted in a Neubauer chamber to determine the number of conidia per milliliter. Inoculum viability was detected by determination of the number of CFU in YPG agar.
Adult male Taconic mice were used. The Institutional Animal Care Committee of the Universidad Nacional Autónoma de México approved the procedures performed with the animals. Ten animals were infected by intraperitoneal injection of 1 ml of the concentrated suspension or dilutions of the suspension. Ten mice were inoculated with sterile saline as controls. The deaths of the animals were recorded until day 45 of infection. The data were subjected to probit analysis, and the LD50s were calculated in a Windows environment with software specially designed by Alberto Pérez Arista.
High (<1 × 108 conidia/ml), medium (0.11 × 109 to 1 × 109 conidia/ml), and low (>1 × 109 conidia/ml) virulences were established on the basis of the LD50s.
Isolation of S. schenckii DNA.
Whole-cell DNA was isolated from 7-day-old mycelia from YPG broth kept at 28°C and shaken at 120 rpm. Approximately 25 mg of mycelium was ground with a mortar and pestle in liquid nitrogen and suspended in 500 μl of sterile lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 100 mM EDTA) with 50 μl of 10% sodium dodecyl sulfate, and the tubes were gently shaken and then centrifuged at 18,300 × g for 5 min. The supernatant was treated with 5 μl of 10 mg of RNase (Qiagen, Hilden, Germany) per ml at 37°C for 1 h and then subjected to phenol-chloroform-isoamyl alcohol (25:24:1) extraction by adding an equal volume of this mixture. Nucleic acids were precipitated by adding 10 μl of 3 M sodium acetate and 2 volumes of absolute ethanol. The pellet was washed with 70% ethanol, dried, and resuspended in water. The DNA was quantified fluorometrically and checked against standard DNA concentrations by agarose gel electrophoresis.
RAPD analysis-PCR assay.
The RAPD analysis-PCR assay was performed with a 25-μl reaction mixture containing 10 ng of the required DNA sample; 2.5 mM MgCl2; 200 μM each dATP, dTTP, dCTP, and dGTP (Perkin-Elmer Cetus, Norwalk, Conn.); 100 pmol of each primer (primers OPBG-01, OPBG-14, and OPBG-19 [kit B; Operon Technologies Ltd.]); sterile, distilled MilliQ water (Millipore, Molsheim, France) to make up the final volume; and 1 U of Taq DNA polymerase (Perkin-Elmer Cetus). Controls, which contained all of the components listed above except DNA, were also set up and run with each set of reactions. Optimal amplification conditions for S. schenckii RAPD analyses were found to be 45 cycles of 1 min at 94°C (denaturation), 1 min at 35°C (annealing), and 1 min at 72°C (extension). A final 7-min cycle at 72°C ensured full extension of all amplified products, and the samples were maintained at 4°C. The polymorphic amplified patterns revealed by RAPD analysis-PCR assays were viewed, and digital images of ethidium bromide (0.5 μg/ml)-stained agarose gels (2%) were captured with a documentation system (GeneCam; Syngene, Cambridge, Mass.) and printed on a digital graphic printer (Sony Electronics Inc., Park Ridge, N.J.). RAPD analysis-PCR assays were repeated independently three times with each primer with all 44 isolates studied, with consistent gel patterns detected from one assay to another.
The amplification products for the different isolates were compared with each other and were screened for the presence or absence of specific bands. These data on the presence or absence of specific bands were then analyzed to obtain an estimate of similarity for each pair of isolates. The similarity was calculated by use of the Jaccard (14) coefficient (Sj), which is equal to a/(n − d), where a is the number of positive matches (1, 1), n is the total sample size, and d is the number of negative matches (0, 0). The Jaccard coefficient does not score negative matches as informative.
The similarity matrix was the basis for the construction of both a phenogram by the unweighted pair-group method with arithmetic averages (UPGMA) as the relationship among isolates in a multidimensional diagram by principal-component analysis (PCA). To measure the extent to which the phenogram result corresponds to the original similarity matrix, we computed a cophenetic correlation coefficient (31). Multivariate statistical methods were carried out with the NTSYS-PC program (version 2.0; Exeter Software) (29).
