Abstract
A novel, highly purified 10% intravenous immunoglobulin (IVIG) formulation was evaluated for both therapeutic efficacy and safety profile in adult patients diagnosed with persistent or chronic primary immune thrombocytopenia (ITP). This phase III, multicenter, open-label, single-arm clinical trial enrolled Chinese adult patients diagnosed with persistent or chronic ITP presenting with baseline platelet counts below 30 × 109/L. Participants received intravenous administration of 10% IVIG at a standardized dosage of 1 g/kg/day for two consecutive days. The primary efficacy endpoint was defined as the proportion of subjects achieving both a platelet count elevation to ≥ 30 × 109/L and a minimum two-fold increase from baseline values within a 7-day post-treatment observation period following the first dose administration. Seventy-two patients were enrolled and sixty patients completed the study. 52 (72.2%; 95% CI: 60.4, 82.1) patients achieved platelet count ≥ 30 × 109/L and experienced a ≥ twofold increase from baseline within 7 days, and 52 (72.2%; 95% CI: 60.4, 82.1) patients achieved complete response (CR) or response (R) within 7 days. 64 patients (88.9%; 95% CI: 79.3, 95.1) achieved platelet count ≥ 50 × 109/L within 7 days with a median time of 3 days. 71 patients completed the ITP bleeding scale assessment after 7 days, showing a decrease of 0.6 ± 1.07 from baseline. A total of 66 patients (91.7%) reported treatment-emergent adverse events (TEAEs) during the study, and 37 patients (51.4%) reported adverse drug reactions (ADRs). The most prevalent ADRs with an incidence exceeding 5% included headache (n = 12, 16.7%), fever (n = 10, 13.9%), decreased white blood cell count (n = 5, 6.9%), and nausea (n = 5, 6.9%). The therapeutic regimen of 10% IVIG administered at a dosage of 1 g/kg/day for two consecutive days demonstrated both favorable safety profiles and clinical efficacy. These robust findings provide substantial evidence supporting the clinical application of this novel 10% IVIG formulation in the management of adult patients with ITP.
Keywords: Intravenous immunoglobulin, Immune thrombocytopenia, Response, Platelet count, Bleeding assessment
Introduction
Primary immune thrombocytopenia (ITP) is classified as an acquired autoimmune hematologic disorder characterized by isolated thrombocytopenia resulting from immune-mediated platelet destruction and impaired megakaryocyte production, in the absence of other identifiable underlying etiologies [1]. The global annual incidence of primary immune thrombocytopenia (ITP) in adult populations is estimated to range between 50 and 100 cases per million person-years, as supported by epidemiological studies [2, 3]. However, population-based epidemiological characteristics and demographic distribution patterns of ITP within the Chinese population remain to be systematically investigated and comprehensively elucidated [1].
Intravenous immunoglobulin (IVIG), a sterile preparation derived from purified human plasma through extensive fractionation processes [4], has been utilized in clinical practice since its initial therapeutic applications in the 1930s [5]. Its pivotal role in autoimmune disorders was first established in 1981, marking a significant advancement in immunomodulatory therapy [6]. Current Chinese clinical guidelines endorse IVIG as a first-line therapeutic intervention for adult ITP, with recommended dosing regimens of 1 g/kg administered over 1–2 days [1].
While the clinical efficacy of IVIG in ITP management is well-established in numerous clinical studies [7], its precise mechanisms of action remain incompletely elucidated. The pathophysiology of ITP is primarily mediated by antiplatelet autoantibodies [8], with IgG subtype being the predominant antibody class identified in patient sera [9]. The disease mechanism involves the binding of platelet-specific autoantibodies to membrane glycoproteins, followed by Fc receptor-mediated recognition and subsequent phagocytosis by the monocyte-macrophage system [10], leading to accelerated platelet destruction.
The investigational product in this study is a 10% IVIG formulation (National Medical Products Administration approval number: 2018L03309), manufactured by Taibang Biological Ltd. through advanced purification processes. This phase III clinical investigation evaluated both the therapeutic efficacy and safety profile of this high-purity IVIG preparation in adult patients with persistent or chronic primary ITP.
