Abstract
SARS-CoV-2 hijacks multiple organelles for virion assembly, of which the mechanisms have not been fully understood. Here, we identified a SARS-CoV-2-driven membrane structure named the 3a dense body (3DB). 3DBs are unusual electron-dense and dynamic structures driven by the accessory protein ORF3a via remodeling a specific subset of the trans-Golgi network (TGN) and early endosomal membrane. 3DB formation is conserved in related bat and pangolin coronaviruses but was lost during the evolution to SARS-CoV. During SARS-CoV-2 infection, 3DB recruits the viral structural proteins spike (S) and membrane (M) and undergoes dynamic fusion/fission to maintain the optimal unprocessed-to-processed ratio of S on assembled virions. Disruption of 3DB formation resulted in virions assembled with an abnormal S processing rate, leading to a dramatic reduction in viral entry efficiency. Our study uncovers the crucial role of 3DB in maintaining maximal SARS-CoV-2 infectivity and highlights its potential as a target for COVID-19 prophylactics and therapeutics.
Subject terms: SARS-CoV-2, Golgi, Endosomes
Hartmann et al. discovered that SARS-CoV-2 constructs a group of dynamic membrane structures named the 3DB. 3DB regulates the processing of the viral spike protein to assemble a highly infectious virus, highlighting the potential of 3DB as a novel COVID-19 therapeutic target.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a positive-sense single-stranded RNA virus that causes coronavirus disease 2019 (COVID-19). To date, more than 700 million cases of COVID-19 have been reported, resulting in more than 7 million reported deaths1. SARS-CoV-2 is genetically similar to the previously discovered SARS-CoV responsible for the 2002–2003 SARS outbreak2,3. SARS-CoV and SARS-CoV-2 both form interconnected double-membrane vesicles (DMVs) derived from the endoplasmic reticulum (ER) to serve as replication organelles where viral RNA replication occurs4–9. In addition, ERGIC-derived structures were proposed to be important for the assembly of mature SARS-CoV-2 virions10. The Golgi apparatus, mitochondria, and peroxisomes were also proposed to be remodeled by SARS-CoV-24. Cryo-electron tomography (cryo-ET) has revealed the presence of single-membrane vesicles (SMVs) containing assembled virions, suggesting that these may be the virion assembly site11. However, how these membrane structures coordinate to orchestrate virion assembly has not been fully characterized.
One intriguing question regarding virion assembly is how viral structural proteins undergo coordinated trafficking, processing, and post-translational modifications (PTMs) to form mature virions with optimal infectivity. SARS-CoV-2 has four structural proteins, spike (S), membrane (M), envelope (E), and nucleocapsid (N). During virion assembly, trimeric S is incorporated into the viral lipid envelope and protrudes from the virion12. After release from host cells, virions rely on S for binding to the human angiotensin-converting enzyme 2 (hACE2) receptor on new host cells to mediate viral entry. S protein contains two subunits, S1 and S2. S1 mediates binding to the ACE2 receptor, whereas S2 mediates viral fusion with the host cellular membrane13. During virion assembly, S undergoes furin-mediated cleavage into S1 and S2 subunits. The effects of S processing on viral entry and infectivity have been extensively studied using S mutations associated with circulating variants of concern (VOCs), furin depletion, and extracellular furin treatment14–16. While many studies found that S mutations resulting in higher processing rate are associated with enhanced infectivity14,17, other studies observed the opposite effects that are also highly dependent on cell types and entry pathways18–20. The complex outcome of S processing was also evident when furin was depleted from virus-producing cells, which resulted in decreased viral replication and TMPRSS2-mediated cell-to-cell fusion, but increased S-pseudovirus entry16. These results suggest that S processing needs to be tightly controlled in an optimal range for maximizing viral entry efficiency, although the detailed mechanisms to achieve this during virion assembly still remain to be fully defined.
Besides structural proteins, SARS-CoV-2 also encodes a number of accessory proteins that facilitate viral replication, immune response evasion, and pathogenesis10. A previous study showed that ORF3a is the most important accessory protein in SARS-CoV-2 virulence in a K18-hACE2 transgenic mouse model of infection21. A mutant virus deficient in ORF3a (Δ3a) exhibited the highest improvement in lung pathology and survival compared to those infected with wild type (WT) or mutant viruses deficient in other accessory ORF proteins21. Reduced virulence in animals correlated with a defect in Δ3a viral transmission as indicated by reduced plaque size21. A number of mammalian coronaviruses share similar genomic sequences with SARS-CoV and SARS-CoV-2. Together, these viruses form the group of SARS-related coronaviruses (SARSr-CoVs)10,22. ORF3a is conserved among SARSr-CoVs, but not with other human coronaviruses (HCoVs) such as Middle East respiratory syndrome coronavirus (MERS-CoV), HCoV-NL63, or HCoV-229E10,23. ORF3a was previously proposed to be a viral small ion channel protein (viroporin)24,25, although its ion channel activity has remained controversial26,27. Recent studies have proposed the involvement of ORF3a in lysosomal exocytosis-mediated viral egress, autophagy, and late endosome/lysosome trafficking26,28–39. However, whether ORF3a has other virulence-driving mechanisms, specifically, whether it influences virion assembly to promote virion entry efficiency, has remained unknown.
Here, we identified a membrane structure assembled by ORF3a during SARS-CoV-2 infection, which we termed the 3a dense body (3DB). 3DBs are giant electron-dense spherical structures with dynamic inner structures derived from the host trans-Golgi network (TGN) and early endosomal membrane. 3DB mediates the trafficking of S and M and plays a crucial role in maintaining the optimal conformation ratio of S on virions. As a result, virions assembled in the absence of 3DB are significantly defective in viral entry efficiency. Our findings highlight the potential of targeting ORF3a for the rational development of live-attenuated vaccines to combat SARS-CoV-2 and future emerging HCoVs, given the highly conserved nature of this remodeling activity in bat progenitor coronaviruses. Screening of inhibitors targeting 3DB, such as those that bind to or modify the seven key residues in ORF3a that are essential for 3DB assembly, may provide promising directions for the discovery of innovative COVID-19 therapeutics.
Results
Identification of 3aCoV2-driven dense bodies
The TGN serves as the major sorting compartment and the center for terminal processing and modifications of newly synthesized proteins40. Our previous work discovered that several microbial factors, including the bacterial ionophore nigericin, induce TGN disassembly into vesicles without dispersing the cis/medial-Golgi or other organelles41. The dispersed TGN vesicles then serve as a signaling platform for the assembly and activation of the NLRP3 inflammasome41. This indicates that the TGN can be specifically remodeled through host-bacteria interactions. However, whether viral proteins possess similar remodeling activity remains unknown. We hypothesized that viroporins might be potential TGN-remodeling factors. We selected a group of viroporins derived from phylogenetically diverse groups of DNA and RNA viruses (Supplementary Fig. 1a). To prevent the interference of other viral factors, we developed an individual expression system, in which the viroporin genes were cloned into a lentiviral vector for stable expression in HeLa cells. Either N-terminal or C-terminal tagging was used based on previous literature or our pilot experiments to ensure optimal expression and localizations of viroporins. The cells were then fixed and immunostained for TGN46 (also known as TGOLN2 or TGN38), a marker for TGN. We defined TGN remodeling as a ≥3-fold increase in the average surface area containing TGN46-positive structures with p value < 0.01 (Student’s two-sided t-test) compared to that of the parental HeLa cells. For simplicity, ORF3a proteins from SARS-CoV and SARS-CoV-2 are hereinafter referred to as 3aCoV1 and 3aCoV2, respectively. Out of the ten viroporins that were successfully expressed, seven (2B from poliovirus, M2 from influenza A virus, NSP4 from rotavirus, VP4 from human rhinovirus, Vpu from HIV, 3aCoV1, and 3aCoV2) showed at least partial colocalization with TGN46 (Fig. 1a and Supplementary Fig. 1b). However, only 3aCoV2 induced dramatic dispersion of TGN46-positive structure from an intact cluster (no visible spherical structures) into multiple spherical structures (Fig. 1a, 0.60 ± 0.22 μm in diameter), as quantified by a ~ 10-fold increase in TGN46-positive area (287.1 ± 105.2 μm2 vs 26.4 ± 9.4 μm2). 3aCoV2 was mainly localized on these TGN46-positive spherical structures (Fig. 1a, Zoom-in) besides additional cytosolic aggregates and plasma membrane (PM) localization. The ability of 3aCoV2 to induce TGN46-positive spherical structures is highly efficient, with 100% penetrance in the stable cell line. Surprisingly, this remodeling activity was not observed for 3aCoV1, which was expressed at a comparable level based on immunoblotting (Fig. 1a). 3aCoV1 was predominantly localized on the intact TGN cluster, besides additional cytosolic aggregates and PM localization (Fig. 1a). For both 3aCoV2 and 3aCoV1, only the C-terminal but not the N-terminal tagging could be detected via immunostaining (Supplementary Fig. 2a–c). The N-terminal tagging did not affect the expression of both 3a proteins or the remodeling ability of 3aCoV2 (Supplementary Fig. 2b, c), suggesting that the N-terminus of 3aCoV1 and 3aCoV2 is likely processed during or after translation. We therefore used C-terminally tagged ORF3a for the rest of this study.