RESULTS
The S. schenckii isolates studied were from patients with fixed or lymphocutaneous sporotrichosis from MX (n = 17), GT (n = 7), and CO (n = 10); environmental isolates (n = 10) were only from Mexico. Data for each isolate are shown in Table 1. The fungal isolates were identified according to the following criteria: development of the conventional colonial morphology, the presence of typical microscopic conidia, conversion of cells from the mycelium to the yeast form at 37°C, and the formation of precipitin lines by exoantigen production. All isolates studied presented with a moist, flat, and finely wrinkled or folded surface; they were initially cream colored but gradually turned dark brown, depending on the medium; the sympodial conidia were in radial flower-like clusters and/or presented as thick-walled, dark brown conidia borne laterally along the hyphae in a sleeve-like manner; the yeast cells had ovoid budding at 37°C; and all isolates had the precipitin lines of an identity reaction with a reference specific anti-S. schenckii rabbit serum prepared with reference strain EH-143 (2).
The phenotypic characteristics studied, conidium size (in micrometers) and thermotolerance at 35 and 37°C (reported as percent GI) for all isolates and LD50s (numbers of conidia per milliliter) for selected isolates, are shown on Table 1. The widths of the conidia of all isolates varied from 1.2 to 3.5 μm, while the lengths varied from 1.2 to 5.4 μm, with the largest conidia detected from isolates from CO. The mean conidial length for isolates from CO (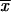 = 4.03 ± 1.04 μm) was significantly different (α = 0.01) from the mean conidial lengths for isolates from MX (
= 4.03 ± 1.04 μm) was significantly different (α = 0.01) from the mean conidial lengths for isolates from MX (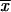 = 2.06 ± 0.53 μm) and GT (
= 2.06 ± 0.53 μm) and GT (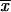 = 2.68 ± 0.83 μm), as well as the environmental isolates from MX (
= 2.68 ± 0.83 μm), as well as the environmental isolates from MX (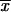 = 2.03 ± 0.77 μm) (Table 2).
= 2.03 ± 0.77 μm) (Table 2).
TABLE 2.
Mean conidial length and percent GI for clinical and environmental isolates from MX and clinical isolates from GT and COa
| Isolate | Conidial length (μm) | % GI at:
|
|
|---|---|---|---|
| 35°C | 37°C | ||
| MX (clinical) | 2.06 ± 0.53 | 37.7 ± 12.2 | 62.7 ± 8.67 |
| MX (environmental) | 2.03 ± 0.77 | 33.0 ± 10.9 | 59.1 ± 10.8 |
| GT (clinical) | 2.68 ± 0.83 | 27.6 ± 9.2 | 69.9 ± 4.0 |
| CO (clinical) | 4.03 ± 1.04 | 50.1 ± 15.9 | 72.7 ± 10.9 |
The values are means ± standard deviations (α = 0.01).
The isolates also had different thermotolerances at both temperatures. At 35°C, isolates from clinical sources in MX had from 23.1 to 55.4% GI and those from environmental sources in MX had from 23.6 to 53.9% GI, isolates from GT had from 16.4 to 39.2% GI, and isolates from CO had from 28.0 to 71% GI. At the other temperature tested, 37°C, isolates from clinical sources in MX had from 47.6 to 75.9% GI and those from environmental sources in MX had from 24.7 to 70.6% GI, isolates from GT had from 66.2 to 77.0% GI, and isolates from CO had from 63.7 to 81.8% GI. Fungal isolates from CO were the most sensitive to both temperatures tested (P < 0.05). The mean percentages of GI at both 35 and 37°C are given in Table 2.
The LD50s for the isolates selected for virulence studies (21 of 44 isolates) are reported in Table 1. Environmental isolates from MX had the highest virulence, ranging from 1.4 × 107 to 29.5 × 107 conidia/ml (Table 1). The lowest virulence was observed for isolate 6514 from CO (290 × 107 conidia/ml). Mice that presented signs of the disease developed subcutaneous nodules on the tail and orchitis, and cultures of liver and spleen tissue specimens were positive for S. schenckii.
