Abstract
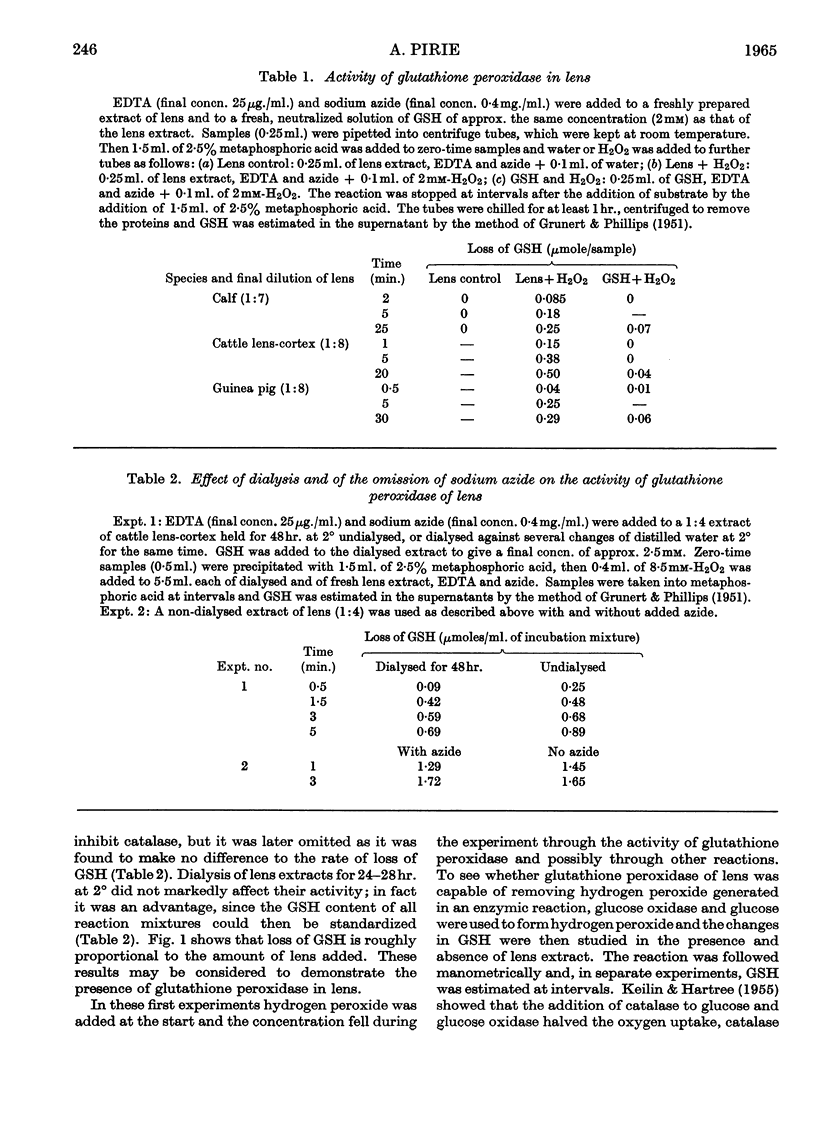
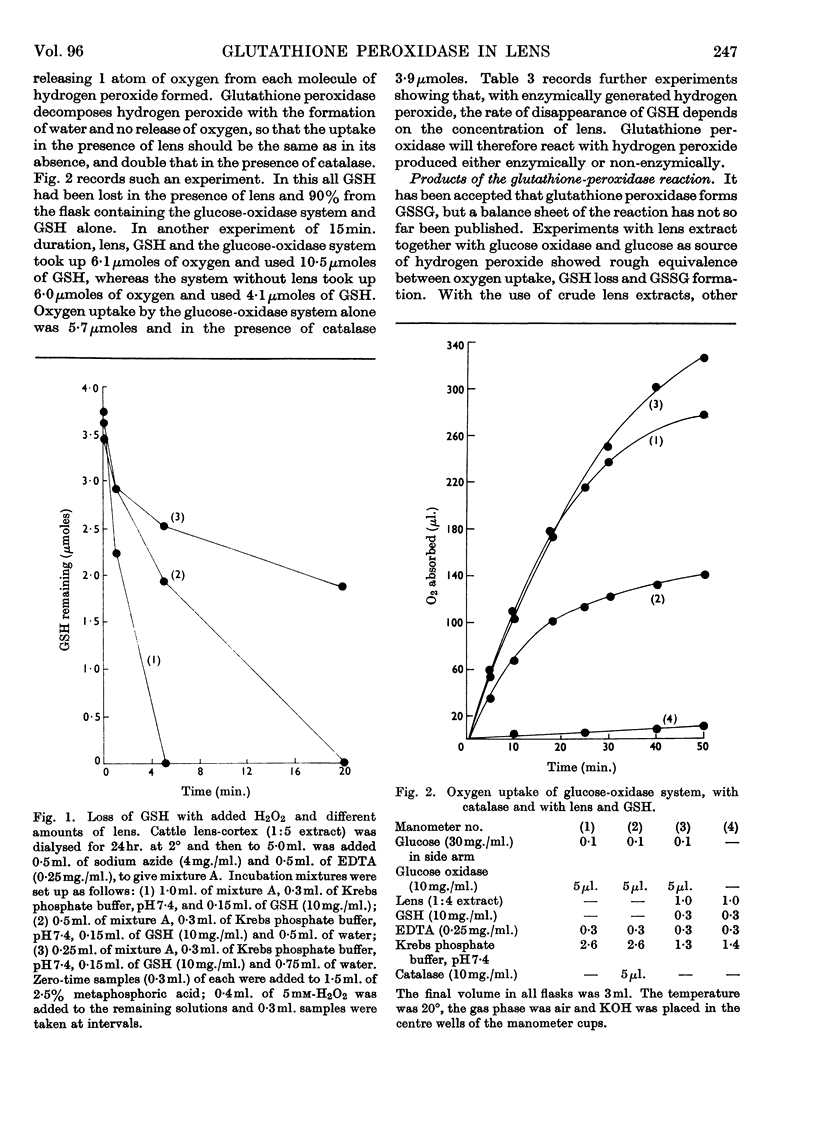
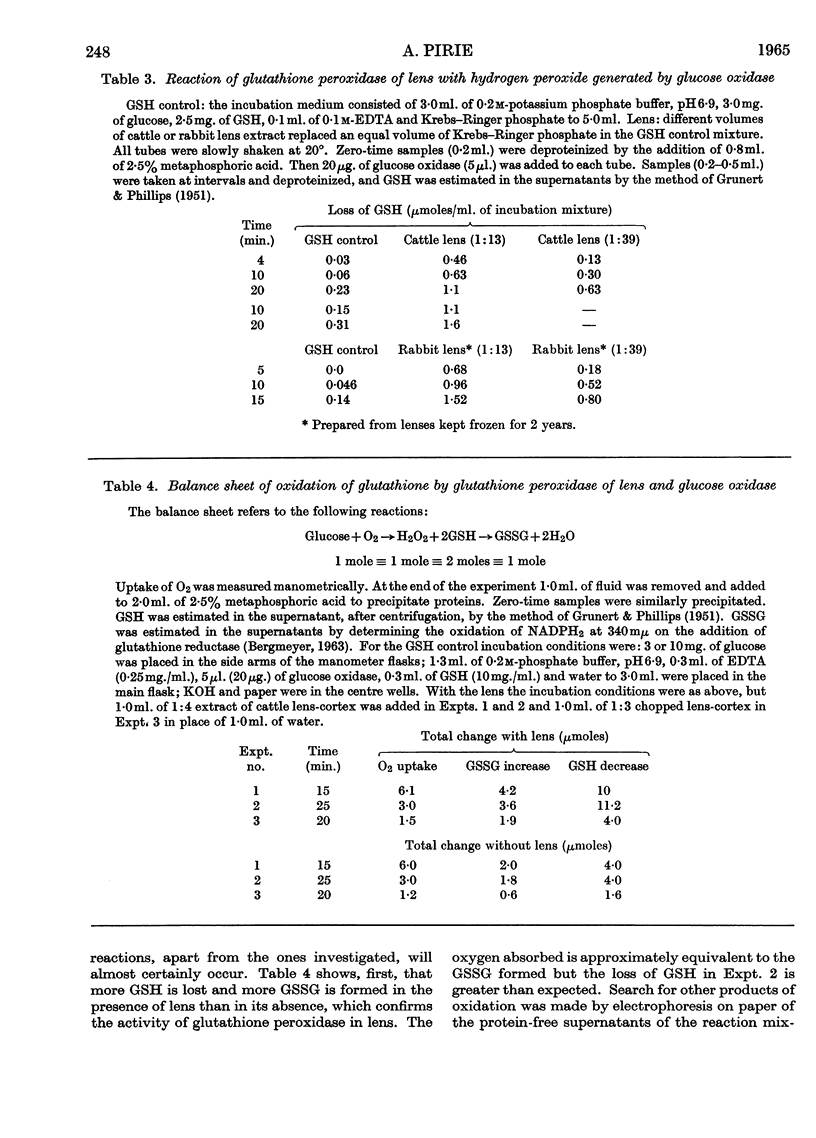
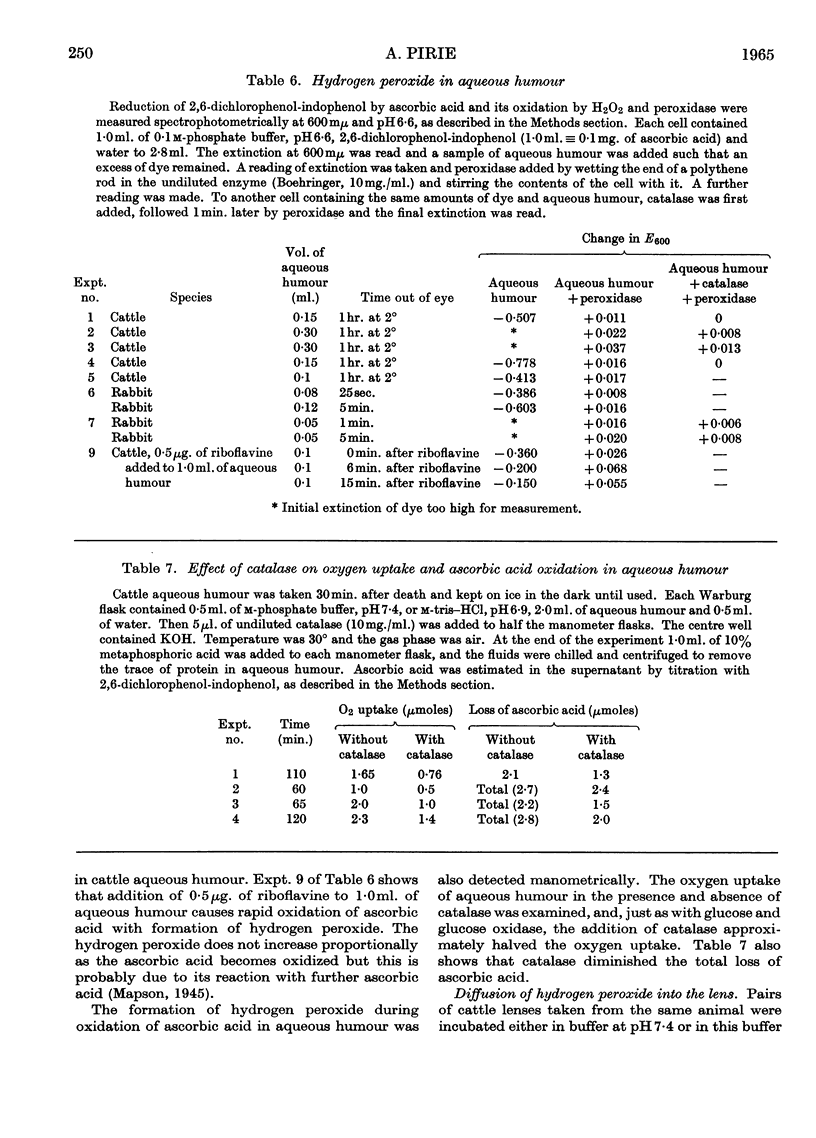
1. Glutathione peroxidase has been demonstrated in cattle, rabbit and guineapig lenses. 2. The enzyme will oxidize GSH either with hydrogen peroxide added at the start of the reaction or with hydrogen peroxide generated enzymically with glucose oxidase. 3. No product other than GSSG was detected. 4. Oxidation of GSH can be coupled with oxidation of malate through the intermediate reaction of glutathione reductase and NADPH2. 5. Traces of hydrogen peroxide are present in aqueous humour: it is formed when the ascorbic acid of aqueous humour is oxidized. 6. Hydrogen peroxide will diffuse into the explanted intact lens and oxidize the contained GSH. The addition of glucose to the medium together with hydrogen peroxide maintains the concentration of lens GSH. 7. Glutathione peroxidase in lens extracts will couple with the oxidation of ascorbic acid. 8. It is suggested that, as there is only