Fig. 1. 3aCoV2 but not 3aCoV1 induces giant dynamic dense bodies.
a HeLa cells stably expressing 3aCoV1-GFP or 3aCoV2-GFP were immunostained for TGN46, a TGN marker. The parental HeLa cells (labeled with ‘/‘) were used as controls. A magnified region of HeLa 3aCoV2-GFP cells is shown to highlight the spherical structures positive with 3aCoV2-GFP and TGN46. Areas containing TGN46-positive structures were measured with ImageJ (n = 40 cells/sample; mean ± s.d.; two-sided t-test; NS, not significant; black line indicates median value). GFP immunoblotting was performed to show that the 3a-GFP proteins were expressed at comparable levels. Representative data from at least three independent experiments are shown. b The indicated cell lines stably expressing 3aCoV1-Flag or 3aCoV2-Flag were imaged with phase contrast microscopy. For HeLa, Vero E6, and MEF series, the number of spherical structures per cell (visible on the current focal plane with a clear DAPI signal) was quantified. For the A549-hACE2 series, because the massive formation of spherical structures made it challenging to quantify the spherical structures, the ratio of spherical structure area to whole-cell area was measured instead. n = 40 cells/sample; mean ± s.d.; two-sided t-test; black line indicates median value. Representative data from at least three independent experiments are shown. c Upper panel: Vero E6 parental cells or cells stably expressing 3aCoV2-GFP were imaged with transmission electron microscopy (TEM). Three pictures (pic 1–3) are shown to highlight different morphologies of 3a dense bodies (3DBs) labeled with*. Mito mitochondria. Bottom panel: five subtypes of 3DB based on their morphological features are shown. (i) consisting of several membranous sub-compartments; (ii) consisting of dense pebble-like substructures and membranous sub-compartments; (iii) consisting of dense pebble-like substructures; (iv) highly electron-dense structures; (v) similar to (iv), but fused to one or multiple electron-lucent vesicle-like structures. Representative images from >40 cells per subtype are shown. Source data is provided as a Source Data file.
The different effects of 3aCoV2 and 3aCoV1 on TGN46-positive structures were recapitulated in a variety of cell lines, including two that are routinely used for SARS-CoV-2 infection studies42,43: (1) Vero E6 (Supplementary Fig. 3a), an African green monkey kidney epithelial cell line; (2) A549-hACE2 (Supplementary Fig. 3b), a human lung epithelial cell line stably expressing hACE23. 3aCoV2-induced spherical structures can be detected with phase contrast microscopy, which also showed that 3aCoV2 was localized on these giant spherical structures (Supplementary Fig. 3c). These structures were driven by the expression of 3aCoV2, but not 3aCoV1, in a variety of human, monkey, and mouse cell lines, with visible number ranging from ~20 to a few hundred per cell, while cells without 3aCoV2 expression usually had 0–3 spherical structures that likely represent other types of vesicles (Fig. 1b).
To confirm that the remodeling was not caused by overloading the TGN with overexpressed 3aCoV2, we established a series of A549-hACE2 cell lines stably expressing 3aCoV1-GFP or 3aCoV2-GFP at different levels through lentivirus titrations. Strikingly, even at a much lower expression level than 3aCoV1-GFP, 3aCoV2-GFP still potently induced massive spherical structure formation (Supplementary Fig. 3d). These results indicate that even a low amount of 3aCoV2 is sufficient to promote robust remodeling. Cells expressing 3aCoV1 or 3aCoV2 were morphologically healthy and could be maintained as stable cell lines for a long period of time, at least two months before frozen, indicating that the remodeling does not affect the basal cell survival.
Surprisingly, when imaged with transmission electron microscopy (TEM), 3aCoV2-induced structures appeared as giant spherical electron-dense bodies with highly dynamic inner compositions (Fig. 1c, upper panel). These structures can be grouped into five subtypes based on their morphological features (Fig. 1c, lower panel): (i) consisting of several membranous sub-compartments; (ii) consisting of dense pebble-like substructures and membranous sub-compartments; (iii) consisting of dense pebble-like substructures; (iv) highly electron-dense structures; (v) similar to (iv), but fused to one or multiple electron-lucent vesicle-like structures. These five subtypes likely represent different maturation stages and/or different sections of the structures. While all five subtypes were observed at high frequencies, (i) and (ii) were the most abundant ones, suggesting that they may be the mature or most stable forms. These structures are distinct from nigericin-induced TGN vesicles or SARS-CoV/SARS-CoV-2-induced DMVs, with the latter two appearing as electron-lucent vesicles4,41 (Supplementary Fig. 3e). They are also dramatically different from lipid droplets44 (Supplementary Fig. 3e), multivesicular bodies (MVBs)45,46, autophagosomes and related structures47, endosomes45, and lysosomes45. Besides Vero E6 in Fig. 1c, similar 3aCoV2-driven structures were also observed in HeLa cells (Supplementary Fig. 3f). Given the unusual dense nature of their inner compositions, we named these structures the 3a dense bodies (3DBs).
3aCoV2 specifically remodels a subset of the TGN membrane
Besides the dramatic difference in morphology revealed by TEM, 3DBs and nigericin-induced TGN vesicles also differ in number and diameter (Supplementary Fig. 4a), thus raising the question as to whether these two remodeling events are of different nature. We previously discovered that several NLRP3 inflammasome stimuli, including nigericin, disperse the entire TGN into vesicle structures as indicated by multiple TGN markers41. After that, the negatively-charged phospholipid PtdIns4P on the dispersed TGN binds to a polybasic region on NLRP3 to mediate NLRP3 recruitment and inflammasome complex assembly41. Interestingly, while nigericin treatment triggered the dispersion of all five TGN markers tested, 3aCoV2 only dispersed TGN46 (Fig. 2a). 3aCoV2 also failed to disperse the PtdIns4P-positive TGN structures as detected by the PtdIns4P-binding protein OSBPPH-GFP48 (Supplementary Fig. 4b). Consistent with our previous finding that the dispersed PtdIns4P-positive TGN structures are required for NLRP3 activation41, 3aCoV2 did not promote NLRP3 puncta formation (Supplementary Fig. 4c) or caspase-1 cleavage (Supplementary Fig. 4d), two hallmarks of NLRP3 inflammasome activation. 3aCoV2 did not prevent nigericin-induced formation of larger TGN46 vesicles, NLRP3 recruitment, or caspase-1 cleavage (Supplementary Fig. 4c, d), suggesting that 3aCoV2-mediated TGN remodeling does not interfere with inflammasome-related TGN remodeling. To examine whether 3aCoV2 activates the NLRP3 inflammasome during viral infection, we established a RAW 264.7 murine macrophage cell line stably expressing hACE2-Flag and ASC, the adapter protein downstream of NLRP3. RAW 264.7 cells express endogenous NLRP3 but not ASC49,50, and therefore, exogenous expression of ASC in this cell line is often used to reconstitute the inflammasome pathway41,51. The expression of hACE2-Flag allowed this cell line to be infected with SARS-CoV-2. As expected, nigericin treatment resulted in dramatic formation of ASC specks (Supplementary Fig. 4e), a hallmark of inflammasome activation52,53. In contrast, cells infected with SARS-CoV-2 (USA-WA1) had a minimal level of ASC speck formation (Supplementary Fig. 4e). Our data indicate that 3aCoV2 remodels the TGN in a manner distinct from previously characterized NLRP3 inflammasome stimuli, and as a result, is not a potent NLRP3 stimulus either expressed alone or during viral infection.
Fig. 2. 3DB derives from a specific subset of TGN and early endosomal membrane.
a HeLa cells were treated with 1.5 μL/mL DMSO solvent (‘Mock’) or nigericin (Nig, 10 μM = 1.5 μL/mL dissolved in DMSO) for 80 min (min). Together with HeLa cells stably expressing 3aCoV2-GFP, these cells were immunostained for the indicated TGN markers. Areas containing TGN-marker-positive structures were measured with ImageJ (n = 40 cells/sample; mean ± s.d.; one-sided t-test; NS, not significant; black line indicates median value). Representative data from at least three independent experiments are shown. b HeLa cells stably expressing 3aCoV2-GFP were immunostained for the indicated organelle markers. For EEA1 immunostaining, a magnified region is shown to highlight EEA1 on a subset of 3DBs. Colocalization of 3DBs with these organelle markers was quantified with the Pearson correlation coefficient using the Coloc 2 plugin of ImageJ (n = 20 cells/sample; threshold regression: Costes). Organelle makers with strong and weak colocalization with 3DBs are labeled in blue and pink, respectively. Control organelle imaging of HeLa parental cells and measurement of areas containing GM130/giantin-positive structures are in Supplementary Fig. 5. Representative data from at least three independent experiments are shown. Source data is provided as a Source Data file.
To examine whether 3aCoV2 hijacks membranes from other organelles, we imaged a series of organelle markers. 3aCoV2 did not disperse the cis- or medial-Golgi (Fig. 2b, parental cell controls and quantification in Supplementary Fig. 5), again highlighting its specificity. In addition, 3DBs did not contain organelle markers GM130 (cis-Golgi), giantin (cis/medial-Golgi), calnexin (ER), ERGIC-53 (ERGIC), TOM20 (mitochondria), Rab7 (late endosome), LAMP1 (lysosome), or LC3 (autophagosome) (Fig. 2b, parental cell controls in Supplementary Fig. 5). These results indicate that 3DB formation is a previously uncharacterized function of 3aCoV2, distinct from its known ability to regulate late endosome/lysosome trafficking and autophagy26,28–39. Interestingly, EEA1, an early endosome marker, was recruited to a subset of 3aCoV2 structures (Fig. 2b). Our results indicate that 3aCoV2 hijacks a specific subset of TGN and early endosomal membranes either directly from these organelles, or indirectly through the cargo exchange between the TGN and early endosomes.
