Abstract
2,3-dihydroquinazolin-4(1H)-one derivatives are recognized as vital compounds in medicinal chemistry due to their diverse biological activities, making them valuable for pharmaceutical research and therapeutic applications. Hence, the rapid and accurate synthesis of these derivatives need efficient nano-catalysts. The study outlines the preparation of nano-SiO2-SO3H through the covalent attachment of sulfonic acid groups to silica nanoparticles. Then, this material is applied as a catalyst for the efficient one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives. The reaction involves isatoic anhydride, an aromatic aldehyde, and ammonium acetate, directed under solvent-free conditions at a temperature of 110 °C. Also, the prepared catalyst characterized using Field Emission Scanning Electron Microscopy (FE-SEM) and Transmission Electron Microscopy (TEM) for morphological analysis, Fourier Transform Infrared Spectroscopy (FT-IR) for functional group identification, and Energy Dispersive X-ray Spectroscopy (EDAX) for its elemental composition. The presence of sulfonic acid groups as a Brønsted acids, enhance the catalytic properties of the silica nanoparticles and facilitate the reaction between the reactants in synthesis process and make the designed catalyst as a suitable candidate for promoting the 2,3-dihydroquinazolin-4(1H)-one synthesis. This synthesis protocol offers a sustainable and eco-friendly approach, eliminating the use of toxic solvents. It provides significant advantages compared to previously reported methods, such as using of nontoxic, green, and stable nanocatalyst. Additionally, the catalyst is easily recoverable and reusable, the reactions proceed under solvent-free conditions, and the process characterized by short reaction times (5–20 min). Furthermore, it achieves excellent yields ranging from 85 to 98% and features a simple workup procedure, making it highly relevant for advancing environmental chemistry practices.
Keywords: Nano-SiO2-SO3H; 2,3-dihydroquinazolin-4(1H)-ones; Solvent free conditions; Nanocatalyst; Advanced nanomaterials
Subject terms: Nanoscience and technology, Nanoscale materials
Introduction
Homogeneous acids are essential in facilitating chemical reactions with industrial applications. However, their application is frequently limited due to their corrosiveness properties and negative environmental impact. To overcome these drawbacks, scientists are investigating the potential of heterogeneous catalysts. These alternatives not only simplify material separation and recycling, but also minimize the amount of catalyst required. As a result, there is increasing focus on developing and refining solid catalysts to address these challenges1–3.
Silica-based materials are valued for being cost-effective, eco-friendly, and thermal stability, which makes them ideal for various applications. These materials, which have multiple hydroxyl (OH) groups on their surface, can be modified with various functional groups. This process improves their chemical and physical properties, making them more suitable for specific applications such as catalysis, adsorption, or biomedical uses4–6. Recent studies have explored the covalent attachment of sulfonic acid as an acidic functional group to mesoporous silica-based materials using grafting and co-condensation methods lead to enhancing of their role in organic synthesis and production of important reagents in medical chemistry7,8.
These functionalized groups are used as efficient heterogeneous catalysts for various organic reactions, including esterification, acylation, and condensation9,10. Among the various functional groups, sulfonic acid acts as a strong Brønsted acid catalyst on silica-based materials and provide better performance compared to traditional catalysts11. These functionalized nano-catalysts have several advantages, such as easy separation, regeneration, and reusability without significant loss of their activity12. Alo, these materials have hydrophobic nature and crystalline pore structure9. Additionally, the functionalized silica-based catalysts demonstrate good hydrothermal stability and can be recovered and reused multiple times, making them attractive for industrial and pharmaceutical applications10,11. For example, sulfonic acid groups functionalized silica (SiO2-Pr-SO3H) and metal-organic frameworks (MOFs) have been successfully applied in various organic synthesis and demonstrated high catalytic activity and reusability13,14. For example, sulfonic acid-functionalized silica-coated magnetic nanoparticles, exhibit high catalytic activity and reusability in synthesizing 2-substituted benzimidazole and bis indole methane derivatives, with effective recovery using an external magnet over six cycles15.