Materials and methods
This prospective, open-label, single-arm, multicenter phase III clinical trial was conducted across ten tertiary hospitals in China between July 2020 and August 2021 to evaluate the therapeutic efficacy and safety profile of a novel 10% IVIG formulation in adult patients diagnosed with primary ITP presenting with baseline platelet counts below 30 × 109/L.
The treatment protocol consisted of IVIG administration at a dose of 1 g/kg/day for two consecutive days, initiated at an infusion rate of 0.5 mg/kg/min (0.005 ml/kg/min) for the first 15 min, followed by gradual titration to a maximum tolerated rate of 8 mg/kg/min (0.08 mL/kg/min), with continuous monitoring of vital signs and potential adverse reactions.
The primary efficacy endpoint was defined as the proportion of subjects achieving both a platelet count elevation to ≥ 30 × 109/L and a minimum two-fold increase from baseline values within a 7-day post-treatment observation period following the initial administration of the investigational product.
Study patients
Following written informed consent acquisition, potential participants underwent comprehensive screening procedures. Eligible subjects included both male and female patients meeting the following inclusion criteria: 1) age between 18 and 65 years; 2) confirmed diagnosis of primary ITP for a minimum duration of 3 months preceding study enrollment; 3) baseline platelet count below 30 × 109/L.
The exclusion criteria were rigorously defined as follows: 1) secondary thrombocytopenia attributable to other underlying pathologies; 2) documented hypersensitivity to immunoglobulin preparations or plasma-derived products; 3) concurrent autoimmune hemolytic anemia; 4) administration of intravenous immunoglobulin, anti-D immunoglobulin, blood products, immunosuppressive agents, or immunomodulatory investigational therapies within 4 weeks prior to consent; 5) treatment with rituximab or live-attenuated vaccines within 8 weeks preceding screening; 6) use of recombinant human thrombopoietin, thrombopoietin receptor agonists, or other platelet-enhancing investigational agents within 2 weeks prior to enrollment; 7) hepatic dysfunction evidenced by alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels exceeding threefold the upper limit of normal (ULN), or total bilirubin > 1.5 × ULN; 8) poorly controlled hypertension (grade II or higher); 9) renal impairment defined as serum creatinine ≥ 1.5 × ULN or creatinine clearance < 60 mL/min (calculated using Cockcroft-Gault formula); 10) pregnancy or lactation status.
Measurements
Blood samples were collected via standardized venipuncture from the antecubital vein using sterile, single-use systems. For platelet counts, whole blood was collected in EDTA tubes, while plasma samples for biomarker analysis were collected in sodium citrate tubes (3.2% or 3.8%), gently inverted 8–10 times post-collection. EDTA samples were analyzed within 2 h, and citrated plasma was centrifuged at 1500 × g for 15 min at 4 °C within 30 min, with the supernatant stored at − 80 °C. Platelet counts were determined using automated hematology analyzers (Beckman Coulter UniCel DxH), and biomarker analyses were performed via ELISA, flow cytometry, or mass spectrometry, with quality control measures including duplicate readings and calibration with known standards.
Efficacy outcome
The primary efficacy endpoint was defined as the proportion of subjects achieving both a platelet count elevation to ≥ 30 × 109/L and a minimum two-fold increase from baseline values within a 7-day post-treatment observation period following the initial administration of the investigational product. The secondary efficacy outcomes of the study included the followings: 1) The proportion of patients attaining platelet counts ≥ 50 × 109/L and the corresponding median time-to-response during the 7-day post-treatment period; 2) The percentage of patients meeting dual criteria within 7 days post-infusion: a) platelet count ≥ 30 × 109/L, and b) ≥ twofold increase from baseline platelet count;3) Response rates and median time-to-response, where Response (R) was defined as achieving both platelet count ≥ 30 × 109/L and ≥ twofold increase from baseline, and Complete Response (CR) was defined as platelet count normalization to ≥ 100 × 109/L, within the 7-day evaluation window; 4) The proportion of patients experiencing relapse, defined as platelet count decrease below response criteria, within 28 days following initial treatment;5) Longitudinal changes in ITP-specific bleeding scale scores at days 7 and 14 post-treatment initiation.