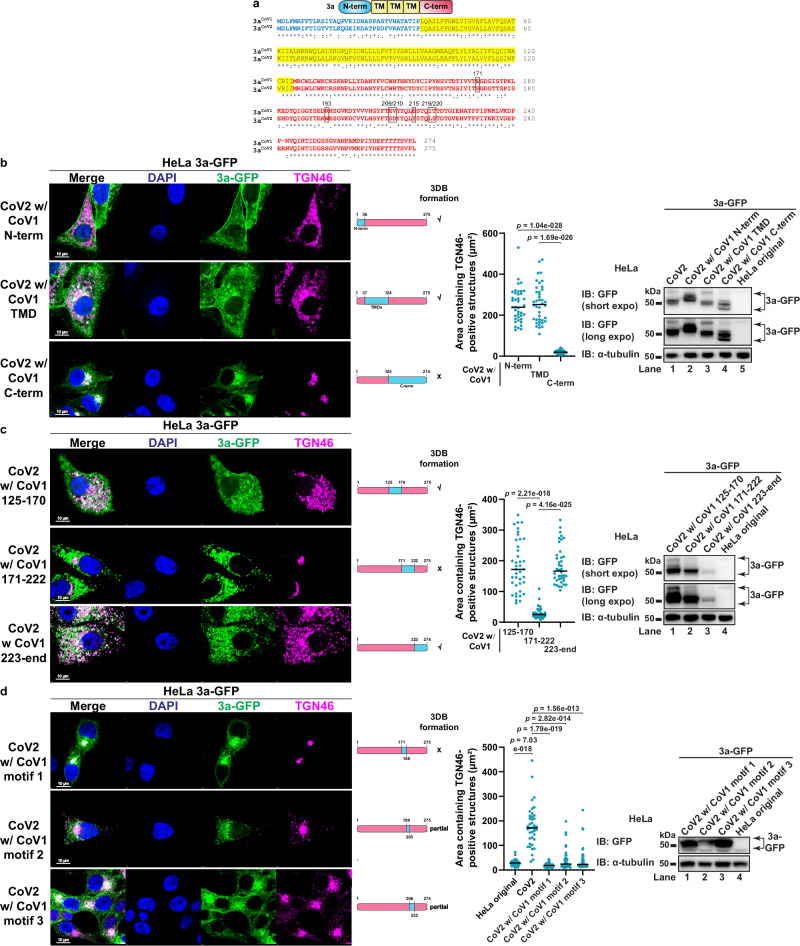
The C-terminal region of 3aCoV2 is critical for 3DB formation
3aCoV2 and 3aCoV1 share a similar domain structure: an N-terminal region (N-term), a transmembrane-domain region (TMD) and a C-terminal region (C-term)27,54, with ~72% amino acid (aa) identity (Fig. 3a). We performed a series of domain swapping to identify the region critical for 3DB formation. Replacing N-term (aa 1–36) or TMD (aa 37–124) of 3aCoV2 with the corresponding regions in 3aCoV1 did not affect 3DB formation (Fig. 3b). In contrast, replacing C-term (aa 125–end) completely abolished the activity, while still maintaining comparable expression level and strong colocalization with the TGN similar to 3aCoV1 (Fig. 3b). Consistently, swapping C-term of 3aCoV1 with that of 3aCoV2 promoted 3DB formation comparable to that caused by 3aCoV2 (Supplementary Fig. 6a). These data indicate that the C-term of 3aCoV2 is crucial for the remodeling activity.
Fig. 3. Identification of ORF3a motifs critical for 3DB formation.
a Alignment of 3aCoV1 and 3aCoV2 protein sequences with Clustal Omega. N-term, TMD, and C-term are labeled in blue, yellow, and red, respectively. The seven key residues are highlighted in black frames. b–d HeLa cells stably expressing the indicated 3a swapping mutants were immunostained for TGN46. The blue and pink bars represent sequences derived from 3aCoV1 and 3aCoV2, respectively. Areas containing TGN46-positive structures were measured with ImageJ (n = 40 cells/sample; mean ± s.d.; two-sided t-test; black line indicates median value). GFP immunoblotting was performed to compare the expression levels of 3a-GFP proteins. Representative data from at least three independent experiments are shown. Source data is provided as a Source Data file.
To further narrow down the key region, we divided the C-term into three smaller regions. Swapping aa 171–222 in 3aCoV2 completely abolished 3DB formation (Fig. 3c), while swapping the corresponding region in 3aCoV1 restored the activity (Supplementary Fig. 6b). Swapping the other two smaller regions in the C-term (aa 125–170 and aa 223–end) of 3aCoV2 did not affect 3DB formation (Fig. 3c), despite one of them (aa 223–end) being expressed at a much lower level than the other mutants (Fig. 3c, immunoblotting). This is consistent with our observation that 3aCoV2 is capable of robust remodeling even at low expression. Consistently, swapping aa 125–170 or aa 223–end in 3aCoV1 failed to restore the activity (Supplementary Fig. 6b). These results indicate that aa 171–222 of 3aCoV2 is crucial for 3DB formation.
We further dissected aa 171–222 into three regions with lengths of 17–18 aa, referred to as motif 1 (aa 171–188), motif 2 (aa 189–205), and motif 3 (aa 206–222). Swapping motif 1 in 3aCoV2 completely abolished the remodeling, while swapping motif 2 or motif 3 resulted in partial defects (Fig. 3d). Motif 2 swapping resulted in decreased expression (Fig. 3d, immunoblotting), although the level was still above what was sufficient to cause robust 3DB formation in 3aCoV2. Swapping motif 1, 2, or 3 individually in 3aCoV1 was not sufficient to restore 3DB formation (Supplementary Fig. 6c). These results indicate that multiple residues spanning all three motifs are important.
The remodeling activity is conserved in ORF3a from multiple but not all SARSr-CoVs
To test whether 3DB formation is conserved in other SARSr-CoVs, we examined ORF3a derived from three SARSr-CoVs using the individual expression system in HeLa (Fig. 4a): (1) Bat-CoV-RaTG13, a horseshoe bat coronavirus that is one of the closest related coronaviruses to SARS-CoV-23; (2) Pangolin-CoV-GX-P4L, a pangolin SARSr-CoV evolutionarily close to SARS-CoV-255–57; (3) Civet-CoV-007/2004, a civet SARSr-CoV proposed to be the intermediate species for SARS-CoV58. Consistent with their evolutionary distance from SARS-CoV-2 and SARS-CoV, the bat and pangolin ORF3a induced profound 3DB formation, while the civet ORF3a behaved similarly to 3aCoV1 (Fig. 4b).
Fig. 4. ORF3a-mediated 3DB assembly is conserved in multiple but not all SARSr-CoVs.
a A phylogenetic tree of ORF3a proteins was constructed with Clustal Omega. ORF3a proteins with or without 3DB formation activity are labeled in red and blue, respectively. GFP immunoblotting was performed to compare the expression levels of 3a-GFP proteins. Representative data from at least three independent experiments are shown. b HeLa cells stably expressing the indicated 3a-GFP proteins were immunostained for TGN46. Areas containing TGN46-positive structures were measured with ImageJ (n = 40 cells/sample; mean ± s.d.; one-sided t-test; NS, not significant; black line indicates median value). Representative data from at least three independent experiments are shown. c The bat cell line R-06E was transduced with lentivirus to stably express the indicated 3a-GFP proteins. 3DB formation was examined with phase contrast microscopy, while the 3a-GFP levels were examined with fluorescence microscopy. The number of 3DBs per cell (visible on the current focal plane with clear DAPI signal) was quantified (n = 50 cells/sample; mean ± s.d.; two-sided t-test; NS, not significant; black line indicates median value). Representative data from two independent experiments are shown. d, e Similar to (a, b), except four additional bat SARS-CoV ORF3a proteins were examined. Representative data from at least three independent experiments are shown. f Model: two possible routes for the loss of 3DB during evolution to SARS-CoV: (1) ORF3a proteins in all bat SARSr-CoVs possess the activity to form 3DB, but the activity was lost during/after spillover from bat to civet; or (2) the 3DB formation activity was lost in a yet unidentified bat SARSr-CoV that is more closely related to SARS-CoV than Bat-CoV-WIV16. The intermediate host for SARS-CoV-2 has not been fully confirmed, and therefore it is labeled as ‘pangolin or other mammals’. Source data is provided as a Source Data file.
The observation that 3aBat RaTG13 induced robust 3DB formation raised the question as to whether this remodeling activity occurs in bats, the host organisms for progenitor coronaviruses of both SARS-CoV-2 and SARS-CoV10. We adapted the individual expression system to R-06E, an Egyptian fruit bat (Rousettus aegyptiacus) embryonal cell line59. The R. aegyptiacus TGN46 protein sequence is significantly different from the human one and thus cannot be recognized by immunostaining. Instead, we used the phase contrast microscopy method to detect 3DB formation. A large number of 3DBs were formed in R-06E cells expressing 3aCoV2-GFP or 3aBat RaTG13-GFP, but not in those expressing 3aCoV1-GFP (Fig. 4c). Our results confirm that the cellular mechanisms supporting 3DB formation is conserved in bat cells.
The absence of 3DB formation in 3aCivet-CoV-007/2004 made us wonder whether the watershed event for ORF3a to acquire or lose this activity preceded the spillovers from bats to other animal hosts. We characterized four additional bat SARSr-CoV ORF3a homologs (Fig. 4d) in HeLa, chosen based on their varied evolutionary distance to 3aCoV1 and 3aCoV2. These bat ORF3a proteins were expressed at varied levels, and all of them were lower than 3aCoV2 (Fig. 4d), probably due to the suboptimal adaptation to human codons. Nevertheless, all four bat ORF3a promoted robust 3DB formation (Fig. 4e). Unexpectedly, this included ORF3a from Bat-CoV-WIV16, a close relative to SARS-CoV60. These results suggest that 3DB formation is highly conserved in bat SARSr-CoVs. However, this activity was lost either (1) during/after spillover from bat to civet, or (2) in a yet unidentified bat SARS-CoV that is more closely related to SARS-CoV than Bat-CoV-WIV16 (Fig. 4f).