Solvent-free reactions, also known as solid-state or dry-phase reactions, represent an innovative approach in organic chemistry that completely eliminates the use of various solvents16. This technique aligns with the principles of green chemistry by drastically reducing hazardous waste and minimizing environmental impact. In conventional organic synthesis, solvents are often critical but can pose environmental hazards and contribute to pollution. By omitting solvents, chemists can adopt more sustainable methods while enhancing the efficiency of synthetic processes. Consequently, solvent-free reactions have become increasingly popular in modern organic chemistry17. Typically, various methods such as grinding, using of ultrasonic waves, and microwaves are employed to carry out reactions under solvent-free conditions. Grinding of the reactants under solvent-free conditions can lead to effective mixing and increased reaction yield18.
2,3-dihydroquinazolin-4(1H)-one derivatives are recognized as crucial compounds in medicinal chemistry due to their diverse biological activities, making them valuable for pharmaceutical research and therapeutic applications. These nitrogen-containing heterocyclic compounds, demonstrates notable pharmacological properties, including anticancer, antimicrobial, and anti-inflammatory effects19,20. The flexibility of quinazoline-based compounds is evident in their potential applications in treating various diseases, such as diabetes, viral infections, and oxidative stress-related conditions. Also, their importance in drug discovery is further emphasized by the presence of quinazoline/quinazolinone moieties in several marketed drugs. The ease of synthetic accessibility and flexibility in structural modifications make these compounds particularly attractive for medicinal chemists21.
Recently, various catalysts such as heteropoly acids22, ZnCl223, [C12Py][FeCl3Br] ionic liquid24, and InBr325 have been applied for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives under various experimental conditions,. Although these catalysts and methods have some advantages, most of them suffer from one or more limitation such as difficulty in preparation, recovery and reusability of the catalyst, low yields, long reaction times and using of toxic solvents.
Following our previous studies in the synthesis and design of green and eco-friendly catalysts26,27, this study focuses on the preparation of nano-SiO2-SO3H, for the one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones. The process involves the reaction of isatoic anhydride, aromatic aldehydes, and ammonium acetate under solvent-free conditions. This nano-catalyst with high surface area and a lot of acidic active sites, facilitates strong interactions between the reactants and allowing the reaction to proceed under milder reaction conditions and shorter reaction times. In addition, this catalyst can be easily recovered and reused several times and retains its catalytic activity with only a minimal decrease in its efficiency, presenting a cost-efficient and environmentally friendly alternative (Scheme 1).
Scheme 1.
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones in the presence of nano-SiO2-SO3H as a catalyst under solvent free conditions.
Experimental
Materials
Nano-SiO2, ClSO3H, isatoic anhydride, aromatic aldehyde, ammonium acetate, n-Hexane, EtOH, and ZnO were purchased from Sigma. All the mentioned materials were utilized in their original state without undergoing additional purifications.
Instruments
FTIR spectroscopy was applied to investigate the functional groups of nanostructures and synthesis compounds over a scanned range of 400 to 4000 cm−1 using a WQF-510 A spectrophotometer. The shape and morphology of the membranes were examined using FESEM (HITACHI S-4160) and TEM (Philips BioTwin, the Netherlands), while EDAX was applied to exhibit the alteration and distribution of particles in the prepared nano-catalyst.
Preparation of the catalyst
The preparation of the silica-based nano-catalyst (nano-SiO2-SO3H) involved functionalizing nano-silica particles with sulfonic acid groups to enhance their acidic sites and catalytic activity. 60 g of silica nanoparticles were functionalized via a reaction involving 0.7 mol (81.13 g) of chloro-sulfonic acid (ClSO3H). The procedure was conducted in a 50 mL suction flask equipped with a constant-pressure dropping funnel. The ClSO3H was added dropwise at room temperature over a period of 30 min. A gas tube was used to direct the emitted hydrochloride gas into an adsorption solution. After the modification process was complete, the mixture was stirred for an additional 30 min, resulting in the formation of a white solid product, nano-SiO2-SO3H (Scheme 2).