Safety evaluation
Following informed consent acquisition, safety assessments were conducted and documented in accordance with the study protocol, including longitudinal monitoring of vital signs, clinical symptomatology, adverse event profiles, and serial laboratory evaluations.
Statistical analysis
The sample size calculation was based on the following parameters: the anticipated overall response rate within 7 days post-treatment was estimated at 77%, with a predefined threshold of 60% (corresponding to the lower bound of the two-sided 95% confidence interval). Assuming a type I error rate (α) of 0.05 (two-sided) and statistical power (1-β) of 80%, a minimum of 60 evaluable cases was required. To account for potential attrition, the sample size was increased by 20%, resulting in a planned enrollment of 72 participants.
Continuous variables were expressed as mean ± standard deviation (SD) with median (range) values, while categorical variables were presented as frequencies and percentages. All statistical analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
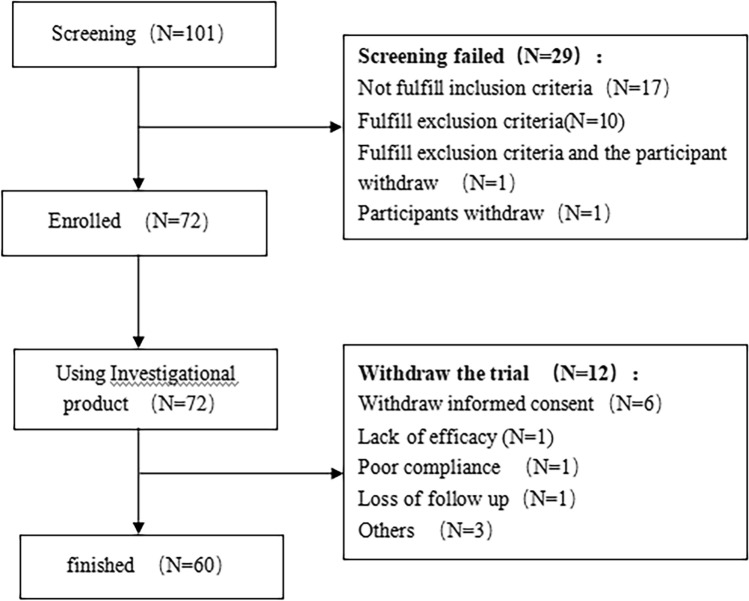
A total of 101 patients were screened, of which 72 patients were enrolled and 60 patients completed the study (Fig. 1). All 72 patients met the criteria of the full analysis set.
Fig. 1.
Diagram of study flow
The medication compliance of all enrolled patients during the treatment period was in the range of 80–120%. The average cumulative dose of the investigational product per patient was 126.27 g (median of 121.50 g).
Patients
Patients age ranged from 19 to 65 years (mean 41.0 ± 12.33 years) (Table 1 Comparison of Baseline Characteristics). Twenty-four patients were male (33.3%). The weight ranged from 43 to 95 kg (mean 63.13 ± 12.164), and the mean of body mass index was 23.69 ± 3.406. The mean time of ITP diagnosis was 54.12 ± 58.390 months (median of 31.20 months). Twenty-seven patients had a history of bleeding. Two patients had refractory ITP defined as the failure to achieve a response or loss of response after splenectomy. A total of 68 patients (94.4%) had been previously treated for ITP.
Table 1.
Comparison of baseline characteristics
| Baseline | Results |
|---|---|
| N | 72 |
| Age (years) | |
| Mean ± SD | 41.0 ± 12.33 |
| Median (range) | 41.5 (19 ~ 65) |
| Body mass index (kg/m2) | |
| Mean ± SD | 23.69 ± 3.406 |
| Median (range) | 23.20 (15.9 ~ 34.1) |
| Sex | |
| Male n (%) | 24 (33.3) |
| Female n (%) | 48 (66.7) |
| History of ITP | |
| Time from diagnosis (months) | |
| Mean ± SD | 54.12 ± 58.390 |
| Median (range) | 31.20 (3 ~ 282.4) |
| Phase | |
| Refractory n (%) | 2 (2.8) |
| Persistent n (%) | 23 (31.9) |
| Chronic n (%) | 47 (65.3) |
| Treatment history | |
| Newly diagnosed without prior treatment n (%) | 4 (5.6) |
| Previous treatment history n (%) | 68 (94.4) |
| Bleeding | |
| Yes n (%) | 45 (62.5) |
| No n (%) | 27 (37.5) |
Efficacy
All 72 enrolled patients were included in the full analysis set (FAS), while 5 patients were excluded from the per-protocol set (PPS) due to protocol deviations.