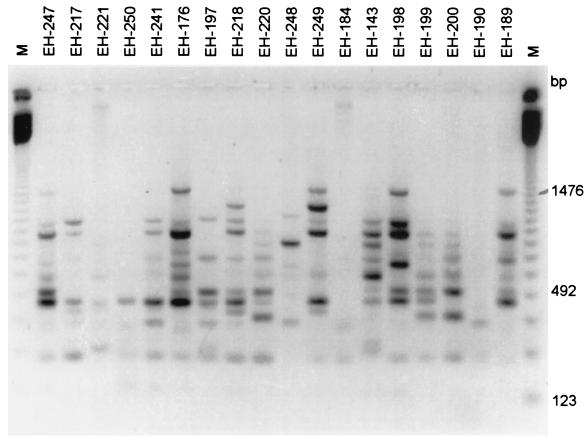
The three primers tested yielded 52 clearly scorable, repeatable, polymorphic banding patterns among the isolates. The patterns obtained by RAPD analysis-PCR with primer OPBG-14 with clinical isolates from MX are depicted in Fig. 1. The similarity between isolate pairs was highly variable and ranged from 0.941 to 0.031. The mean genetic similarity was 0.317.
FIG. 1.
RAPD analysis-PCR profiles of 18 isolates of S. schenckii from Mexican patients generated with primer OPBG-14. Lanes M, 123-bp molecular size marker ladder. The gel was stained with ethidium bromide. The image was inverted with GeneSnap software (Syngene) for the best resolution. Image analysis was performed as described in Material and Methods.
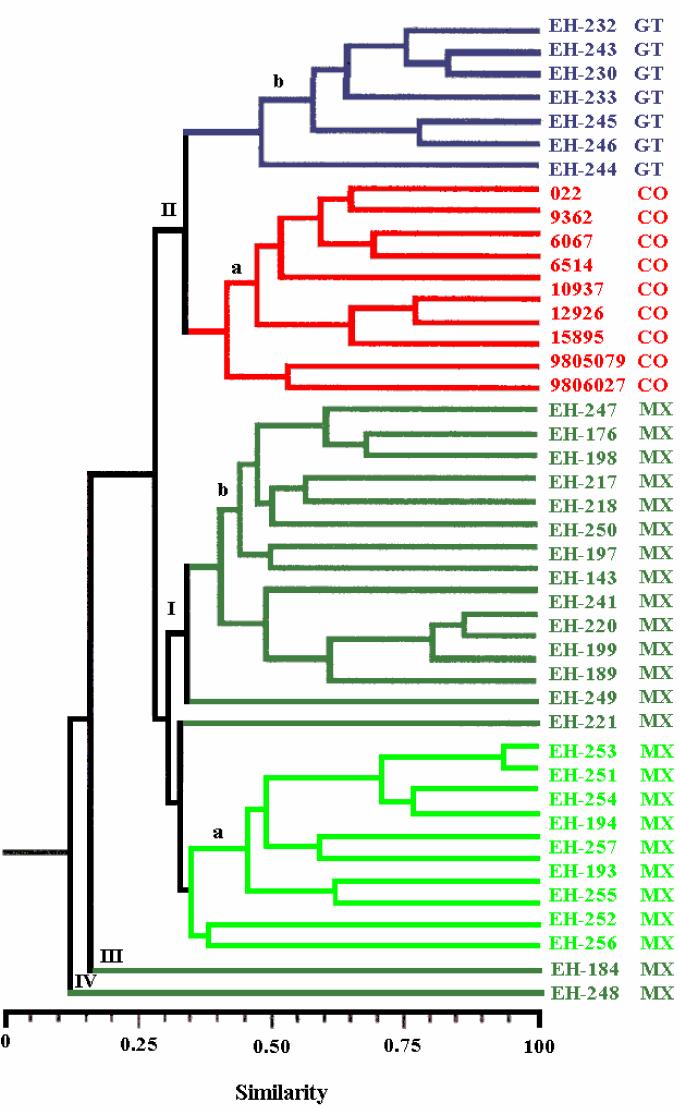
The phenogram for the S. schenckii isolates yielded four clusters (Fig. 2). The first cluster (group I) included 25 (of 27) isolates from Mexico with 31% similarity and showed two subgroups. Subgroup Ia included 10 environmental isolates and 1 clinical isolate (isolate EH-221) with 35% similarity, and subgroup Ib included only isolates from patients, with the same percent similarity as subgroup Ia. The second cluster (group II) also contained two subgroups with 33% similarity. Subgroup IIa consisted of isolates from CO with 42% similarity. Subgroup IIb consisted of isolates from GT. Group III consisted of only one clinical isolate (isolate EH-184) from MX, and lastly, group IV consisted of individual clinical isolate (isolate EH-248) from MX.