Scheme 2.
Modification of nano-SiO2 with sulfonic acid groups.
General procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-one’s derivatives
In a test tube, a mixture of isatoic anhydride (0.5 mmol, 0.08 g), aromatic aldehyde (0.5 mmol), ammonium acetate (1 mmol, 0.077 g), and nano-SiO2-SO3H (0.02 g) was stirred at 110 °C for an appropriate period of time. The reaction progress was monitored through TLC paper utilizing in a mixture of ethyl acetate and n-hexane (1:1). Once the reaction completed, hot ethanol was added to the mixture, and the catalyst was separated through filtration. The pure final products were then isolated by recrystallizing in ethanol.
Results and conditions
Characterization of the prepared nano-catalyst
The prepared catalyst characterized using FT-IR, FE-SEM, TEM and EDAX techniques. The FT-IR spectra of nano-SiO2-SO3H is shown in Fig. 1. The peaks in 809 cm−1 and 1105 cm−1 are related to the symmetric and unsymmetric vibrations of Si-O-Si and S-O bonds, respectively. The band in 470 cm−1 is belong to the bending frequency of Si-O-Si bonds. The broadband peak in 3419 cm−1 belongs to O-H stretching frequency of silanol groups and SO3H moiety in the surface of prepared catalyst. Also, the peak observed at 1628 cm−¹ corresponds to the H-O-H bending vibration, which is characteristic of both free and adsorbed water molecules.
Fig. 1.
FT-IR spectra of prepared nano-SiO2-SO3H.
The FE-SEM images typically reveal the surface morphology and particle size distribution of the nano-SiO2-SO3H. These images show that the silica nanoparticles are uniformly distributed, with a relatively small size, which is essential for their application in catalysis and other fields. The presence of the -SO3H functional group on the silica surface enhances the material’s catalytic properties, making it suitable for various chemical reactions. The images often depict a rough surface texture, indicating a high surface area that is beneficial for catalytic activity and adsorption processes (Fig. 2).
Fig. 2.
FE-SEM images of prepared nano-SiO2-SO3H.
The TEM images of prepared nano-SiO2-SO3H are presented in Fig. 3, providing a detailed visualization of the material’s morphology and structural characteristics. The images show that nano-SiO2-SO3H particles exhibit a uniform size distribution, less than 100 nm in diameter.
Fig. 3.
TEM images of nano-SiO2-SO3H.
Also, EDAX analysis of nano-SiO2-SO3H is shown in Fig. 4. The presence of Si, O, and S confirm the successful modification of nano-SiO2 with SO3H functional groups.
Fig. 4.

EDAX analysis of nano-SiO2-SO3H.
The acidity of the prepared catalyst was determined using two methods. First, the pH of catalyst using litmus paper was approximately 2. Secondly, titration with 0.01 M sodium hydroxide revealed that 0.05 g of the catalyst corresponded to 0.0185 moles of H+ (Fig. 5).
Fig. 5.

Titration of the catalyst with sodium hydroxide.
Optimization of the reaction condition
At first, the reaction conditions were optimized using a mixture of isatoic anhydride (0.5 mmol, 0.08 g), 4-chlorobenzaldehyde (0.5 mmol, 0.07 g), and ammonium acetate (1 mmol, 0.077 g), under various parameters. Then, 0.02 g of different catalysts, such as Fe2O3-MCM-41-SO3H, ZnO, KCC-1/ZnO, and nano-SiO2-SO3H were employed for the model reaction in ethanol and under solvent-free conditions at temperatures of 70 °C and 110 °C (Scheme 3).
Scheme 3.
A model reaction for optimization of the reaction conditions.