The primary efficacy endpoint was achieved in 52 patients (72.2%; 95% confidence interval [CI]: 60.4–82.1%), demonstrating both a platelet count elevation to ≥ 30 × 109/L and a minimum two-fold increase from baseline values within the 7-day post-treatment observation period. (Table 2).
Table 2.
Efficacy outcomes (full analysis set)
| Efficacy variable | Results |
|---|---|
| N | 72 |
| Platelet | |
| Maximum platelet count (109/L) | |
| Mean ± SD | 185.7 ± 138.90 |
| Median (range) | 143.5 (18 ~ 771) |
| Time to maximum platelet count (days) | |
| Mean ± SD | 6.0 ± 2.54 |
| Median (range) | 6.0 (1 ~ 14) |
| Platelet ≥ 30 × 109/L and ≥ twofold increase from baseline within 7 days | |
| Yes n (%)[95 CI] | 52 (72.2)[60.4,82.1] |
| No n (%) | 20 (27.8) |
| Platelet ≥ 50 × 109/L within 7 days | |
| ≥ 50 × 109/L n (%) | 64 (88.9)[79.3,95.1] |
| Median time of platelet ≥ 50 × 109/L (days) | 3[NA, NA] |
| < 50 × 109/L n (%) | 8 (11.1) |
| Platelet ≥ 30 × 10^9/L and at least tripled from the baseline within 7 days | |
| Yes n (%) | 66 (91.7)[82.7,96.9] |
| No n (%) | 6 (8.3) |
| Response/complete response/no response/relapse | |
| Percentage | |
| Complete response n (%)[95% CI] | 23 (31.9)[21.4,44.0] |
| Response n (%)[95% CI] | 29 (40.3)[28.9,52.5] |
| No response n (%)[95% CI] | 4 (5.6)[1.5,13.6] |
| Relapse n (%)[95% CI] | 16 (22.2)[13.3,33.6] |
| Response or complete response within 7 days | |
| Response/complete response n (%)[95% CI] | 52 (72.2)[60.4,82.1] |
| Median time of response/complete response (days) [95% CI] | 3[3.0,4.0] |
| No response/relapse n (%) | 20 (27.8) |
| Achieving response/complete response and experiencing relapse within 28 days | |
| N | 52 |
| Relapse n (%)[95% CI] | 38 (73.1)[59.0,84.4] |
| No relapse n (%) | 14 (26.9) |
| ITP bleeding scale score | |
| Baseline | |
| N | 72 |
| Mean ± SD | 1.1 ± 1.18 |
| Median (range) | 1.0 (0 ~ 6) |
| 7 days | |
| N (N Miss) | 71 (1) |
| Mean ± SD | 0.4 ± 0.74 |
| Median (range) | 0 (0 ~ 3) |
| 14 days | |
| N (N Miss) | 68 (4) |
| Mean ± SD | 0.4 ± 0.94 |
| Median (range) | 0 (0 ~ 5) |
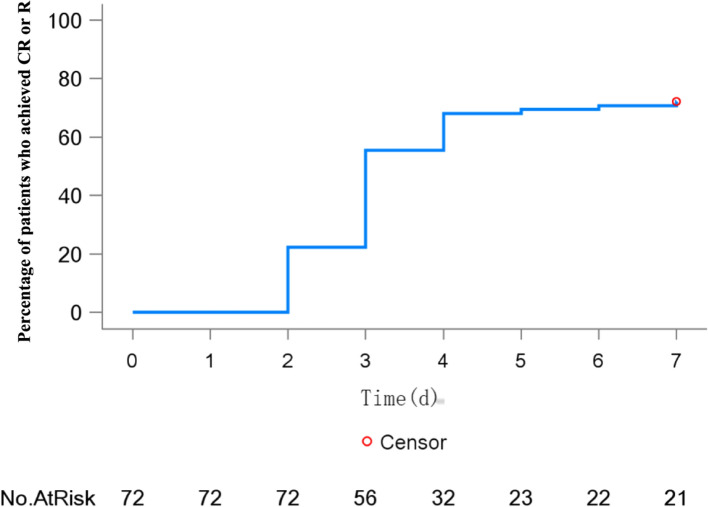
A total of 23 patients (31.9% of patients; 95% CI:21.4, 44.0) achieved CR, 28 patients (40.3% of patients; 95% CI: 28.9, 52.5) achieved R, 4 patients (5.6% of patients; 95% CI: 1.5, 13.6) had no response, and 16 patients (22.2% of patients; 95% CI: 13.3, 33.6) experienced relapse within 7 days (Table 2). 52 (72.2%) patients achieved CR or R within 7 days of the initial investigational product and the median time was 3 days (95% CI:3.0, 4.0). The percentage of patients who achieved CR or R at each time point during treatment is shown in Fig. 2. Totally, 38 patients (73.1%) who had achieved R or CR experienced relapse within 28 days.
