Abstract
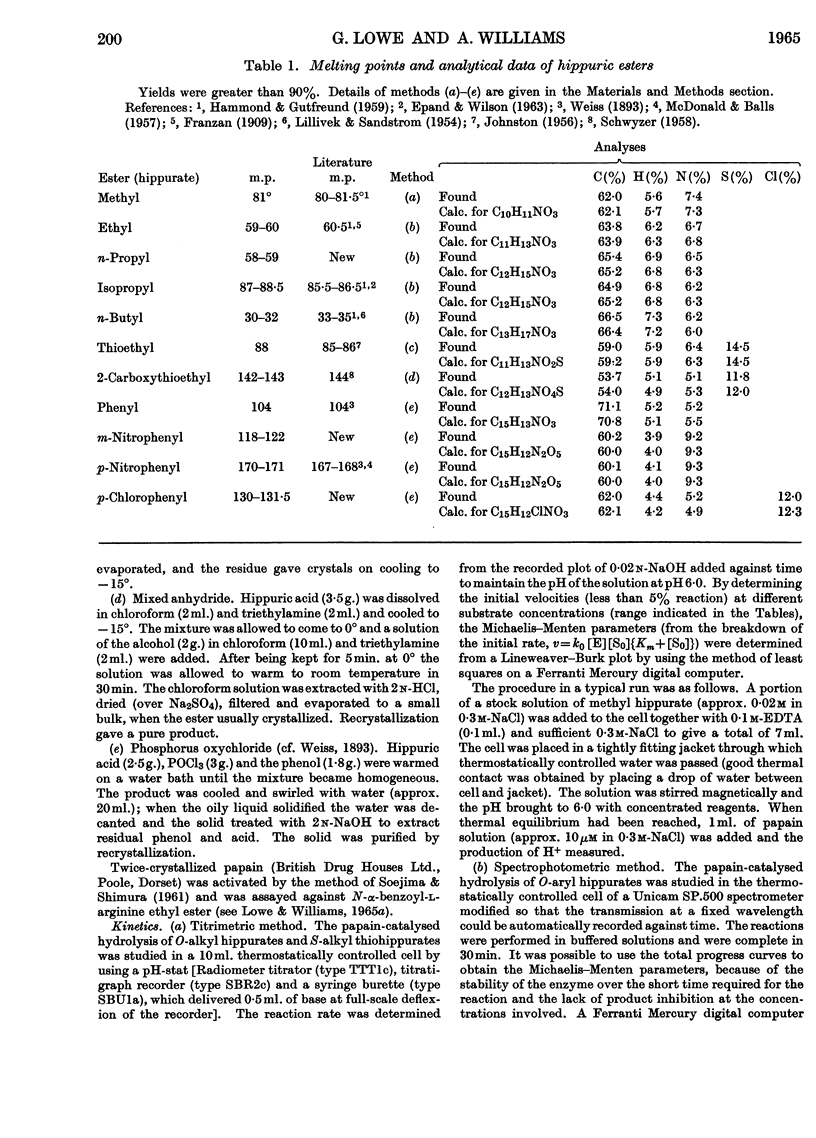
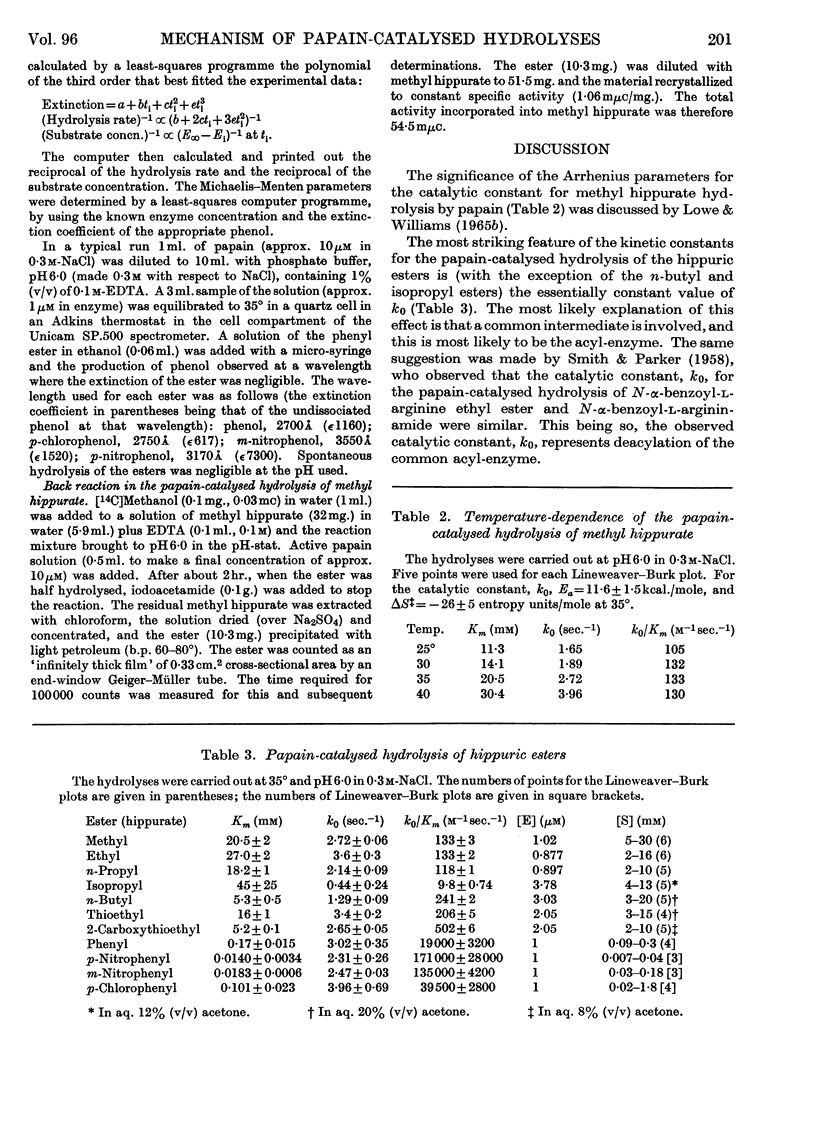
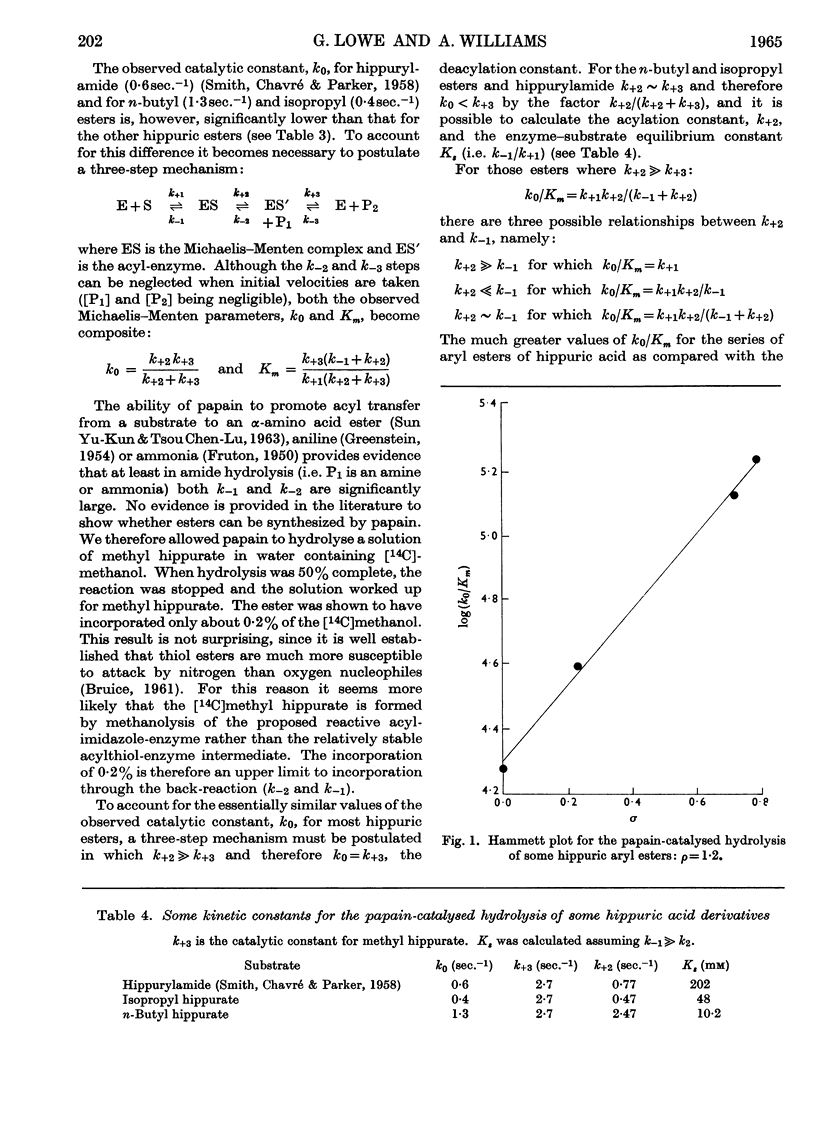
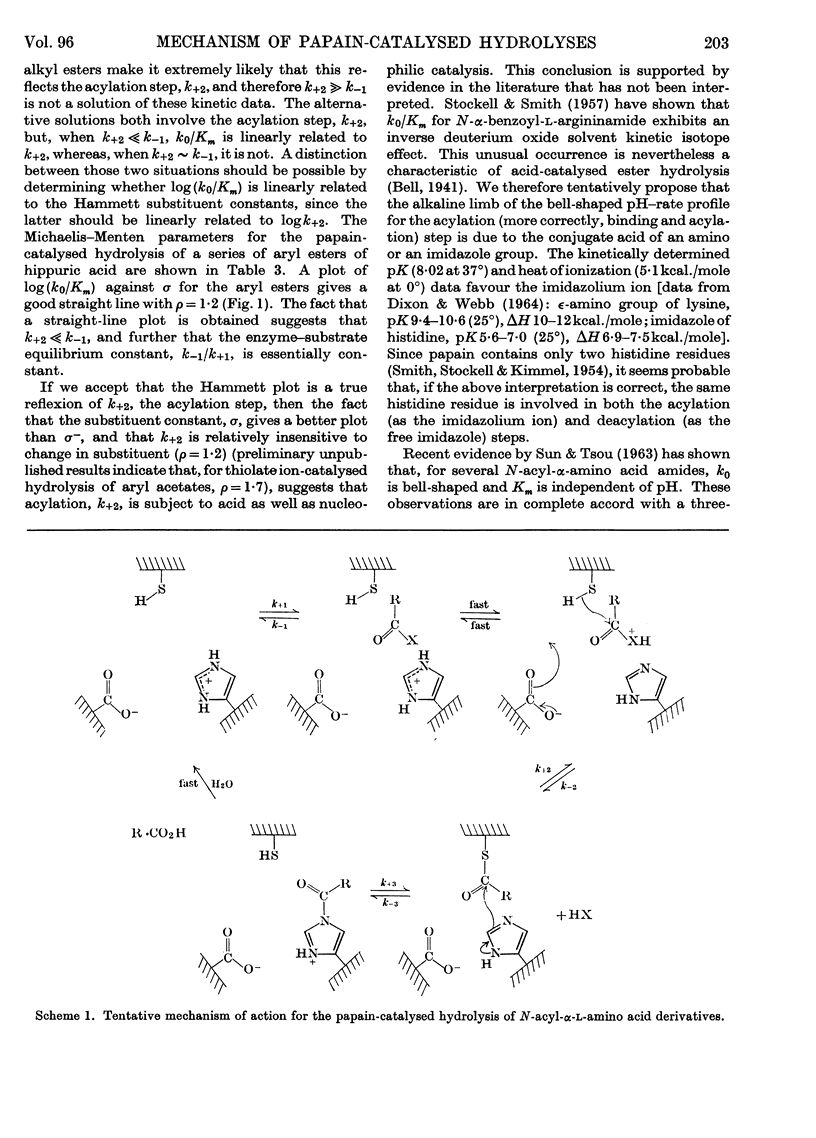
1. The Michaelis–Menten parameters for the papain-catalysed hydrolysis of a number of alkyl, aryl and alkyl-thiol esters of hippuric acid have been determined. 2. For all the aryl esters and most of the alkyl esters studied, the catalytic constant, k0, is 2–3sec.−1 and most probably represents deacylation of the common intermediate, hippuryl-papain. 3. Two alkyl esters and hippurylamide, however, have catalytic rate constants, k0, less than 2–3sec.−1. It is possible to interpret all the available kinetic data in terms of a three-step mechanism in which an enzyme–substrate complex is first formed, followed by acylation of the enzyme through an essential thiol group, followed by deacylation of the acyl-enzyme. 