FIG. 2.
Relationship of S. schenckii isolates. The phenogram was generated from genetic similarity coefficients obtained by determination of the presence and absence of a total of 52 DNA bands from 44 isolates and is based on UPGMA. Dark green, clinical isolates from MX; light green, environmental isolates from MX.
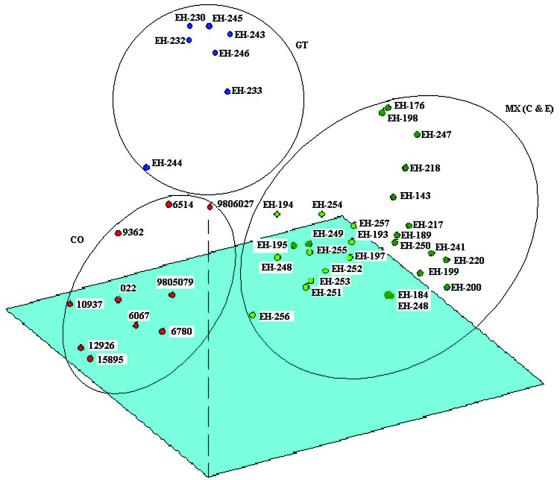
The similarity matrix produced from the phenogram and the original similarity matrix yielded a good cophenetic correlation (r = 0.8; P < 0.001), which suggests that the phenogram accurately represents the original genetic similarities between isolates. PCA (Fig. 3) shows three discrete groups that depended on the geographical origins of the isolates (MX, CO, and GT). Isolates EH-184 and EH-248 from patients in MX, which formed two separate groups (groups III and IV, respectively) in the phenogram, were included in the MX group. The first three principal components explain 50% of the variation observed. These findings largely correspond to the clustering obtained by UPGMA (Fig. 2).
FIG. 3.
PCA. The first three components explain 50% of the observed variation. Each of the three groups formed represents isolates from the three geographical regions studied (MX, GT, and CO). The group formed with isolates from MX includes clinical (C) and environmental (E) isolates.
DISCUSSION
The present report is the first one that associates a phenotypic characteristic of S. schenckii to different geographical origins, although genotypes have been related to geographic distribution by some previous investigators (12, 13; Ishizaki et al., Abstr. 14th Congr. Int. Soc. for Hum. Anim. Mycol.; Kawasaki et al., Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol.). A high degree of similarity of the mean conidial length was observed among isolates from MX (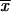 = 2.06 μm) and GT (
= 2.06 μm) and GT (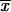 = 2.68 μm) (Table 2) compared with the mean length reported by Dixon et al. (9) for clinical isolates from an outbreak of sporotrichosis in the United States (
= 2.68 μm) (Table 2) compared with the mean length reported by Dixon et al. (9) for clinical isolates from an outbreak of sporotrichosis in the United States (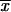 = 2.4 μm). In this work, the largest conidial length of the fungus was found among isolates from CO (
= 2.4 μm). In this work, the largest conidial length of the fungus was found among isolates from CO (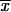 = 4.03 μm), which were statistically different (P < 0.01) from those for the other isolates. Differences in morphological structures related to geographical distribution have been observed in other human pathogens, i.e., Histoplasma capsulatum, in which the sizes of the yeast cells for isolates from the African continent (7 to 15 μm), corresponding to H. capsulatum var. duboisii, are different from those from the American continent (2 to 4 μm), corresponding to H. capsulatum var. capsulatum (16, 27); however, a reasonable explanation for these findings would be far-fetched at this point. Another interesting phenotypic characteristic found was the high thermosensitivity at 35 and 37°C, revealed in isolates from CO, in contrast to those for isolates from MX and GT (P < 0.01). Thermotolerance results were reported on the basis of a mathematical formula based on percent GI, and a statistical analysis showed statistically significant differences among the isolates from the three different geographical origins studied. Previously, Albornoz et al. (1) and Kwon-Chung (15) referred only to Sporothrix isolates from fixed dermal lesions as growing poorly at temperatures above 35°C and isolates from extracutaneous, lymphocutaneous, or some fixed-type lesions growing at 37°C. Tachibana et al. (33) recently demonstrated that heating of the footpads of BALB/c mice by use of a heated cage floor after subcutaneous inoculation of S. schenckii reduced the magnitude of fungal proliferation compared with the magnitude for mice kept on a nonheated floor. This effect shows the preference of Sporothrix to infect subcutaneous tissues, where the temperature is significantly lower than the body temperature. Our results point out a probable interaction between the well-defined thermosensitivity findings and the clinical manifestations of disease, considering that isolates from CO had the highest percentages of GI at 35 and 37°C (Tables 1 and 2), and these isolates could be associated with superficial skin lesions in patients with the fixed clinical forms of sporotrichosis. Furthermore, the fixed clinical form is the most frequent form of sporotrichosis in CO (36, 37), whereas the lymphocutaneous forms prevail in MX and GT (3, 6, 17, 21, 22). Interestingly, the most common manifestations of African histoplasmosis also occur in bone and skin, and the distinctive phenotypic characteristic of the yeasts that cause histoplasmosis in Africa is their increased size compared with the size of the yeasts that cause histoplasmosis on the American continent. As a consequence, the same association between the size of the fungal structure and the clinical manifestation of infection is shared by both fungi.