Fig. 2.
Kaplan–Meier curve depicting the percentage of patients achieving R or CR at each time point during treatment (full analysis set)
Platelet assessments
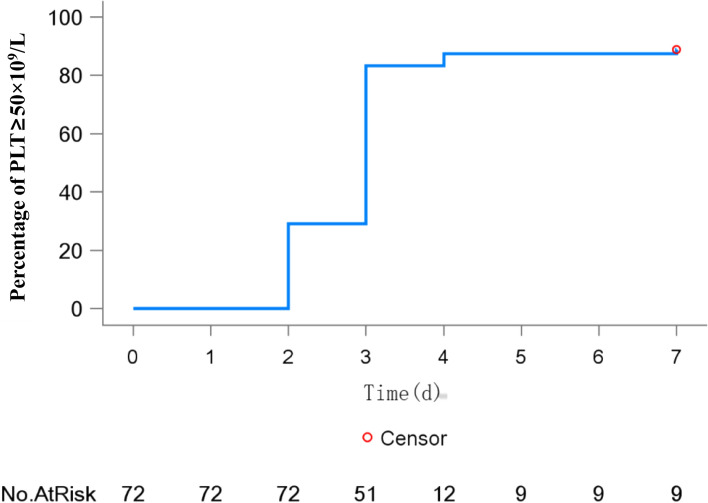
The peak platelet count across the study population reached a mean of 185.7 × 109/L (median: 143.5 × 109/L), with a median time-to-peak of 6 days (range: 1–14 days) (Table 2). Notably, 64 patients (88.9%; 95% CI: 79.3–95.1) achieved platelet counts ≥ 50 × 109/L within 7 days (median time: 3 days), with temporal response patterns illustrated in Fig. 3. Furthermore, 66 patients (91.7%; 95% CI: 82.7–96.9) attained platelet counts ≥ 30 × 109/L on at least one occasion during the 7-day evaluation period (Fig. 3).
Fig. 3.
Kaplan–Meier curve depicting the percentage of patients with PLT ≥ 50 × 109/L at each time point during treatment (full analysis set)
Bleeding assessments
Seventy-one patients completed the ITP bleeding scale assessment after 7 days, showing a decrease of 0.6 ± 1.07 from baseline. Additionally, sixty-eight patients completed the ITP bleeding scale assessment at day 14 after treatment, also showing a decrease of 0.6 ± 1.14 from baseline. Specific values are presented in Table 2.
Safety
A total of 66 patients (91.7%) reported treatment-emergent adverse events (TEAEs) during the study, and 37 patients (51.4%) reported adverse drug reactions (ADRs). None subject quit the trial because of TEAEs.
Serious Adverse Events (SAEs) were observed in 9 patients (12.5%) with a total of 11 times, including rashes, purpura, oral bleeding, gastrointestinal bleeding, thrombocytopenia, anemia, acute cholecystitis, and pregnancy. Except one subject died of severe gastrointestinal bleeding due to refractory thrombocytopenia, all other SAEs have recovered or resolved.
The most prevalent ADRs with an incidence exceeding 5% included headache (n = 12, 16.7%), fever (n = 10, 13.9%), decreased white blood cell count (n = 5, 6.9%), and nausea (n = 5, 6.9%). Notably, all ADRs have been resolved or recovered. Specific ADRs are detailed in Table 3.