S171 and W193 are key residues for 3DB formation
We have now identified two distinct groups of ORF3a based on whether they possess (Group I) or lack (Group II) the ability to form 3DBs (Supplementary Fig. 7a). Interestingly, alignment of motif 1–3 revealed that motif 3 sequences (orange residues) are 100% identical in Group II ORF3a and 3aBat WIV16, suggesting that while motif 3 is important for maintaining high remodeling activity in 3aCoV2, other motifs can support 3DB formation in 3aBat WIV16. We noticed that aa E171 and R193, located in motif 1 and motif 2, respectively, are the only two residues that exclusively appear in Group II but not Group I ORF3a, suggesting that these two residues may be important in defining the difference. Consistent with this hypothesis, swapping aa 171 in 3aCoV2 to that of 3aCoV1 (S171E) completely abolished 3DB formation, while swapping aa 193 (W193R) partially reduced the activity (Supplementary Fig. 7b). As expected, swapping both residues (S171E/W193R) caused complete defect similar to S171E (Supplementary Fig. 7b). Swapping of aa 171 and 193 in 3aCoV1 at the same time (E171S/R193W), but not individually (E171S or R193W), restored 3DB formation (Supplementary Fig. 7c). These results indicate that aa 171 in motif 1 and aa 193 in motif 2 are both important and work together to support the remodeling. It also explains why swapping motif 1 and motif 2 individually in 3aCoV1 did not restore 3DB formation (Supplementary Fig. 6c), as swapping both is essential for restoring the activity.
Engineering of a recombinant SARS-CoV-2 mutant defective in 3DB formation
We aimed to engineer a SARS-CoV-2 mutant virus specifically defective in 3DB assembly to investigate its functions during viral infection. Because motif 3 only contains five residues (aa 209, 210, 215, 219, and 220) that are different between 3aCoV2 and 3aCoV1, we designed a mutant with these five residues plus aa 171 and 193 swapped with 3aCoV1 (3aCoV2 7 aa swap) to disrupt any residual remodeling activity. 3aCoV2 7 aa swap had complete defect in 3DB formation, while still retaining strong expression and localization pattern similar to 3aCoV1 (Supplementary Fig. 7c). Because SARS‑CoV/SARS‑CoV-2 chimeric viruses are classified as select agents by the Centers for Disease Control and Prevention (CDC)61 due to concerns of potential gain of functions, we designed another 3aCoV2 mutant with these seven residues mutated to alanine (3aCoV2_7Ala) (Supplementary Fig. 7d). Similar to 3aCoV2 7 aa swap, 3aCoV2_7Ala was expressed at comparable level to 3aCoV2, shared similar localization pattern with 3aCoV1, and exhibited a significant defect in 3DB formation (Supplementary Fig. 7e–g). Consistently, the giant 3DB structures under TEM disappeared in cells expressing this mutant (Supplementary Fig. 3f). Furthermore, 3aCoV2_7Ala and 3aCoV1 colocalized with each other in a co-expression system (Supplementary Fig. 7h), further confirming that 3aCoV2_7Ala can be used to mimic the replacement with 3aCoV1 to study the impacts of losing 3DB assembly during viral infection.
Using a bacterial-artificial-chromosome (BAC)-based reverse genetic system62–64, we engineered two recombinant SARS-CoV-2 (rSARS-CoV-2) viruses based on the genomic sequence of USA-WA1 strain: one with a Flag-tag inserted at the C-terminus of WT 3aCoV2 (referred to as WT-Flag virus), and the other with 3aCoV2 replaced by 3aCoV2_7Ala with a C-terminal Flag-tag (referred to as 7Ala-Flag virus) (Fig. 5a). The C-terminal Flag-tag was added to allow immunoblotting and immunostaining of ORF3a. We confirmed that both viruses contained the intended genomic sequences using next-generation sequencing technology (see Methods), and that WT-Flag virus propagated similarly to a previously characterized rSARS-CoV-2 virus without a Flag-tag62–64, indicating that the Flag tag insertion does not affect viral propagation. 7Ala-Flag virus tended to show lower titers (~3–10-fold reduction) compared to WT-Flag virus when propagated between passages. We normalized the titers for these two viruses by adjusting the volumes with additional infection medium for infection experiments with similar multiplicity of infection (MOI). We confirmed that once normalized, WT-Flag and 7Ala-Flag virus titers stayed comparable over the entire study (Supplementary Fig. 8a). The titers were always verified at the time of infection experiments.
Fig. 5. 3DB is assembled during SARS-CoV-2 infection.
a Schematic for the generation of rSARS-CoV-2. Vero E6 cells were transfected with the indicated BAC plasmids before virus-containing supernatants were collected and propagated to generate virus stocks. WT-Flag but not 7Ala-Flag virus can form 3DB. b Vero E6 cells were infected with the indicated rSARS-CoV-2 virus at an MOI of 0.1 for 24 h. Areas containing 3a-Flag-positive structures (3DB in WT-Flag virus-infected cells or the perinuclear cluster in 7Ala-Flag virus-infected cells) were measured with ImageJ (n = 40 cells/sample; mean ± s.d.; two-sided t-test; black line indicates median value). Representative data from at least three independent experiments are shown. c Vero E6 cells were infected as in (b) and immunostained for Flag and EEA1. For cells infected with WT-Flag virus, colocalization of 3DB with organelle markers (images in Fig. 5c and Supplementary Fig. 9b) was quantified with the Pearson correlation coefficient using the Coloc 2 plugin of ImageJ (n = 20 cells/sample; threshold regression: Costes). Organelle makers with strong and weak colocalization with 3DB are labeled in blue and pink, respectively. Representative data from two independent experiments are shown. d Vero E6 cells were infected as in (b) and imaged with TEM. Four pictures (pic 1–4) are shown to highlight different subtypes of 3DBs (labeled with * and yellow outline). Insets: higher magnification of two 3DBs. Mito mitochondria, Nu nucleus, DMV double-membrane vesicle. Red arrowheads indicate several of the extracellular virions. Blue arrowhead indicates an intracellular virion in an SMV. Representative images from two independent experiments (>40 cells per condition) are shown. e Left panel: primary human bronchial/tracheal epithelial cells were mock-infected or infected with WT-Flag virus at an MOI of 5 for 72 h. The cells were fixed and immunostained for Flag. Middle panel: for a WT-Flag virus-infected cell, Z-stack images were acquired and 3D reconstructed to highlight 3DBs viewed from different rotation angles. Right panel: the diameter of 3DB (n = 60) was measured with ImageJ (mean ± s.d.; N.D. not detectable; black line indicates median value). Representative data from three independent experiments are shown. Source data is provided as a Source Data file.
3DBs are assembled during SARS-CoV-2 infection
Previous studies have shown that SARS-CoV-2 infection leads to a complete fragmentation of the Golgi apparatus, including the cis-Golgi4,43. The Golgi fragmentation was proposed to be induced by multiple viral factors other than 3aCoV2 65. Consistent with these studies, we observed that (1) SARS-CoV-2 induced dramatic dispersion of TGN46-positive structures (Supplementary Fig. 8b, c), but the effect was not dependent on the presence of ORF3a (Supplementary Fig. 8c); (2) SARS-CoV-2 infection also induced the fragmentation of the cis-Golgi (Supplementary Fig. 8d), in contrast to the lack of effect on the cis-Golgi morphology by 3aCoV2 in the individual expression system. Therefore, dispersion of TGN46-positive structures is not a suitable hallmark for studying 3aCoV2-mediated remodeling during viral infection due to the interference of other viral factors. Instead, we focused on monitoring 3DBs via Flag immunostaining. We infected Vero E6 cells with WT-Flag or 7Ala-Flag virus at an MOI of 0.1 and imaged at 24 hours post-infection (hpi). As shown in Fig. 5b, WT-Flag virus infection led to the formation of multiple giant 3DBs (1.81 ± 0.65 μm in diameter) positive with 3aCoV2-Flag. In contrast, in 7Ala-Flag virus-infected cells, the formation of 3DBs was abolished, and 3aCoV2_7Ala-Flag was instead enriched on a perinuclear cluster (Fig. 5b), recapitulating the localization of this mutant in the individual expression system. Similar results were also observed in A549-hACE2 cells (Supplementary Fig. 9a). These results indicate that 3aCoV2 drives 3DB formation during viral infection in a way dependent on the seven key residues.
Colocalization between 3DBs and TGN46 was observed in infected cells, but was less prominent than the individual expression system, probably due to the additional TGN fragmentation caused by other viral factors. Consistent with the individual expression system, 3DBs formed during infection were not positive with organelle markers of the cis-Golgi, ER, ERGIC, or lysosome (images in Supplementary Fig. 9b, quantification in Fig. 5c). CD63, a marker of MVBs, exosomes, late endosomes, and lysosomes66,67, was not detected on 3DBs either (images in Supplementary Fig. 9b, quantification in Fig. 5c). In contrast, the early endosome marker EEA1 was highly enriched on 3DBs (Fig. 5c). These results again support the TGN and early endosomal origin of 3DBs. When imaged with TEM, the electron-dense 3DBs were only detected in cells infected with WT-Flag virus, but not in those infected with 7Ala-Flag virus (Fig. 5d). More than 90% of WT-Flag virus-infected cells (n > 80 cells in two biological repeats) showed at least one 3DB in the current cut section. In contrast, DMVs and budding virions were detected for both viruses (Fig. 5d). To examine whether 3DB formation occurs in primary human cells, we infected primary bronchial/tracheal epithelial cells from healthy human donors with WT-Flag virus at an MOI of 5 for 72 h. These higher MOI and longer infection time were necessary for these primary cells to be infected by SARS-CoV-2, consistent with previous findings using the same source of primary bronchial/tracheal cells68. Flag immunostaining revealed the assembly of multiple giant 3DB structures upon infection (Fig. 5e and Supplementary Movie 1). The diameter range of 3DBs in primary cells (quantified in Fig. 5e) matched that of 3DBs in Vero E6 (1.81 ± 0.65 μm). Interestingly, 3DBs formed during SARS-CoV-2 infection in both cell lines and primary cells appeared larger in size but lower in number compared to those formed in the 3aCoV2 expression system. This is likely due to constant fusion and engulfment of 3DBs during SARS-CoV-2 infection (discussed below).