= 4.03 μm), which were statistically different (P < 0.01) from those for the other isolates. Differences in morphological structures related to geographical distribution have been observed in other human pathogens, i.e., Histoplasma capsulatum, in which the sizes of the yeast cells for isolates from the African continent (7 to 15 μm), corresponding to H. capsulatum var. duboisii, are different from those from the American continent (2 to 4 μm), corresponding to H. capsulatum var. capsulatum (16, 27); however, a reasonable explanation for these findings would be far-fetched at this point. Another interesting phenotypic characteristic found was the high thermosensitivity at 35 and 37°C, revealed in isolates from CO, in contrast to those for isolates from MX and GT (P < 0.01). Thermotolerance results were reported on the basis of a mathematical formula based on percent GI, and a statistical analysis showed statistically significant differences among the isolates from the three different geographical origins studied. Previously, Albornoz et al. (1) and Kwon-Chung (15) referred only to Sporothrix isolates from fixed dermal lesions as growing poorly at temperatures above 35°C and isolates from extracutaneous, lymphocutaneous, or some fixed-type lesions growing at 37°C. Tachibana et al. (33) recently demonstrated that heating of the footpads of BALB/c mice by use of a heated cage floor after subcutaneous inoculation of S. schenckii reduced the magnitude of fungal proliferation compared with the magnitude for mice kept on a nonheated floor. This effect shows the preference of Sporothrix to infect subcutaneous tissues, where the temperature is significantly lower than the body temperature. Our results point out a probable interaction between the well-defined thermosensitivity findings and the clinical manifestations of disease, considering that isolates from CO had the highest percentages of GI at 35 and 37°C (Tables 1 and 2), and these isolates could be associated with superficial skin lesions in patients with the fixed clinical forms of sporotrichosis. Furthermore, the fixed clinical form is the most frequent form of sporotrichosis in CO (36, 37), whereas the lymphocutaneous forms prevail in MX and GT (3, 6, 17, 21, 22). Interestingly, the most common manifestations of African histoplasmosis also occur in bone and skin, and the distinctive phenotypic characteristic of the yeasts that cause histoplasmosis in Africa is their increased size compared with the size of the yeasts that cause histoplasmosis on the American continent. As a consequence, the same association between the size of the fungal structure and the clinical manifestation of infection is shared by both fungi.
Virulence studies with selected S. schenckii isolates from each country and source revealed no association between virulence and either geographical origin or the clinical form of sporotrichosis (only isolates from patients with fixed or lymphocutaneous sporotrichosis were studied) since different levels of virulence, that is, low, medium, and high levels of virulence (Table 1), were found among the isolates studied. However, high levels of virulence were mostly related to environmental Sporothrix isolates from MX. Other data from in vivo LD50 assays from our laboratory (unpublished data) have also revealed that isolates from the environment always have high degrees of virulence. Although Dixon et al. (9) did not determine LD50s, they assayed the virulence of different clinical and environmental S. schenckii isolates in NYLAR male mice following intravenous injection of 106 to 108 conidia into each mouse. All S. schenckii isolates, both clinical and environmental, produced 100% mortality between 12 and 24 days after injection (9). The results obtained by the quantitative in vivo LD50 assay showed that environmental isolates are the most virulent for Taconic mice. The environmental isolates were also the most thermotolerant at 37°C, with GI ranging from 24.7 to 70.6% (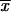 = 59.1 ± 10.8; Table 2); in contrast, at the same temperature the GI of clinical isolates ranged from 47.6 to 81.8% (Table 1), with means of 62.7% ± 8.67%, 69.9% ± 4.0%, and 72.7% ± 10.9% GI for isolates from MX, GT, and CO, respectively (Table 2). In another model of pathogenic fungus, H. capsulatum, the same observation of higher levels of virulence of environmental Histoplasma isolates was detected by the in vivo LD50 assay (35).