Table 3.
Summary of adverse drug reactions (safety analysis set)
| Adverse drug reactions | Results | |
|---|---|---|
| N (%) | Times | |
| N | 72 | |
| Total n (%) | 37 (51.4) | 80 |
| Nervous system disorders n (%) | 13 (18.1) | 18 |
| Headache n (%) | 12 (16.7) | 16 |
| Dizziness n (%) | 2 (2.8) | 2 |
| General disorders and administration site conditions n (%) | 13 (18.1) | 15 |
| Fever n (%) | 10 (13.9) | 10 |
| Fatigue n (%) | 3 (4.2) | 3 |
| Discomfort at the infusion site n (%) | 1 (1.4) | 1 |
| Chest discomfort n (%) | 1 (1.4) | 1 |
| Investigations n (%) | 10 (13.9) | 13 |
| Decreased white blood cell count n (%) | 5 (6.9) | 5 |
| Decreased neutrophil count n (%) | 2 (2.8) | 3 |
| Increased heart rate n (%) | 1 (1.4) | 1 |
| Elevated blood bilirubin n (%) | 1 (1.4) | 1 |
| Increased platelet count n (%) | 1 (1.4) | 1 |
| Elevated blood pressure n (%) | 1 (1.4) | 2 |
| Gastrointestinal disorders n (%) | 8 (11.1) | 12 |
| Nausea n (%) | 5 (6.9) | 5 |
| Vomiting n (%) | 3 (4.2) | 5 |
| Cheilitis n (%) | 1 (1.4) | 1 |
| Gingival bleeding n (%) | 1 (1.4) | 1 |
| Blood and lymphatic system disorders n (%) | 5 (6.9) | 7 |
| Anemia n (%) | 2 (2.8) | 3 |
| Hemolytic anemia n (%) | 2 (2.8) | 2 |
| Hemolysis n (%) | 1 (1.4) | 1 |
| Acholuric jaundice n (%) | 1 (1.4) | 1 |
| Hepatobiliary disorders n (%) | 4 (5.6) | 4 |
| Abnormal liver function n (%) | 3 (4.2) | 3 |
| Hyperbilirubinemia n (%) | 1 (1.4) | 1 |
| Vascular disorders n (%) | 3 (4.2) | 4 |
| Hypotension n (%) | 3 (4.2) | 4 |
| Skin and subcutaneous tissue disorders n (%) | 2 (2.8) | 2 |
| Rash n (%) | 2 (2.8) | 2 |
| Metabolism and nutrition disorders n (%) | 1 (1.4) | 1 |
| Anorexia n (%) | 1 (1.4) | 1 |
| Infections and infestations n (%) | 1 (1.4) | 1 |
| Upper respiratory tract infection n (%) | 1 (1.4) | 1 |
| Musculoskeletal and connective tissue disorders n (%) | 1 (1.4) | 1 |
| Limb pain n (%) | 1 (1.4) | 1 |
| Respiratory, thoracic and mediastinal disorders n (%) | 1 (1.4) | 1 |
| Cough n (%) | 1 (1.4) | 1 |
| Cardiac disorders n (%) | 1 (1.4) | 1 |
| Sinus arrhythmia n (%) | 1 (1.4) | 1 |
Discussion
Primary ITP represents a prevalent acquired hemorrhagic disorder characterized by isolated thrombocytopenia [1]. IVIG has been accepted as one of the effective treatments for ITP in many guidelines [1, 11–13]. The immunomodulatory mechanisms of IVIG in ITP treatment are multifactorial, including: 1) Fc receptor blockade in splenic macrophages, preventing phagocytosis of antibody-opsonized platelets; 2) modulation of autoantibody production and binding capacity, coupled with inflammatory cytokine suppression; and 3) inhibition of complement-mediated platelet destruction.
While IVIG formulations are commercially available at various concentrations (typically 5% or 10%), only 5% preparations are currently marketed in China. This study investigates a novel, highly purified 10% IVIG formulation, which has received clinical trial authorization from the National Medical Products Administration (NMPA) for the treatment of persistent or chronic ITP [13].