3DBs are loaded with viral S and M
Notably, 3DBs were loaded with S, as confirmed by immunostaining with two antibodies recognizing the S1 subunit and S2 subunit of S, respectively, in both Vero E6 and A549-hACE2 cells (Fig. 6a, b and Supplementary Fig. 10a, b). While all S-positive spherical structures had 3aCoV2 signal, only a subset of 3DBs were loaded with S. In addition, in 7Ala-Flag virus-infected cells, the giant spherical structural localization of S disappeared (Fig. 6a, b and Supplementary Fig. 10a, b). These results suggest that 3aCoV2 forms 3DBs to recruit S. The viral structural protein M is incorporated into the viral lipid envelope and serves as a scaffold for virion assembly69. We found that M was also recruited to 3DBs in a manner dependent on the seven key residues of 3aCoV2 (Fig. 6c, d). In contrast, the N protein, a viral structural protein that encapsulates the viral RNA70, did not localize to 3DBs (Supplementary Fig. 10c, quantification in Fig. 6e). This is consistent with a previous study showing that N shares limited colocalization with other structural proteins, including S and M, indicating that N uses a different trafficking route for virion assembly43. Double-stranded RNA (dsRNA), a product of SARS-CoV-2 viral genome replication and mRNA transcription71, was not detected on 3DBs either (Supplementary Fig. 10d, quantification in Fig. 6e). The lack of N, dsRNA, and ER/ERGIC markers on 3DBs suggests that 3DBs are distinct from the previously characterized replication organelles. We propose that 3DBs are membrane structures specifically involved in the trafficking of S and M before their incorporation into mature virions during virion assembly. Consistent with this hypothesis, 3DBs underwent constant fusion and/or fission events, as well as engulfment of smaller 3DBs (Fig. 6f). These observations suggest that 3DBs are highly dynamic and constantly exchange the loaded cargos S and M. While 3DBs were deprived of N, dsRNA, ER marker and ERGIC marker, 3DBs were in proximity to these structures, suggesting that 3DBs may be highly interconnected with these membrane structures to facilitate viral protein trafficking and virion assembly.
Fig. 6. 3DBs are loaded with S and M and undergo dynamic cargo exchange.
a, b Vero E6 cells were infected with the indicated rSARS-CoV-2 virus at an MOI of 0.1 for 24 h, before being immunostained for Flag and spike S1. The percentage of 3DBs loaded with spike was quantified (n = 40 infected cells/sample; mean ± s.d.; two-sided t-test; black line indicates median value). Representative data from at least three independent experiments are shown. c, d Similar to (a, b), except the cells were immunostained for Flag and membrane (M). The percentage of 3DBs loaded with M was quantified with methods similar to (b). Representative data from at least three independent experiments are shown. e For Vero E6 cells infected with WT-Flag virus, colocalization of 3DB with S, M, N, and dsRNA (images in Fig. 6a, c and Supplementary Fig. 10c, d) was quantified with Pearson correlation coefficient using the Coloc 2 plugin of ImageJ (n = 20 cells/sample; threshold regression: Costes). Strong and weak colocalization with 3DBs are labeled in blue and pink, respectively. Representative data from at least three independent experiments are shown. f A magnified image of Vero E6 infected with WT-Flag virus and immunostained for Flag and spike S1, as in (a), is shown. Three regions are highlighted: I. and II. show large 3DBs containing smaller 3DBs; III. shows a fusion or fission event between 3DBs. Representative data from at least three independent experiments are shown. Source data is provided as a Source Data file.
To study the kinetics of 3DBs formation, we imaged infected Vero E6 cells at an MOI of 0.1 for 5 h, 8 h, and 15 h. These time points were chosen to represent the three stages of viral protein expression43. Both WT 3aCoV2 and 3aCoV2_7Ala became detectable in a small subset (~1%) of infected cells at 5 hpi, before S and M became detectable (Supplementary Fig. 11a, b). At this early time point, WT 3aCoV2 was localized on tubular and punctate structures. At 8 hpi, WT 3aCoV2 formed small 3DBs that were clustered together, which recruited S and M (Supplementary Fig. 11a, b). At 15 hpi, 3DBs became larger. In contrast, while N became detectable as early as 5 hpi, it did not localize on 3DBs during the entire time course (Supplementary Fig. 11c). These data indicate that 3aCoV2 is one of the early-synthesized viral proteins and forms 3DBs between 5 and 8 hpi. The growth in 3DB size may be a result of the constant fusion.
3DB is required for maximal infectivity of virions
rSARS-CoV-2 Δ3a produced reduced plaque size21, suggesting that 3aCoV2 is important for optimal viral infectivity. To study the contributions of 3DB formation, we compared the plaque size of WT-Flag, 7Ala-Flag, and Δ3a virus. 7Ala-Flag virus consistently showed significantly smaller plaques compared to WT-Flag virus, although the average plaque size was still larger than that of Δ3a virus (Fig. 7a). These data indicate that 3aCoV2 possesses both 3DB-dependent and -independent functions to facilitate viral spread, with the latter likely contributed by the previously characterized functions of ORF3a in lysosomal exocytosis-mediated viral egress, autophagy, and late endosome/lysosome trafficking26,28–39.
Fig. 7. 3DB is essential for optimal viral infectivity.
a Vero E6 cells were infected for 72 h for the plaque assay. The plaque size was measured with ImageJ (n = 34, 50, and 28 plaques for WT-Flag, 7Ala-Flag, and Δ3a virus, respectively; mean ± s.d.; two-sided t-test; black line indicates median value). b, c Vero E6 cells were infected at an MOI of 0.1 for 24 h. The cells (n = 1.0 × 106 cells/sample at the time of infection) were stained for spike S2 antibody and sorted by flow cytometry to quantify spike-positive cells. Forward scatter height (FSC-H) vs side scatter height (SSC-H) gating was used to remove cell debris. SSC-H vs side scatter area (SSC-A) gating was used to remove cell clumps. d Vero E6 cells were infected at the same MOI (0.1) for 1 h before being washed to remove extracellular virus. The cells (n = 2.5 × 106 cells/sample at the time of infection) were then incubated in fresh medium for another 23 h (for a total of 24-h infection), and the medium and lysate were collected to determine the extracellular and cell-associated virion titers, respectively. Mean ± s.d.; two-sided t-test. e Virus stock immunoblotting: 3 × 105 PFU of virus was concentrated for immunoblotting of N protein. Two technical replicates are shown for each virus stock. ‘x’, an empty well. expo, exposure time. f Particle-to-PFU ratio measurement: viruses normalized to the same titer were subjected to viral RNA extraction and RT. Real-time qPCR was performed to measure the N RNA level. The results were then converted to numbers of viral genome copies. The particle-to-PFU ratio was then calculated (mean ± s.d.; two-sided t-test). Representative data from at least three independent experiments are shown for all experiments above. Source data is provided as a Source Data file.
Plaque assays only quantify viral spread starting from day 3 post-infection due to the small plaque size in the first two days. To examine viral spread in the first 24 h, we infected Vero E6 with WT-Flag virus and 7Ala-Flag virus at an MOI of 0.1. At 24 hpi, the cells were fixed and stained with S antibody followed by Alexa Fluor 568, before being analyzed by flow cytometry to quantify the percentage of infected cells (spike+). The populations were analyzed by size and morphology to ensure that no significant cytotoxicity occurred at this time point. To exclude effects caused by titer decrease during storage or freezing/thawing, we re-measured the titers at the same time to confirm that the two viruses were maintained at the same titer. 7Ala-Flag virus consistently infected a lower percentage (~50% decrease) of cells compared to WT-Flag virus at 24 hpi (Fig. 7b, c).
Next, we examined the infectivity of both extracellular (i.e., virions released into the medium) and cell-associated (i.e., both intracellular and cell-bound virions) virus (Fig. 7d). Vero E6 was infected with WT-Flag virus and 7Ala-Flag virus at a similar MOI (0.1) for 1 h, before the medium was removed and the cells were washed with PBS. The cells were then incubated in fresh medium for another 23 h for a total of 24 h of infection. The supernatant and cell lysate were then collected separately for plaque assay to measure the extracellular and cell-associated viral titer, respectively. For both types, 7Ala-Flag virus consistently showed ~10-fold reduction in viral titers (Fig. 7d). While the measurement of spike+ cells in Fig. 7c reflects a snapshot of infection efficiency at 24 hpi, the measurement of extracellular and cell-associated viral titers reflects the capacity of the virus to continue infecting cells beyond 24 h. The enhanced defect in the latter (~10-fold reduction in Fig. 7d vs ~50% decrease in Fig. 7c) indicates that the contribution of 3DBs for infectivity increases as infection progresses.
In line with the results above, 7Ala-Flag virus contained significantly higher levels of N protein than WT-Flag virus at the same titer (Fig. 7e), suggesting that virions assembled in the absence of 3DB require a higher number to achieve the same infection efficiency. To further validate this conclusion, we determined the particle-to-plaque-forming unit (PFU) ratio for both viruses, defined as the number of viral particles (determined by viral genome copy number) needed to form one plaque (determined by plaque assay). WT-Flag virus had a particle-to-PFU ratio close to 103 (Fig. 7f), consistent with previous literature72,73. In contrast, 7Ala-Flag virus consistently showed a > 10-fold increase in particle-to-PFU ratio (Fig. 7f), confirming that it has a lower capacity to initiate successful infections. Together, these results demonstrate that 3DB is essential for maximal infectivity.