= 59.1 ± 10.8; Table 2); in contrast, at the same temperature the GI of clinical isolates ranged from 47.6 to 81.8% (Table 1), with means of 62.7% ± 8.67%, 69.9% ± 4.0%, and 72.7% ± 10.9% GI for isolates from MX, GT, and CO, respectively (Table 2). In another model of pathogenic fungus, H. capsulatum, the same observation of higher levels of virulence of environmental Histoplasma isolates was detected by the in vivo LD50 assay (35).
The molecular studies clearly grouped the S. schenckii isolates on the basis of their geographical origins. Molecular analysis of the isolates in group I, which included all isolates from MX, also discriminated isolates from clinical and environmental sources in MX (Fig. 2 and 3). Group II contained two well-defined subgroups: subgroup IIa, with isolates from CO, and subgroup IIb, with isolates from GT. The isolates in groups III and IV, with only one isolate each, differed from the remaining isolates from Mexican patients. The cluster analysis showed the relevance of the RAPD analysis method in this type of study, especially when it is performed under standardized and well-controlled experimental conditions. This point of view is reinforced by PCA, by which the isolates in group III (EH-184) and group IV (EH-248) were clustered in the group of isolates from MX by PCA.
Even though the isolates studied were grouped by their geographical origins, a high degree of genotypic variability was observed among the isolates. This diversity may be due to different ecological processes and/or genetic factors and arises and is maintained through the interplay of both ecological processes and genetic factors: without variation there can be no evolution and no divergence. Until now no sexual state has been reported for S. schenckii, even though molecular analysis of its 18S rRNA has provided indirect evidence that its sexual form is probably Ophiostoma stenoceras (4) and that it is related to the ascomycetes, based on evidence of the presence of three chitin synthase genes in S. schenckii (8). There may be several explanations for the genotypic variability in the polymorphic patterns observed in S. schenckii isolates. For example, nonmeiotic recombination may occur in a parasexual cycle, as observed in vitro in other entomopathogenic fungi such as Paecilomyces fumosoroseus (26) and Metarhizium anisopliae (24), which show high degrees of genotypic variability and which, until recently, have not been reported to have a sexual state. Molecular evidence for recombination and for a cryptic state have already been proposed for another fungus pathogenic for humans, Coccidioides immitis, for which, until recently, no sexual state has been reported (7).
Another explanation for the polymorphic pattern variability in Sporothrix could be the presence of mutations, although the latter occur at a low frequency in nature. Meiotic recombination and mutation occur in fungal populations, as does nonmeiotic recombination. The fungi are capable of making progeny with new genetic combinations, and these fungi have been used in laboratory genetic analyses, but the generation of variations by mutation, although essential, is the ultimate resource in nature.
Acknowledgments
We thank Maria Lucia Taylor for valuable and appropriate criticisms to improve this paper and Aldo Valera for valuable help in the laboratory. We gratefully acknowledge Ingrid Mascher for editorial assistance. We are indebted to P. Lavalle, A. Espinosa Texis, Roberto Arenas, A. Bonifaz, D. Martínez, and Heidi Logemann for the donation of Sporothrix isolates that enriched our collection and research.
This work was supported mainly by grant DGAPA-UNAM-IN201394 from the Dirección General de Asuntos del Personal Académico.