In this study, 72.2% patients achieved platelet count ≥ 30 × 109/L and experienced a ≥ twofold increase from baseline after the initial investigational product administration within 7 days, which was similar to the other 10% IVIG reported by clinical trial [14–17]. The other 10% IVIG showed a response rate of 75.7% for GC5107A [14], 63.2% (95% CI: 46.0, 78.2) for IQYMUNE® [15], 52.9% (95% CI: 35.1, 70.2) for Yimmugo® [16], 80.7% (95% CI: 69.2, 89.3) for Privigen® [17], 80·6% (95 CI: 63·98, 91·81%) for Panzyga® [18], and so on. The median time to achieve platelet count ≥ 50 × 109/L, was 3 days, which was similar to the other 10% IVIG reported by clinical trial [17].
Safety analysis revealed that while 91.7% of patients experienced TEAEs, the majority were mild to moderate in severity. ADRs were reported in 51.4% of subjects, consistent with established safety profiles of other 10% IVIG formulations [17], with all events resolving without sequelae. Nine serious adverse events (SAEs) were documented, including one mortality case attributed to disease progression and subsequent massive gastrointestinal hemorrhage. Notably, the fatal event occurred approximately 3 months post-infusion, with no causal relationship to the investigational product, as evidenced by the subject's positive initial treatment response and absence of infusion-related adverse effects. The investigational 10% IVIG demonstrated favorable tolerability, with an adverse event profile comparable to existing IVIG products. The most frequently observed ADRs included headache (20.8%), pyrexia, leukopenia, and nausea, consistent with established safety data [18]. All headache events were mild and self-limiting, with severity and incidence rates within expected parameters.
Manufactured by Taibang Biological Ltd [19]. the investigational product undergoes rigorous viral safety measures, including low-temperature ethanol fractionation, caprylic acid precipitation, ion-exchange chromatography, low pH incubation, and pasteurization. Comprehensive viral safety was confirmed through serological testing, with all patients testing negative for HBsAg, anti-HCV, anti-HIV, and anti-Treponema pallidum antibodies pre- and post-treatment, and no reported viral transmissions [20].
This novel 10% IVIG formulation offers distinct pharmacokinetic advantages, including a 34% reduction in infusion time compared to standard 5% formulations while maintaining comparable therapeutic efficacy and safety profiles [19]. The established dosing regimen of 1 g/kg/day for two consecutive days demonstrated both clinical efficacy and favorable safety, supporting its therapeutic application in adult ITP management.
Based on the current findings, we hypothesize that a single-dose IVIG regimen (1 g/kg administered once) may demonstrate comparable therapeutic efficacy to the conventional two-dose regimen (2 × 1 g/kg over two days), as our phase III trial established that the novel 10% IVIG formulation exhibits comparable efficacy and safety profiles to existing IVIG therapies in adult primary ITP patients. The rationale for exploring a single-dose regimen is supported by IVIG's rapid immunomodulatory effects (typically within 24–48 h post-infusion), preliminary clinical evidence suggesting satisfactory therapeutic response in some patients with a single dose, and potential healthcare optimization through reduced hospitalization duration and treatment costs. However, a well-designed randomized controlled trial (RCT) comparing single-dose versus standard two-dose regimens is essential to establish non-inferiority or potential superiority of the simplified approach, with future investigations incorporating expanded multicenter participation to ensure robust implementation. If non-inferiority is established, the single-dose regimen could emerge as a more cost-effective and patient-friendly treatment paradigm, potentially influencing guideline updates and clinical practice, while demonstrating inferior efficacy would reinforce the necessity of the two-dose regimen as the standard of care for optimal platelet recovery.
Author contributions
H.C. and J.W. wrote the main manuscript text; Z.Y. and H.Z. prepared figures and tables; Z.L. and F.Y. conducted data analysis; P.G. and D.G. performed literature review; J.J. and Y.Z. provided critical revisions; S.W. supervised the study. All authors reviewed the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and informed consent
Approval for the study was obtained from Peking Union Medical College Hospital of ethics committee (Date 2018; No.2018L03309). All of the patients had consented to research authorization for record review, and the study was approved by the institutional review board.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. (A copy of the written consent is available for review by the Editor-in-Chief of this journal).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huacong Cai and Jishi Wang contributed equally to this work.