3DB is crucial for assembled virions to achieve optimal entry efficiency
To examine which stage of the viral infection cycle is defective for virions assembled in the absence of 3DB, we designed a series of assays as shown in Fig. 8a–d. In the viral entry assay (Fig. 8a), we infected Vero E6 cells with WT-Flag and 7Ala-Flag virus with the same number of viral particles. The viral particle number used was equivalent to MOI 0.1 for WT-Flag virus, but >10-fold lower MOI for 7Ala-Flag virus due to the latter’s higher particle-to-PFU ratio. The cells were incubated with the indicated virus at 4 °C for 1 h to allow virus binding to cells but prevent viral entry. After the unbound virus was removed with washing, the cells were switched to 37 °C to initiate synchronized viral entry. At both 1 h and 2 h post entry, cells infected with 7Ala-Flag virus showed a significant reduction (~10-fold) in intracellular viral RNA level compared to those infected with WT-Flag virus (Fig. 8a), indicating that 7Ala-Flag virus has lower viral entry efficiency with the same number of viral particles.
Fig. 8. 3DB regulates S processing rate and is essential for the optimal viral entry efficiency of assembled virions.
a Viral entry assay: Vero E6 cells were incubated with the same number of virions for 1 h at 4 °C before being washed and incubated at 37 °C to start synchronized viral entry. At 1 h and 2 h post-entry, the cells were lysed for RNA extraction and RT-qPCR to measure intracellular N RNA level. The results are presented as fold change to the baseline detection limit (Ct = 40) (mean ± s.d.; two-sided t-test; N.D., not detectable). b Viral RNA replication assay: Vero E6 cells were infected at the same MOI (0.05) for 1 h before being washed, incubated with fresh medium, and measured similarly to (a). The results are presented as fold change to the WT-Flag virus-infected cells at 1 hpi (mean ± s.d.; two-sided t-test; NS not significant). c Viral protein synthesis assay: Vero E6 cells were infected at the same MOI (0.1) for the indicated time length before being lysed for immunoblotting. d Egressed virion imaging: Vero E6 cells were infected at the same MOI (0.1) for 24 h before TEM imaging. Red arrows, virions. The number of visible virions per cell (n = 20 cells/sample) and the diameter of virions (n = 40 virions/sample) were measured with ImageJ (mean ± s.d.; two-sided t-test; NS, not significant; black line indicates median value). e Virion immunoblotting assay: Vero E6 cells were infected at the same MOI (0.3) for 1 h, before being washed and incubated for another 23 h. The supernatant was collected and concentrated for immunoblotting. FL, full-length. The band intensity of the processed S protein was quantified using ImageJ and presented as fold change to WT-Flag virus samples. f Model: ORF3a from SARS-CoV-2 hijacks a specific subset of TGN and early endosomal membranes to build 3DBs. 3DBs are loaded with the viral structural proteins S and M and negatively regulate the furin-mediated processing of S during virion assembly. As a result, 3DB is essential for maintaining the optimal processing ratio of S on assembled virions and promoting their entry efficiency. Representative data from at least three independent experiments are shown for all experiments above. Source data is provided as a Source Data file.
We did not observe a significant increase in intracellular viral RNA level from 1 h to 2 h post entry for either WT-Flag or 7Ala-Flag virus (Fig. 8a), suggesting that viral RNA replication has not significantly contributed to the measurements at these early times points, and therefore did not contribute to the observed defect for 7Ala-Flag virus. To directly examine the effects on viral RNA replication without the interference of the difference in viral entry efficiency, we designed another assay in which cells were infected with the same MOI instead of the same viral particle number (Fig. 8b). This ensured that equal numbers of cells were initially infected (i.e., similar entry capacity) by both viruses. Compared to 1 hpi, the intracellular viral RNA level at 6 and 24 hpi increased by ~10 and ~104-fold, respectively (Fig. 8b), indicating robust viral RNA replication. Interestingly, 7Ala-Flag virus did not show any detectable reduction during the entire 24-h infection time course (Fig. 8b), indicating that it is not defective in viral RNA replication. We next performed a viral protein synthesis assay with similar settings (i.e., using equal MOI), in which infected cells were lysed for immunoblotting of intracellular viral proteins (Fig. 8c). 7Ala-Flag virus infection led to comparable protein levels of intracellular S and ORF3a with WT-Flag virus (Fig. 8c), indicating that viral protein synthesis is not directly affected.
In Fig. 7d, both extracellular and cell-associated infectivity showed a similar level of defect for 7Ala-Flag virus (both with ~10-fold reduction), suggesting that viral egress did not further exaggerate the difference. To directly examine the viral egress efficiency of virions assembled without 3DB, we infected Vero E6 cells with WT-Flag and 7Ala-Flag virus at the same MOI and examined egressed virions with TEM. 7Ala-Flag virus did not show any significant difference in egressed virion number or diameter when compared to WT-Flag virus (Fig. 8d), indicating that viral egress is not directly affected. These data also highlight that the 7Ala mutations do not impair the previously reported function of ORF3a in lysosome-mediated viral egress29, consistent with our observation that 7Ala-Flag virus showed a lower defect than Δ3a virus in the plaque size assay (Fig. 7a). Therefore, this mutant virus serves as a great tool for specifically studying the effects of 3DB formation.
3DB maintains the optimal S processing rate
Despite being expressed at high levels inside the infected host cells (Fig. 8c), ORF3a was undetectable in the egressed virions (Supplementary Fig. 12), suggesting that it is not incorporated into the virions. In addition, our time course infection experiment has shown that ORF3a expression was only detectable in a small subset of cells starting from 5 hpi, and that 3DB assembly did not start until 8 hpi (Supplementary Fig. 11), indicating that neither ORF3a protein nor 3DB structures directly account for the defect observed in the viral entry assay at early time points (1–2 hpi, Fig. 8a). Therefore, we hypothesized that the absence of 3DB during virion assembly altered the virion composition to indirectly impact the viral entry efficiency of these virions. Based on the observation that 3DB mediates the trafficking of S protein (Fig. 6a, f), the key viral protein in viral entry, we focused on the effects of 3DB on S. Vero E6 cells were infected with WT-Flag or 7Ala-Flag virus at the same MOI for 24 h. At this point, the cytopathic effect was undetectable, thus preventing the interference of intracellular virions and viral proteins not incorporated into virions since they were not released into the medium. We collected the medium containing egressed virions and analyzed their protein compositions with immunoblotting. As expected, N protein in the egressed virions did not show any significant difference between WT-Flag and 7Ala-Flag virus samples (Fig. 8e and Supplementary Fig. 12), again confirming that 3DB does not directly affect the level of viral egress. S on WT-Flag virions was predominantly in the full-length form (Fig. 8e), consistent with previous findings using USA-WA1 infection in Vero E614. Strikingly, however, S underwent significantly higher processing rate on 7Ala-Flag virions (~10-fold higher), as confirmed by both S2 (Fig. 8e) and S1 (Supplementary Fig. 12) immunoblotting. This dramatic difference in S processing rate is highly reproducible, as confirmed with three independent batches of WT-Flag and 7Ala-Flag viruses propagated from individually prepared passage (P)0. This suggests that 3DB may negatively regulate the processing rate of recruited S to prevent its premature inactivation, which is crucial for the viral entry efficiency of the assembled virions. Our results thus uncovered a previously uncharacterized mechanism of ORF3a to fine-tune S processing during virion assembly through the 3DB structures.
In summary, our study has unveiled a group of dense bodies, 3DBs, assembled by ORF3a of SARS-CoV-2 (Fig. 8f). The 3DB assembly is driven by the reorganization of a specific subset of the host TGN and early endosomal membrane. This remodeling activity is conserved in ORF3a derived from bat and pangolin SARSr-CoVs but was lost during the evolution to SARS-CoV. 3DBs undergo dynamic fusion and engulfment, which facilitates the exchange of the loaded cargo S and M proteins. 3DB maintains the optimal unprocessed-to-processed ratio of S on assembled virions. As a result, disruption of 3DB distorts the S processing rate on virions and diminishes the viral entry efficiency of these virions, highlighting the potential of targeting the 3DB for developing COVID-19 vaccines and therapeutics.
Discussion
Here, we identified and characterized the 3DB, an ORF3a-driven membrane structure assembled during SARS-CoV-2 infection. The unusual electron-dense nature and membranous sub-compartments of 3DBs distinguish them from other organelles, such as nigericin-induced TGN vesicles, DMVs, and MVBs. Electron-dense nature with TEM is usually correlated with a large amount of proteins and lipids, but can also indicate the presence of metal elements, phosphate, or other chemicals74,75. 3DBs show several different morphologies ((i–v) in Fig. 1c). In both the individual expression system and infection system, (i) and (ii) were the most abundant forms, indicating that they may be the mature or most stable forms. The membranous sub-compartments observed in these two forms may be related to the small 3DBs engulfed in giant 3DBs (Fig. 6f). In contrast, (iv) and (v), the most electron-dense 3DB structures, were relatively smaller than the other three forms in the individual expression system (Fig. 1c), and were rarely detectable in the infection system at 24 hpi, suggesting that they may represent either the early stages or the less stable end stages of 3DBs.