weak catalase activity in lens, glutathione peroxidase may act as one link between the oxygen of the aqueous humour and NADPH2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWNESS J. M., MORTON R. A., SHAKIR M. H., STUBBS A. L. Distribution of copper and zinc in mammalian eyes. Occurrence of metals in melanin fractions from eye tissues. Biochem J. 1952 Jul;51(4):521–530. doi: 10.1042/bj0510521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. A modification of the nitroprusside method of analysis for glutathione. Arch Biochem. 1951 Feb;30(2):217–225. [PubMed] [Google Scholar]
- JOCELYN P. C. OXIDATION OF GLUTATHIONE AND OTHER THIOLS BY THE XANTHINE OXIDASE AND HYPOXANTHINE OF RAT LIVER HOMOGENATES. Nature. 1964 Jun 13;202:1115–1115. doi: 10.1038/2021115a0. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Catalase, peroxidase and metmyoglobin as catalysts of coupled peroxidatic reactions. Biochem J. 1955 Jun;60(2):310–325. doi: 10.1042/bj0600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINOSHITA J. H. Carbohydrate metabolism of lens. AMA Arch Ophthalmol. 1955 Sep;54(3):360–368. doi: 10.1001/archopht.1955.00930020366005. [DOI] [PubMed] [Google Scholar]