Initially, the reaction conducted without a catalyst at 120 °C, under solvent-free conditions resulted in an 80% yield within 30 min (entry 1, Table 1)28. The synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives has traditionally depended on the use of various types of Lewis acids and acidic catalysts. Considering this, the effectiveness of ZnO nanoparticles (0.02 g) was evaluated in combination with a eutectic solvent in a model reaction. Findings revealed that at 70 °C, the reaction achieved a 35% yield after 65 min (entry 2). In contrast, employing ZnO nanoparticles (0.02 g) under solvent-free conditions at 110 °C allowed the reaction to be completed in just 10 min with excellent yield (98%), highlighting a remarkable improvement in both efficiency and reaction time (entry 3). Also, ZnO particles were embedded within dendritic fibrous nano silica (KCC-1) to enhance their recovery and reusability. These modified particles were subsequently employed as a catalyst for the solvent-free synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives at 70 °C and 110 °C. Nevertheless, the results were not satisfactory at all (entries 4 and 5), possibly due to insufficient preparation of the catalytic substrate. Alos, Fe2O3-MCM-41-SO3H (0.02 g) was tested for the model reaction which the excellent yield (95%) was obtained in 15 min (entry 6).
Table 1.
Optimization of reaction conditions using various catalysis.
| Entry | Solvent | Catalyst | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | Solvent free, 120 °C | - | 30 | 8028 |
| 2 | ChCl: EG, 70 °C | ZnO | 65 | 35 |
| 3 | Solvent free, 110 °C | ZnO | 10 | 98 |
| 4 | Solvent free, 70 °C | KCC-1/ZnO | 20 | Trace |
| 5 | Solvent free, 110 °C | KCC-1/ZnO | 30 | Trace |
| 6 | Solvent free, 110 °C | Fe2O3-MCM-41-SO3H | 15 | 95 |
| 7 | Ethanol, reflux | Nano-SiO2-SO3H | 90 | 60 |
| 8 | Solvent free, 110 °C | Nano-SiO2-SO3H (0.02 g) | 8 | 98 |
| 9 | Solvent free, 110 °C | Nano-SiO2-SO3H (0.03 g) | 8 | 98 |
The acidic characteristics of silica nanoparticles, as well as their easy preparation, recyclability and sustainability, were demonstrated by evaluating the performance of nano-SiO2-SO3H (0.02 g) in a model reaction conducted under reflux in ethanol (entry 7). However, the reaction was incomplete after 90 min, yielding only 60% of the desired product. The study demonstrated that performing the reaction under solvent-free conditions at 110 °C provided the most favorable results (entry 8). Optimization of catalyst quantity revealed that 0.02 g of nano-SiO2-SO3H was the most effective, producing the highest yield (98%) in the shortest time (8 min). Increasing the amount of catalyst beyond this did not improve the reaction yield (entry 9). This catalyst proves to be a more economical, safer, and recyclable option compared to other commonly used acidic catalysts.
The research highlights an efficient method for the synthesizing 2,3-dihydroquinazolin-4(1H)-one derivatives by employing aromatic compounds with diverse substituents, encompassing both electron-donating and electron-withdrawing groups. This technique sets itself apart by providing higher product yields and significantly reducing reaction times compared to previous approaches (Table 2).
Table 2.
Synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives using nano-SiO2-SO3H under solvent free conditions.
| Entry | Product | Time (min) | Yield (%) | m. p. (°C) found | m. p. (°C) reported | Ref. |
|---|---|---|---|---|---|---|
| 1 |

|
8 | 98 | 203–205 | 203–206 | 29 |
| 2 |

|
10 | 85 | 200–202 | 195–198 | 29 |
| 3 |
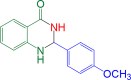
|
10 | 94 | 178–180 | 180–183 | 29 |
| 4 |
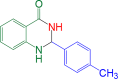
|
8 | 96 | 216–218 | 219–221 | 29 |
| 5 |

|
20 | 95 | 186–188 | 186–188 | 30 |
| 6 |

|
20 | 92 | 204–205 | 205–208 | 29 |
| 7 |
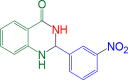
|
20 | 95 | 186–188 | 186–188 | 29 |
| 8 |

|
5 | 92 | 230–231 | 231–232 | 31 |
| 9 |

|
10 | 95 | 210–212 | 210–212 | 29 |
The proposed mechanism is shown in the Scheme 4. The synthesis process in the presence of nano-catalyst involves a three-component coupling reaction. Initially, isatoic anhydride which activated with nano-SiO2-SO3H, reacts with ammonium acetate and this reaction leads to the formation of an intermediate (2-aminobenzamide), which then reacts with aromatic aldehyde and undergoes cyclization to produce the 2,3-dihydroquinazolin-4(1H)-one compounds under solvent-free conditions. The nano-SiO2-SO3H catalyst not only enhances the reaction rate by activation of the reactants, but also offers the advantage of being easily recoverable and reusable, promoting a more sustainable and efficient approach.