In the individual expression system, TGN46 was abundantly localized on most (if not all) 3DBs (Fig. 1a) while EEA1 was only recruited to a subset of 3DBs (Fig. 2b). In contrast, during SARS-CoV-2 infection, EEA1 was recruited to a large amount of 3DBs (Fig. 5c) while TGN46 was only detected on a subset of 3DBs (Fig. 5b). These differences suggest that other viral factors may have additional effects on the membrane remodeling. For example, Golgi fragmentation may reduce the amount of TGN membrane available for 3DB formation. These observations also indicate that the recruitment of TGN46 and EEA1 is not entirely dependent on each other, as they have different recruitment patterns to 3DBs, although we cannot exclude the possible involvement of cargo exchange between the TGN and early endosomes. SARS-CoV-2 uses the Golgi apparatus for virion trafficking and post-translational modifications76. Therefore, one possibility why 3aCoV2 targets a narrow range of host TGN membrane may be to prevent interfering with the Golgi apparatus hijacking by other viral factors. This highlights the complexity and well-coordinated nature of virus-mediated host organelle remodeling.
Another difference between the infection system and the individual expression system is that the number of 3DBs formed during infection is lower, while the diameter is higher (e.g., Fig. 5b vs Supplementary Fig. 3a, both in Vero E6). This was not due to overexpression in the individual expression system, as individually expressed 3aCoV2 induced a large number of small 3DBs even at a barely detectable level (Supplementary Fig. 3d). One possibility is that the complete Golgi fragmentation by other viral factors reduced the amount of TGN membrane 3aCoV2 can hijack during infection, resulting in a lower number of 3DBs. The increase in 3DB diameter during infection may be caused by constant fusion and/or fission events (Fig. 6f), which can also reduce the number of 3DBs. Indeed, when 3DBs first appeared at 8 hpi, they were small structures resembling those in the individual expression system, before growing larger at 15 hpi (Supplementary Fig. 11). The growth in size may be facilitated by the loading of S and M or mediated by other viral factors. One interesting observation is that 3aCoV2_7Ala localized on a perinuclear cluster structure during infection (Fig. 5b). This perinuclear cluster resembled the Golgi apparatus and was located in proximity to the dispersed cis-Golgi marker GM130 (Supplementary Fig. 9b), thus raising the question whether a small subset of the Golgi apparatus remains intact during infection. An extensive characterization of Golgi markers during SARS-CoV-2 infection may help answer this question.
In the individual expression system, we noticed a modest level of overlap between 3aCoV2 and some organelle makers (e.g., LAMP1 and LC3) in a subset of cells (Fig. 2b colocalization analysis). These are likely due to two factors. (1) Besides 3DBs, 3aCoV2 is also localized on punctate structures in the cytosol, similar to 3aCoV1. These structures may be related to the lysosomes and autophagosomes that 3aCoV2 was reported to localize to in previous studies26,28–37. (2) 3DBs are highly abundant in the individual expression system, which may have caused artificial overlap with abundant organelle markers such as those of lysosomes. In contrast, in the SARS-CoV-2 infection experiments, 3DBs are in lower number, and have shown a clear separation from other organelle markers, such as the lysosomes (Supplementary Fig. 9b, quantification in Fig. 5c). Therefore, we conclude that these organelle markers are not localized on 3DBs during SARS-CoV-2 infection.
Consistent with our previous discovery that the dispersion of PtdIns4P-positive TGN structure is required for the NLRP3 inflammasome assembly and activation41, neither 3aCoV1 nor 3aCoV2 activates the NLRP3 inflammasome (Supplementary Fig. 4c, d). This is in contrast to other studies proposing that both 3aCoV1 and 3aCoV2 activate the NLRP3 inflammasome77–79. The discrepancies may be due to different cell models and expression systems used. While we observed minimal inflammasome activation in a RAW 264.7 infection model (Supplementary Fig. 4e), we cannot exclude the possibility that SARS-CoV-2 may activate the NLRP3 inflammasome in other cell types or in vivo, as indicated by other studies80–82.
ORF3a homologs in bat and pangolin coronaviruses also have the 3DB formation activity (Fig. 4b). Unexpectedly, this activity was lost in ORF3a from SARS-CoV and a closely related civet coronavirus (Figs. 1a and 4b). While both SARS-CoV and SARS-CoV-2 are highly similar in genome sequence (79% genome sequence identity)23, they differ greatly in transmission rates, pathogenesis, and host immune responses83. Our discovery that ORF3a in these two viruses possesses a strikingly different ability to assemble 3DBs provides a new direction to understand the different features of these two coronaviruses, especially for the highly contagious nature of SARS-CoV-2. Bat coronaviruses serve as reservoirs for a number of important emerging HCoVs. Therefore, close genomic monitoring of bat coronaviruses for changes in 3DB formation activity will provide insights into identifying future pathogenic HCoVs with pandemic potential.
One of the major questions that remains to be answered is how 3aCoV2 hijacks host membranes to form these giant dense bodies. While the viroporin activity of 3aCoV2 has been supported by a previous structural study27, a recent study has suggested that 3aCoV2 is not a viroporin26. It thus remains to be determined whether the viroporin activity of 3aCoV2 exists, and if so, whether it is involved in 3DB assembly. Our immunoblotting results show that ORF3a proteins from SARSr-CoVs often appeared as multiple bands (e.g., Fig. 4a, d), indicating that they may undergo extensive PTMs or proteolytic cleavage. However, we did not observe strong correlations between protein band positions and 3DB formation activity. Therefore, whether the remodeling activity is dependent on particular PTMs or cleavage events still remains to be studied. Finally, it remains to be investigated whether host factors are essential to facilitate the 3DB assembly, or 3aCoV2 alone is sufficient to form these structures. We have demonstrated that a small Flag-tag can be inserted at the C-terminus of 3aCoV2 without disrupting virion assembly or viral propagation. This will allow future identification and characterization of 3aCoV2 PTMs and binding partners during infection using Flag immunoprecipitation coupled to mass spectrometry.
Coronaviruses possess the largest genomes in RNA viruses, and thus, it is technically challenging and time-consuming to engineer recombinant SARS-CoV-2 mutants. We therefore took advantage of the individual expression system for domain swapping to identify the key motifs for 3DB formation, before engineering a mutant virus (7Ala-Flag) defective in 3DB formation. Using this mutant virus, we found that virions assembled in the absence of 3DB do not have direct defects in viral RNA replication, viral protein synthesis, or viral egress (Fig. 8b–e). Strikingly, 7Ala-Flag virus showed a significant defect in viral entry (Fig. 8a). This results in its lower viral infectivity in various assays (Fig. 7a–f). 7Ala-Flag virions showed normal diameter (Fig. 8d), N protein level, and total S protein level (Fig. 8e and Supplementary Fig. 12), but a significantly increased S processing rate (Fig. 8e and Supplementary Fig. 12), resulting in a reduced unprocessed-to-processed ratio of S on virions. These data indicate that 3DB may modify the loaded S protein before it is transported to the ultimate virion assembly site (e.g., SMVs or ER/ERGIC/Golgi structures) (Fig. 8f). There are several possible non-mutually exclusive mechanisms. (1) The S protein enriched on 3DBs may adapt specific complex structures that impact its processing rate. In addition, it remains to be determined whether 3DB-loaded M regulates S processing, as a recent study has suggested a key function of M in facilitating S incorporation into virions84. (2) As 3DB is derived from part of the TGN, it may affect the TGN-localized furin85 cleavage efficiency on S. (3) 3DB may affect the glycosylation patterns of S to indirectly affect its processing rate, as a recent study has shown that S glycosylations can impact its cleavage to regulate the infectivity84. SARS-CoV-2 S and M proteins undergo extensive glycosylations, affecting viral entry, replication, and host recognition86–88, and the TGN is a major organelle for the functions of glycosylation enzymes89,90. While we did not observe significant changes in the overall glycosylation level of S protein in the absence of 3DB based on the molecular weight of the highly glycosylated S protein in immunoblotting (Fig. 8e), we cannot rule out the possibility that individual glycosylation modifications may be altered.
S processing is also key in regulating the prefusion-to-postfusion conformation switch91. Both prefusion and postfusion conformations are detected on assembled and egressed virions92. Several cryo-ET and cryo-electron microscopy (cryo-EM) studies found that the prefusion conformation is the predominant form on virions12,93,94. Another study observed predominant postfusion conformation instead95, although it was believed to be caused by β-propiolactone inactivation of the virus91. The prefusion form is metastable, active, and responsible for binding to the ACE2 receptor, while the postfusion conformation is more stable but inactive96,97. Future cryo-EM and cryo-ET studies of 3DB-localized S and M complexes will determine the impacts of 3DB-mediated regulation of S processing on the prefusion-to-postfusion conformation ratio.
Although there was no significant defect in viral RNA replication or viral protein synthesis at 24 hpi (Fig. 8b, c), flow cytometry experiment showed that 50% less cells were infected by 7Ala-Flag virus at this time point (Fig. 7c). This is because flow cytometry used in Fig. 7c can detect a low level of S in newly infected cells even when viral protein synthesis has not started in significant levels. Therefore, even though WT-Flag virus infected more cells at 24 hpi due to its higher viral entry efficiency to start new rounds of infection, the newly infected cells had not accumulated significant levels of newly synthesized viral RNA or viral proteins to show a difference compared to 7Ala-Flag virus.
It remains to be characterized how 3DBs coordinate with DMVs, SMVs, ERGIC, and other membrane structures for the complex process of virion assembly. While dramatically different in morphologies, organelle origin, and loaded viral components, 3DBs and DMVs share two common features: (1) DMVs appeared in infected cells at 6–8 hpi4,98, which overlaps with the time when 3DBs appeared (5–8 hpi) (Supplementary Fig. 11); (2) both DMVs4 and 3DBs (Fig. 6f) had contacts between individual structures that suggested fusion and/or fission, and they both had larger structures containing smaller ones. While future experiments are needed to explore their relationships, it is possible that these two types of virus-induced structures are closely interconnected to facilitate virion assembly.