- KINOSHITA J. H., MASURAT T. Studies on the glutathione in bovine lens. AMA Arch Ophthalmol. 1957 Feb;57(2):266–274. doi: 10.1001/archopht.1957.00930050276018. [DOI] [PubMed] [Google Scholar]
- KINOSHITA J. H. SELECTED TOPICS IN OPHTHALMIC BIOCHEMISTRY. Arch Ophthalmol. 1964 Oct;72:554–572. doi: 10.1001/archopht.1964.00970020554022. [DOI] [PubMed] [Google Scholar]
- KINSEY V. E. Comparative chemistry of aqueous humor in posterior and anterior chambers of rabbit eye, its physiologic significance. AMA Arch Ophthalmol. 1953 Oct;50(4):401–417. doi: 10.1001/archopht.1953.00920030409001. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- MILLS G. C., RANDALL H. P. Hemoglobin catabolism. II. The protection of hemoglobin from oxidative breakdown in the intact erythrocyte. J Biol Chem. 1958 Jun;232(2):589–598. [PubMed] [Google Scholar]
- Mapson L. W. Influence of halides on the oxidation of ascorbic acid: 2. Action of Cl on the cupric-cuprous system. Biochem J. 1945;39(3):228–236. [PMC free article] [PubMed] [Google Scholar]