Scheme 4.
Proposed mechanism for the synthesis of 2,3-dihydroquinazolin-4(1H)-one compounds.
The study highlights the advantages of the nano-SiO2-SO3H catalyst over other reported methods in the literature. Its high surface area and abundance of acidic sites enable more efficient interactions with reactants, leading to improved yields and shorter reaction times. Additionally, the catalyst is easy to prepare, recyclable, and can be reused multiple times with negligible loss in performance. These features make it both cost-effective and environmentally friendly. Its characteristics align seamlessly with the core tenets of sustainable chemistry, demonstrating its practicality, efficiency, and reduced environmental footprint compared to traditional methods (Table 3).
Table 3.
Comparison of the performance of prepared nanocatalyst in the present work with other catalysts in other reported methods.
| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | Cellulose-SO3H | CH3CN, r.t. | 55 | 90 | 32 |
| 2 | ZrCl4 | EtOH, r.t. | 37 | 91 | 33 |
| 3 | ZnFe2O4@SiO2-ascorbic acid | EtOH, reflux | 45 | 95 | 34 |
| 4 | lactic acid | Solvent free, 70 °C | 30 | 90 | 35 |
| 5 | SBA-16/GPTMS-TSC-CuI | Solvent free, 60 °C | 35 | 95 | 36 |
| 6 | Amberlyst-15 | CH3CN, 80 °C | 60 | 85 | 37 |
| 7 | Br3-TBA‐Fe3O4 | H2O, 70 °C | 40 | 97 | 38 |
| 8 | Cerium (IV) sulfate | Solvent free, 120 °C | 50 | 90 | 39 |
| 9 | nano-SiO2-SO3H | Solvent free, 110 °C | 8 | 98 | This work |
Reusability of the catalyst
The recoverability and reusability of the catalyst is one of the important properties of the catalyst40. The study explored the reusability of the catalyst in a reaction involving isatoic anhydride (0.5 mmol), aromatic aldehyde (0.5 mmol), and ammonium acetate (1 mmol) under optimized conditions. Upon completing the reaction, the catalyst was efficiently recovered from the mixture, washed with ethanol, and reused over five consecutive cycles without significant reduction in catalytic performance (Fig. 6).
Fig. 6.

Reusability of the nano-SiO2-SO3H catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones derivatives.
Conclusion
In conclusion, synthesis of 2,3-dihydroquinazolin-4(1H)-ones can be efficiently achieved using nano-SiO2-SO3H as a catalyst under solvent-free conditions. This approach offers several advantages, including environmental friendliness, reduced waste, and cost-effectiveness. The use of nano-SiO2-SO3H enhances the reaction’s efficiency due to its high surface area and strong acidic properties, facilitating the formation of the desired product in a shorter time (5–20 min), with higher yields (85–98%). Additionally, conducting the reaction under solvent-free conditions aligns with green chemistry principles, minimizing the use of hazardous solvents and reducing the overall environmental impact. This method is particularly valuable for researchers and industries focused on sustainable and efficient chemical processes.
Acknowledgements
We gratefully acknowledge the support of this work by Urmia University.