REFERENCES
- 1.Albornoz, M. B., M. Mendoza, and E. D. Torres. 1986. Growth temperatures of isolates of Sporothrix schenckii from disseminated and fixed cutaneous lesions of sporotrichosis. Mycopathologia 95:81-83. [DOI] [PubMed] [Google Scholar]
- 2.Arenas, G., and C. Toriello. 1986. Actividad inmunológica de antígenos miceliales-levaduriformes de diferentes fases de crecimiento de Sporothrix schenckii. Rev. Mex. Microsc. 2:131-144. [Google Scholar]
- 3.Arenas, R. 1993. Micología médica ilustrada. Interamericana. McGraw-Hill, México D.F., Mexico.
- 4.Berbee, M. L., and J. W. Taylor. 1992. 18S ribosomal RNA gene sequence characters place the human pathogen Sporothrix schenckii in the genus Ophiostoma. Exp. Mycol. 16:87-91. [Google Scholar]
- 5.Boekhout, T., and A. Belkum. 1997. Variability of kariotypes and RAPD types in genetically related isolates of Cryptococcus neoformans. Curr. Genet. 32:203-208. [DOI] [PubMed] [Google Scholar]
- 6.Bonifaz, A. 2000. Micología médica básica. Méndez Editores, México D.F., Mexico.
- 7.Burt, A., D. A. Carter, G. L. Koenig, T. J. White, and J. W. Taylor. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. USA 93:770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua, S. S., M. Momany, L. Mendoza, and P. J. Szaniszlo. 1994. Identification of three chitin synthase genes in the dimorphic fungal pathogen Sporothrix schenckii. Curr. Microbiol. 29:151-156. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, D. M., I. F. Salkin, R. A. Duncan, N. J. Hurd, J. H. Haines, M. E. Kemna, and F. B. Coles. 1991. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 29:1106-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-Ochoa, A. 1965. Contribuciones recientes al conocimiento de la esporotricosis. Gac. Med. Mex. 95:463-474. [Google Scholar]
- 11.Hajjeh, R., S. McDonnell, S. Reef, C. Licitra, M. Hankins, B. Toth, A. Padhye, L. Kaufman, L. Pasarell, C. Cooper, L. Hutwagner, R. Hopkins, and M. McNeil. 1997. Outbreak of sporotrichosis among tree nursery workers. J. Infect. Dis. 176:499-504. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaki, H., M. Kawasaki, M. Aoki, M. Miyaji, K. Nishimura, and J. A. García Fernández. 1996. Mitocondrial DNA analysis of Sporothrix schenckii in Costa Rica. J. Med. Vet. Mycol. 34:71-73. [PubMed] [Google Scholar]
- 13.Ishizaki, H., M. Kawasaki, M. Aoki, T. Matsumoto, A. A. Padhye, M. Mendoza, and R. Negroni. 1998. Mitocondrial DNA analysis of Sporothrix schenckii in North and South America. Mycopathologia 142:115-118. [DOI] [PubMed] [Google Scholar]
- 14.Jaccard, P. 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44:223-270. [Google Scholar]
- 15.Kwon-Chung, K. J. 1979. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J. Infect. Dis. 139:424-431. [DOI] [PubMed] [Google Scholar]
- 16.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 17.Lavalle, P. 1979. Esporotricosis, p. 115-138. In Instituto Syntex (ed.), Simposio Syntex: desarrollo y estado actual de la micología médica en México. Talleres Gráficos de Línea y Color, México D.F., Mexico.