4. The logarithm of the ratio of the Michaelis–Menten parameters, which reflect the acylation rate constant, for four aryl esters of hippuric acid studied give a linear Hammett plot against the substituent constant, σ. Arguments are presented that indicate acid as well as nucleophilic catalysis in the acylation process and that the most likely proton donor is an imidazolium ion. 5. It is suggested that this imidazolium ion is part of the same histidine residue that has been tentatively implicated in the deacylation process (Lowe & Williams, 1965b). 6. A new mechanism is proposed for the papain-catalysed hydrolysis of N-acyl-α-amino acid derivatives.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRUTON J. S. The role of proteolytic enzymes in the biosynthesis of peptide bonds. Yale J Biol Med. 1950 Jan;22(3):263–271. [PMC free article] [PubMed] [Google Scholar]
- GREENSTEIN J. P. The resolution of racemic alpha-amino acids. Adv Protein Chem. 1954;9:121–202. doi: 10.1016/s0065-3233(08)60206-5. [DOI] [PubMed] [Google Scholar]
- HAMMOND B. R., GUTFREUND H. The mechanism of ficin-catalysed reactions. Biochem J. 1959 Jun;72(2):349–357. doi: 10.1042/bj0720349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. A STUDY OF SOME THIOL ESTER HYDROLYSES AS MODELS FOR THE DEACYLATION STEP OF PAPAIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:194–198. doi: 10.1042/bj0960194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. DIRECT EVIDENCE FOR AN ACYLATED THIOL AS AN INTERMEDIATE IN PAPAIN- AND FICIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:189–193. doi: 10.1042/bj0960189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD C. E., BALLS A. K. Analogues of acetyl chymotrypsin. J Biol Chem. 1957 Aug;227(2):727–736. [PubMed] [Google Scholar]
- SMITH E. L. Active site of papain and covalent high-energy bonds of proteins. J Biol Chem. 1958 Dec;233(6):1392–1397. [PubMed] [Google Scholar]
- SMITH E. L., CHAVRE V. J., PARKER M. J. Kinetics of papain action. II. Effect of pH on hydrolysis of three substrates. J Biol Chem. 1958 Jan;230(1):283–293. [PubMed] [Google Scholar]
- SMITH E. L., PARKER M. J. Kinetics of papain action. III. Hydrolysis of benzoyl-L-arginine ethyl ester. J Biol Chem. 1958 Dec;233(6):1387–1391. [PubMed] [Google Scholar]
- SMITH E. L., STOCKELL A., KIMMEL J. R. Crystalline papain. III. Amino acid composition. J Biol Chem. 1954 Apr;207(2):551–561. [PubMed] [Google Scholar]
- STOCKELL A., SMITH E. L. Kinetics of papain action. I. Hydrolysis of benzoly-L-argininamide. J Biol Chem. 1957 Jul;227(1):1–26. [PubMed] [Google Scholar]