Author contributions
Conceptualization: Nasrin ShadjouData curation: Habyl NaeemiFormal Analysis: Nasrin Shadjou and Mehdi Mahmoudian Investigation: Habyl NaeemiMethodology: Nasrin Shadjou and Mehdi MahmoudianProject administration: Nasrin ShadjouSupervision: Nasrin ShadjouValidation: Nasrin Shadjou and Mehdi Mahmoudian Writing – original draft: Habyl Naeemi, Nasrin Shadjou, and Mehdi Mahmoudian Writing – review & editing: Habyl Naeemi, Nasrin Shadjou, and Mehdi Mahmoudian.
Data availability
Access to the data used in this study is available upon request and may be subject to approval by the data provider. Restrictions may apply to the availability of these data, which were used under license for this study. Interested parties are encouraged to contact the corresponding author for further information on accessing the data. All relevant data supporting the findings of this study are available within the article and its supplementary information files, or from the corresponding author upon reasonable request. Access to some data may be restricted due to privacy or ethical restrictions. Any restrictions to data availability will be disclosed at the time of data request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heravi, M. & Bamoharram, F. Applications of heteropoly acids as heterogeneous catalysts, Heteropolyacids as Highly Efficient and Green Catalysts Applied in Organic Transformations. (2022).
- 2.Li, W. J., Wang, J. B., Liu, Y. H., Zhang, M. & Zhang, Z. H. Molybdenum-doped carbon nitride as an efficient heterogeneous catalyst for direct amination of nitroarenes with arylboronic acids. Chin. Chem. Lett.36, 110001 (2025). [Google Scholar]
- 3.Maheshwari, P. et al. A review on latest trends in cleaner biodiesel production: role of feedstock, production methods, and catalysts. J. Clean. Prod.355, 131588 (2022). [Google Scholar]
- 4.Martínez-Edo, G., Balmori, A., Ponton, I. & Martí del Rio, A. Sánchez-García, D. Functionalized ordered mesoporous silicas (MCM-41): synthesis and applications in catalysis. Catalysts8, 617 (2018). [Google Scholar]
- 5.Li, W. J. et al. Room temperature synthesis of β-ketonitriles catalyzed by metal-organic framework supported Pd nanoparticles. J. Catal.430, 115308 (2024). [Google Scholar]
- 6.Chen, Y. X., Zhang, M., Zhang, S. Z., Hao, Z. Q. & Zhang, Z. H. Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes. Green. Chem.24, 4071–4081 (2022). [Google Scholar]
- 7.Singh, S., Kumar, A., Nebhani, L. & Hazra, C. K. Sustainable sulfonic acid functionalized tubular shape mesoporous silica as a heterogeneous catalyst for selective unsymmetrical Friedel–Crafts alkylation in one pot. JACS Au. 3, 3400–3411 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohns, R., Meyer, R., Wenzel, M., Matysik, J. & Enke, D. Tallarek Ulrich in situ synthesis and characterization of sulfonic acid functionalized hierarchical silica monoliths. J. Sol-Gel Sci. Technol.96, 67–82 (2020). [Google Scholar]
- 9.Kapoor, M. P. et al. An alternate approach to the Preparation of versatile sulfonic acid functionalized periodic mesoporous silicas with superior catalytic applications. J. Mater. Chem. A. 18, 4683 (2008). [Google Scholar]
- 10.Vekariya, R. H., Prajapati, N. & Patel, P. H. MCM-41-anchored sulfonic acid (MCM-41-SO3H): an efficient heterogeneous catalyst for green organic synthesis. Synth. Commun.46, 1713–1734 (2016). [Google Scholar]
- 11.Liu, Y. H., Zhang, Z. H. & Li, T. S. Efficient Conversion of Epoxides into β-Hydroperoxy Alcohols Catalyzed by Antimony Trichloride/SiO2. Synthesis 3314–3318 (2008).