- 18.Lin, D., P. F. Lehmann, B. H. Hamory, A. A. Padhye, E. Durry, R. W. Pinner, and B. A. Lasker. 1995. Comparison of three typing methods for clinical and environmental isolates of Aspergillus fumigatus. J. Clin. Microbiol. 33:1596-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariat, F. 1975. Observation sur l'ecologie de Sporothrix schenckii et de Ceratocystis stenoceras en Corse et en Alsace, provinces françaises indemnes de sporotrichose. Sabouraudia 13:217-225. [PubMed] [Google Scholar]
- 20.Mariat, F., R. G. Garrison, K. S. Boyd, M. A. Rouffaud, and H. Fromentin. 1978. Premières observations sur les macrospores pigmentées de Sporothrix schenckii. C. R. Acad. Sci. (Paris) Ser. B 286:1429-1432. [Google Scholar]
- 21.Mayorga, R., A. Cáceres, C. Toriello, G. Gutiérrez, O. Álvarez, M. E. Ramírez, and F. Mariat. 1978. Etude d'une zone d'endemie sporotrichosique au Guatemala. Sabouraudia 16:185-198. [PubMed] [Google Scholar]
- 22.Mayorga-Rodríguez, J. A., J. Barba-Rubio, V. F. Muñoz-Estrada, A. Rangel-Cortés, A. García-Vargas, and I. Magaña-Camarena. 1997. Esporotricosis en el estado de Jalisco, estudio clínico—epidemiológico (1960-1996). Dermatol. Rev. Mex. 41:105-108. [Google Scholar]
- 23.Melo, A. S., L. P. de Almeida, A. L. Colombo, and M. R. Briones. 1998. Evolutionary distances and identification of Candida species in clinical isolates by randomly amplified polymorphic DNA (RAPD). Mycopathologia 142:57-66. [DOI] [PubMed] [Google Scholar]
- 24.Messias, C. L., and J. L. Azevedo. 1980. Parasexuality in the deuteromycete Metarhizium anisopliae. Trans. Br. Mycol. Soc. 75:473-477. [Google Scholar]
- 25.Reyes-Montes, M. R., M. Bobadilla-del Valle, M. A. Martínez, G. Rodríguez, E. Maravilla, J. Sifuentes, and M. L. Taylor. 1999. Relatedness analyses of Histoplasma capsulatum isolates from Mexican patients with AIDS-associated histoplasmosis by using histoplasmin electrophoretic profiles and randomly amplified polymorphic DNA patterns. J. Clin. Microbiol. 37:1404-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riba, G., and A. M. Ravelojoana. 1984. The parasexual cycle in the entomopathogenic fungus Paecilomyces fumosoroseus (Wize) Brown and Smith. Can. J. Microbiol. 30:922-926. [Google Scholar]
- 27.Rippon, J. W. 1990. Tratado de micología médica. The W. B. Saunders Co., Philadelphia, Pa.
- 28.Rodríguez del Valle, N., M. Rosario, and B. Torres. 1983. Effects of pH, temperature, aeration and carbon source on the development of the mycelial or yeast forms of Sporothrix schenckii from conidia. Mycopathologia 82:83-88. [DOI] [PubMed] [Google Scholar]
- 29.Rohlf, F. J. 1998. Numerical taxonomy and multivariate analysis system. Exeter Software Inc., New York, N.Y.
- 30.Schell, W. A. 1998. Agents of chromoblastomycosis and sporotrichosis, p. 315-336. In M. Sussman et al. (ed.), Topley & Wilson's microbiology and microbial infections, 9th ed., vol. 4. Arnold, London, United Kingdom.
- 31.Sneath, P. H. A., and R. R. Sokal. 1973. Taxonomic structure, p. 188-305. In Numerical taxonomy. W. H. Freeman & Co., San Francisco, Calif.
- 32.Standard, P. G., and L. Kaufman. 1976. Specific immunological test for the rapid identification of members of the genus Histoplasma. J. Clin. Microbiol. 3:191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tachibana, T., T. Matsuyama, and M. Mitsuyama. 1998. Characteristic infectivity of Sporothrix schenckii to mice depending on routes of infection and inherent fungal pathogenicity. Med. Mycol. 36:21-27. [PubMed] [Google Scholar]
- 34.Taylor, M. L., C. B. Chávez-Tapia, and M. R. Reyes-Montes. 2000. Molecular typing of Histoplasma capsulatum isolated from infected bats, captured in Mexico. Fungal Genet. Biol. 30:207-212. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, M. L., M. R. Reyes-Montes, M. A. Martínez-Rivera, G. Rodríguez-Arellanes, E. Duarte-Escalante, and J. J. Flores-Estrada. 1997. Histoplasmosis en México. Aportaciones inmunológicas y moleculares sobre su epidemiología. Cienc. Desarrollo 136:58-63. [Google Scholar]
- 36.Velázquez, J. P., A. Restrepo, and G. Calle. 1976. Experiencia de 12 años con la esporotricosis. Polimorfismo clínico de la entidad. Ant. Med. 26:153-169. [Google Scholar]
- 37.Vélez, H., L. Santamaría, G. Guzmán, and M. Escobar. 1984. Esporotricosis. Aspectos clínicos en 78 pacientes. Act. Med. Col. 9:146-149. [Google Scholar]