- PIRIE A. A LIGHT-CALALYSED REACTION IN THE AQUEOUS HUMOR OF THE EYE. Nature. 1965 Jan 30;205:500–501. doi: 10.1038/205500a0. [DOI] [PubMed] [Google Scholar]
- PIRIE A., VAN HEYNINGEN R. Oxidation of glutathione in extracts of lens in the presence of ethylenediaminetetraacetate. Nature. 1954 May 8;173(4410):873–874. doi: 10.1038/173873a0. [DOI] [PubMed] [Google Scholar]
- Philpot F. J., Pirie A. Riboflavin and riboflavin adenine dinucleotide in ox ocular tissues. Biochem J. 1943 Jul;37(2):250–254. doi: 10.1042/bj0370250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. OXIDATION OF REDUCED TRIPHOSPHOPYRIDINE NUCLEOTIDE BY GUINEA PIG POLYMORPHONUCLEAR LEUCOCYTES. Nature. 1964 Apr 4;202:85–86. doi: 10.1038/202085a0. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN R., PIRIE A. Reduction of glutathione coupled with oxidative decarboxylation of malate in cattle lens. Biochem J. 1953 Feb;53(3):436–444. doi: 10.1042/bj0530436. [DOI] [PMC free article] [PubMed] [Google Scholar]