References
- 1.Thrombosis and Hemostasis Group CSoH. Chinese Medical Association. Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). Chin J Hematol. 2020;41(08):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–80. 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 3.Terrell DR, Beebe LA, Neas BR, et al. Prevalence of primary immune thrombocytopenia in Oklahoma. Am J Hematol. 2012;87(9):848–52. 10.1002/ajh.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapir T, Blank M, Shoenfeld Y. Immunomodulatory effects of intravenous immunoglobulins as a treatment for autoimmune diseases, cancer, and recurrent pregnancy loss. Ann N Y Acad Sci. 2005;1051:743–78. 10.1196/annals.1361.118. [DOI] [PubMed] [Google Scholar]
- 5.Bullowa JG. The serum treatment and its evaluation in lobar pneumonia. Bull N Y Acad Med. 1929;5(4):328–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Imbach P, Barandun S, d’Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1(8232):1228–31. 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017. 10.3390/jcm6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watad A, Amital H, Shoenfeld Y. Intravenous immunoglobulin: a biological corticosteroid-sparing agent in some autoimmune conditions. Lupus. 2017;26(10):1015–22. 10.1177/0961203317696589. [DOI] [PubMed] [Google Scholar]
- 9.Moulinet T, Moussu A, Pierson L, et al. The many facets of immune-mediated thrombocytopenia: principles of immunobiology and immunotherapy. Blood Rev. 2024;63:101141. 10.1016/j.blre.2023.101141. [DOI] [PubMed] [Google Scholar]
- 10.Sun Boyang YR. Pathogenesis of immune thrombocytopenic purpura. Int J Blood Transfus Hematol. 2017;40(03):204–8. [Google Scholar]
- 11.Choi PY, Merriman E, Bennett A, et al. Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand. Med J Aust. 2022;216(1):43–52. 10.5694/mja2.51284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neunert C, Terrell DR, Arnold DM, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–66. 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YH, Kim DY, Kim S, et al. Management of immune thrombocytopenia: 2022 update of Korean experts recommendations. Blood Res. 2022;57(1):20–8. 10.5045/br.2022.2022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong J, Bang SM, Mun YC, et al. Efficacy and safety of a new 10% intravenous immunoglobulin product in patients with primary immune thrombocytopenia (ITP). J Korean Med Sci. 2018;33(19):e142. 10.3346/jkms.2018.33.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodeghiero F, Woszczyk D, Slama B, et al. Efficacy and Safety of IQYMUNE®, a ten percent intravenous immunoglobulin in adult patients with chronic. Primary Immune Thrombocytopenia J Hematol. 2018;7(3):87–95. 10.14740/jh385w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demeter J, Hamed A, László S, et al. Efficacy and safety of BT595 (10% human intravenous immunoglobulin) in adult patients with chronic immune thrombocytopenia. Transfus Med. 2023;33(2):165–73. 10.1111/tme.12943. [DOI] [PubMed] [Google Scholar]
- 17.Robak T, Salama A, Kovaleva L, et al. Efficacy and safety of Privigen, a novel liquid intravenous immunoglobulin formulation, in adolescent and adult patients with chronic immune thrombocytopenic purpura. Hematology. 2009;14(4):227–36. 10.1179/102453309x439773. [DOI] [PubMed] [Google Scholar]
- 18.Arbach O, Taumberger AB, Wietek S, et al. Efficacy and safety of a new intravenous immunoglobulin (Panzyga(®) ) in chronic immune thrombocytopenia. Transfus Med. 2019;29(1):48–54. 10.1111/tme.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasserman RL. Gammaplex(®) 5 and 10% in the treatment of primary immunodeficiency and chronic immune thrombocytopenic purpura. Immunotherapy. 2017;9(13):1071–88. 10.2217/imt-2017-0071. [DOI] [PubMed] [Google Scholar]
- 20.Kriván G, Borte M, Soler-Palacin P, et al. BT595, a 10% human normal immunoglobulin, for replacement therapy of primary immunodeficiency disease: results of a subcohort analysis in children. J Clin Immunol. 2023;43(3):557–67. 10.1007/s10875-022-01397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.