- 12.Mohammadi Ziarani, G., Lashgari, N. & Badiei, A. Sulfonic acid-Functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A: Chem.397, 166–191 (2015). [Google Scholar]
- 13.Juan-Alcañiz, J. & Gielisse, R. et. Al. Towards acid MOFs- catalytic performance of sulfonic acid functionalized architectures. Catal. Sci. Technol.3, 2311–2318 (2013). [Google Scholar]
- 14.Vekariya, R. H. & Patel, H. D. Alumina Sulfuric Acid (ASA), Tungstate Sulfuric Acid (TSA), Molybdate Sulfuric Acid (MSA) and Xanthan Sulfuric Acid (XSA) as Solid and Heterogeneous Catalysts in Green Organic Synthesis: A Review, Rev. & Acc. 70–96 (2015). (2015).
- 15.Alka, U. K., Patel, A. & Agarwal Sustainable Design and Revolutionary Synthesis of Highly Recyclable Sulfonic Acid–Based Magnetic Nanoparticles as a Solid Acid for Synthesis of 2-Substituted Benzimidazole and Bis Indole Methane Derivatives, Appl. Organometal. Chem. e7861 (2024).
- 16.Younis, A. & Osman, A. Solvent-free organic reaction techniques as an approach for green chemistry. J. Turk. Chem. Soc. A: Chem.10, 549–576 (2023). [Google Scholar]
- 17.Nagendrappa, G. Organic synthesis under Solvent-free condition. An environmentally benign procedure -I. Resonance7, 59–68 (2002). [Google Scholar]
- 18.Huertas, D., Florschera, M. & Dragojlovic, V. Solvent-free Diels-Alder reactions of in situ generated cyclopentadiene. Green. Chem.11, 91–95 (2009). [Google Scholar]
- 19.Mishra, S. Quinazolinone and Quinazoline derivatives: synthesis and biological application. Quinazolinone Quinazoline Derivatives (2019).
- 20.Karan, R., Agarwal, P., Sinha, M. & Mahato, N. Recent advances on Quinazoline derivatives: A potential bioactive scaffold in medicinal chemistry. Chem. Eng.5, 73 (2021). [Google Scholar]
- 21.Hameed, A. et al. Quinazoline and Quinazolinone as important medicinal scaffolds: a comparative patent review (2011–2016). Expert Opin. Ther. Pat.28, 281–297 (2018). [DOI] [PubMed]
- 22.Zong, Y. X. et al. Highly efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by heteropoly acids in water. Chin. Chem. Lett.21, 778–781 (2010). [Google Scholar]
- 23.Tang, J. H., Shi, D. X., Zhang, L. J., Zhang, Q. & Li, J. R. Facile and One-Pot synthesis of 1,2-Dihydroquinazolin-4(3H)-ones via tandem intramolecular Pinner/Dimroth rearrangement. Synth. Commun.40, 632–641 (2010). [Google Scholar]
- 24.Dutta, A. et al. Design and synthesis of Quinazolinone-Triazole hybrids as potent Anti-Tubercular agents. ACS Appl. Bio Mater.5, 4413–4424 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Rajaka, L., Penumati, N. R., Nagaiah, K., Poornachandra, Y. & Kumar, C. G. Convenient and scalable synthesis of 2,3-Dihydroquinazolin-4(1H)-one derivatives and their anticancer activities. Synth. Commun.45, 1893–1901 (2015). [Google Scholar]
- 26.Sabri, Z., Shadjou, N. & Mahmoudian, M. Accelerated synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives using dendritic mesoporous nanosilica functionalized by hexamethylenetetramine: a novel nanocatalyst. RSC Adv.14, 2633–2651 (2024). [DOI] [PMC free article] [PubMed]
- 27.Asadi, Sh. Shadjou, N, Metformin functionalized dendritic fibrous nanosilica (KCC-1-nPr-Met) as an innovative and green nanocatalyst for the efficient synthesis of tetrahydro-4H-chromene derivatives. J. Mol. Recognit.35, e2943 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Kumar, G. et al. An eco-friendly, sustainable, and greener approach to the synthesis of dihydroquinazolin-4(1H)-ones using a deep eutectic solvent. J. Heterocycl. Chem.61, 347–357 (2024). [Google Scholar]
- 29.Hosseinzadeh, R., Zarei, S., Valipour, Z. & Maleki, B. A vitamin C-based natural deep eutectic solvent for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives. Heliyon10, e37170 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao, L. et al. An efficient synthesis of 2,3-Dihydroquinazolin 4(1H)-one derivatives under Catalyst-Free and Solvent-Free conditions. J. Heterocycl. Chem.48, 957–960 (2011). [Google Scholar]
- 31.Lodhi, A., Dalai, A. K. & Maheria, K. C. Synthesis of biologically active Dihydroquinazolinone catalyzed by the micro- meso–composite of zeolite H–BEA, catalyst under solvent-free condition. Res. Chem. Intermed. 49, 1983–2004 (2023). [Google Scholar]
- 32.Subba Reddy, B. V., Venkateswarlu, A., Madan, C. & Vinu, A. Cellulose-SO3H: an efficient and biodegradable solid acid for the synthesis of quinazolin-4(1H)-ones. Tetrahedron Lett.52, 1891–1894 (2011). [Google Scholar]
- 33.Abdollahi-Alibeik, M. & Shabani, E. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by zirconium (IV) chloride as a mild and efficient catalyst. Chin. Chem. Lett.22, 1163–1166 (2011). [Google Scholar]
- 34.Khanmohammadi-Sarabi, F., Ghorbani-Choghamarani, A., Aghavandi, H. & Zolfigol, M. A. ZnFe2O4@SiO2-ascorbic acid: green, magnetic, and versatile catalyst for the synthesis of chromeno[2,3-d] pyrimidine-8-amine and Quinazoline derivatives. Appl. Organometal Chem.36, e6768 (2022). [Google Scholar]
- 35.Zhaleh, S., Hazeri, N. & Maghsoodlou, M. T. Green protocol for synthesis of 2,3-dihydroquinazolin-4(1H)-ones: lactic acid as catalyst under solvent-free condition. Res. Chem. Intermed. 42, 6381–6390 (2016). [Google Scholar]
- 36.Erfan, M. A., Akhlaghinia, B. & Ghodsinia, S. An efficient green protocol for synthesis of 2,3-Dihydroquinazolin-4(1H)-ones using SBA-16/GPTMS-TSC-CuI under Solvent-Free conditions. Chem. Select. 5, 2306–2316 (2020). [Google Scholar]
- 37.Bharate, S. B. et al. Efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones using heterogeneous solid acid catalysts: unexpected formation of 2,3-dihydro-2-(4-(tetrahydro-2H-pyran-2-yloxy)butyl)quinazolin-4(1H)-one. ARKIVOCviii, 308–318 (2012). [Google Scholar]
- 38.Shiri, L., Ghorbani-Choghamarani, A. & Kazemi, M. Synthesis and characterization of bromine source supported on magnetic Fe3O4 nanoparticles: A new, versatile and efficient magnetically separable catalyst for organic synthesis. Appl. Organometal Chem.31, e3634 (2017). [Google Scholar]
- 39.Davoodnia, A., Khashi, M. & Tavakoli-Hoseini, N. Cerium (IV) sulfate: A highly efficient reusable heterogeneous catalyst for the one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones under solvent-free conditions. Chin. J. Catal.35, 1054–1058 (2014). [Google Scholar]
- 40.Ghosh, N., Sikder, J. & Halder, G. Harnessing a recoverable supermagnetic biogenic nanocatalyst towards transesterified biodiesel production. Chem. Eng. J.503, 158448 (2025). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the data used in this study is available upon request and may be subject to approval by the data provider. Restrictions may apply to the availability of these data, which were used under license for this study. Interested parties are encouraged to contact the corresponding author for further information on accessing the data. All relevant data supporting the findings of this study are available within the article and its supplementary information files, or from the corresponding author upon reasonable request. Access to some data may be restricted due to privacy or ethical restrictions. Any restrictions to data availability will be disclosed at the time of data request.