Finally, while we focused on the reference strain SARS-CoV-2 USA-WA1 in this study, it will be interesting to investigate whether the key motifs and residues are mutated to affect 3DB formation or function in other variants. A recent study99 highlights a few unique ORF3a mutations in Omicron that are absent in other variants. However, none of these are in the aa 171–222 region, consistent with the importance of 3DB assembly driven by this region for viral transmission. Another study100 found that the S171L mutation was found in ORF3a of some circulating strains. S171L is also linked with the Beta variant of concern according to the Stanford Coronavirus Antiviral & Resistance Database (CoVDB)101. Whether this mutation disrupts the 3DB formation activity and the resulting effects on pathogenicity remain to be determined.
Methods
Antibodies and reagents
Antibodies against TGN46 (TGOLN2/TGN38) (HPA012723), α-tubulin (T9026), Flag (F1804 for mouse host and F7425 for rabbit host), AP1G1 (A4200), and SARS-CoV-2 nucleocapsid (N) (ZMS1075) were from Sigma. Antibodies against SARS-CoV-2 spike (S) S1 region (GTX635654, rabbit host) and S2 region (GTX632604, mouse host) were from GeneTex. Antibody against SARS-CoV-2 membrane (M) (NB100-56569) was from Novus Biologicals. Antibodies against EEA1 (610456), GOLGA4 (611280), GM130 (610822), GGA2 (612612), and GGA3 (612310) were from BD Biosciences. Antibodies against giantin (ab24586) and calnexin (ab219644) were from Abcam. Antibodies against TOM20 (sc-11415), CD63 (sc-5275), ERGIC-53 (sc-365158), Rab7 (sc-376362), LAMP1 (sc-20011), and caspase-1 (sc-515) were from Santa Cruz Biotechnology. Antibody against LC3 (PM036) was from MBL Life Science. Antibodies against NLRP3 (AG-20B-0014) and ASC (AG-25b-0006) were from AdipoGen. Alexa Fluor 488 (A-11001, A-11008), 568 (A-11004, A-11011), and 633 (A-21050, A-21070) were from Thermo Fisher Scientific. CF®568 (20101, 20103) was from Biotium. Antibody against dsRNA (76651) and HRP-conjugated secondary antibodies (7076S and 7074S) were from Cell Signaling Technology. Antibody against GFP (902602) was from BioLegend. LPS (tlrl-3pelps) was from InvivoGen. Nigericin (N7143) and carboxymethylcellulose (C4888) were from Sigma.
Mammalian cell culture
HEK293T, HeLa, RAW 264.7, and Vero E6 were obtained from ATCC. Vero E6-TMPRSS2-T2A-ACE2 (NR-54970) was obtained from BEI. A549-hACE2 cell line was a kind gift from Dr. Benjamin tenOever42. The MEF cell line was immortalized from C57BL/6J mice102. R-06E (ACC 756) was obtained from the DSMZ German Collection of Microorganisms and Cell Cultures and was tested to confirm no contamination with human or monkey cells. No commonly misidentified cell lines (ICLAC version 13) were used in this study. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) cosmic calf serum (CS) (Hyclone), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C with 5% (v/v) CO2. Cells were monitored for contamination, including mycoplasma, with e-Myco Mycoplasma PCR Detection Kit (Bulldog Bio) and regularly maintained with Normocin (an antimicrobial reagent against mycoplasma, bacteria, and fungi) (InvivoGen).
Human normal primary bronchial/tracheal epithelial cells (PCS-300-010, Lot # 70036649) were obtained from ATCC. Cells were cultured with primary cell medium, which contained Airway Epithelial Cell Basal Media (ATCC, PCS-300-030) supplemented with Bronchial/Tracheal Epithelial Cell Growth Kit (ATCC, PCS-300-040), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C with 5% (v/v) CO2.
Plasmids, lentivirus, and stable cell lines
The ORF3a genes were synthesized as gBlock gene fragments (Integrated DNA Technologies) based on these coronaviral genomes: CoV2_USA-WA1 (GenBank MN985325), CoV1_Tor2 (GenBank NC_004718), Bat_RaTG13 (GenBank MN996532), Pangolin_GX-P4L (GenBank MT040333), Civet_007_2004 (GenBank AY572034), Bat_WIV16 (GenBank KT444582), Bat_YNLF_31C (GenBank KP886808), RmYN08 (GenBank MZ081378), and 3a_Bat_ZC45 (GenBank MG772933). Other viroporin genes were also synthesized as gBlock gene fragments, except that E5 and M2 were cloned from plasmids provided by Drs. Laimonis A. Laimins (Northwestern University) and Balaji Manicassamy (University of Iowa), respectively. The non-ORF3a viroporin sequences are: 2B (poliovirus, GenBank V01149.1), Agno (JC polyomavirus, GenBank AB190453.1), E5 (human papillomavirus 16, GenBank NC_001526.4), M (Dengue virus, GenBank U87411.1), M2 (influenza A virus, GenBank NC_002016.1), NSP4 (rotavirus, GenBank NC_011504.2), VP4 (human rhinovirus, GenBank AB079152.1), and Vpu (HIV, GenBank NC_001802.1). OSBPPH-GFP, which consists of an initial methionine residue and the PH domain from OSBP (human, amino acids 87–185), was cloned and verified in our previous study41.
The lentiviral vectors for protein expression, pTY-EF1α-target gene-IRES-puroR/hygroR/zeoR, were engineered as indicated in our previous study41. ORF3a and other genes of interest were inserted into the lentiviral vectors using Gibson Assembly. Mutations and domain swapping were introduced with Gibson Assembly. The full-length gene sequences were confirmed by Sanger sequencing or whole-plasmid sequencing.
Stable cell lines were established through lentiviral infection41. In brief, lentivirus was packaged by co-transfecting HEK-293T cells with the lentiviral vector and packaging plasmids psPAX2 (Addgene #12260) and pMD2.G (Addgene #12259). Medium containing lentivirus was filtered and added to target cells in the presence of polybrene (10 μg/mL). Cells were then selected with the respective antibiotics for at least seven days before protein expression was confirmed by immunoblotting and fluorescence microscopy.
Immunostaining and fluorescence microscopy
For immunostaining, cells were seeded onto coverslips, fixed with 4% paraformaldehyde for 10 min (min) (or 10% formalin for 15 min for SARS-CoV-2 infection experiments), permeabilized with 0.1% saponin in phosphate-buffered saline (PBS) for 5 min and blocked with 10% BSA in PBS containing 0.1% saponin for 30 min. The samples were then incubated with primary antibodies, followed by Alexa Fluor secondary antibodies. Nuclei were stained with DAPI in Vectashield antifade mounting medium (H1200). The choices of spike S1 (rabbit host) and S2 (mouse host) in some experiments were due to the host species of the other antibody for co-imaging (e.g., mouse host S2 antibody + rabbit host ASC antibody). The same applies to the choices of Flag antibodies (mouse host Sigma F1804 and rabbit host Sigma F7425). The specificity of antibodies was verified using one or multiple of these methods: (1) cells lacking expression of the protein of interest (e.g., HeLa parental cell line for HeLa 3a-GFP series; TGN46 KO cell line for TGN46 antibody; EEA1 KO cell line for EEA1 antibody); (2) comparison with tagged proteins of interest using antibodies targeting the tags; and (3) multiple different antibodies targeting the same protein (e.g., two Flag antibodies and two S antibodies).
High-resolution fluorescent images were taken with a Nikon C2 point-scanning confocal microscope equipped with a Ti2-E inverted motorized microscope. Phase contrast and fluorescence imaging of live Vero E6 cells, parental cells, or with 3aCoV1-GFP or 3aCoV2-GFP were taken with the Nikon C2 confocal microscope equipped with a Tokai Hit Incubator System (37 °C with 5% (v/v) CO2) and a 60× objective lens (CFI60 Plan Apochromat Lambda) with phase contrast. Images were analyzed using Nikon NIS-Elements software with the built-in Nikon Denoise.ai module103. Except for what is specified above, other phase contrast and fluorescence imaging of live cells was taken with an EVOS M5000 Cell Imaging System (Thermo Fisher Scientific). For color blindness accessibility of confocal images, the red channel was pseudocolored to magenta; when the DAPI channel was not present, the far-red channel was also pseudocolored to blue.
Human normal primary bronchial tracheal epithelial cells were imaged with a Leica Stellaris 8 microscope using a HC PL ALO 63x/1.4 OIL STEDWHITE objective and Type F immersion oil (Leica Microsystems 11513859). Z-stack images were acquired using a stepsize of 0.06 µm and 3D reconstructed to highlight the 3DB structures viewed from different rotation angles.
TEM
Cells were washed with 0.1 M sodium cacodylate buffer and fixed with 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer at 4 °C for 2 h. The cells were post-fixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h. Cells were then rinsed with 0.1 M sodium cacodylate buffer twice and maleate buffer (pH 5.1) once, before being stained in 1% uranyl acetate in maleate buffer for 1 h. After removing uranyl acetate, cells were washed with maleate buffer three times. Cells were then dehydrated with increasing concentrations of ethanol (25%, 50%, 70%, 95%, and 100%), before infiltrated with propylene oxide (100% propylene oxide 3 × 15 min, 2:1 propylene oxide: spurr resin 2 × 30 min, 1:1 propylene oxide: spurr resin 2 × 30 min and overnight, and 100% spurr resin 6 × 60 min). Cells were then polymerized in a 60 °C oven for 1–2 days. Blocks were sectioned with Leica EM UC6 into 90 nm sections before being post-stained with uranyl acetate and lead citrate. Images were acquired on an FEI Tecnai F30 transmission electron microscope using a voltage of 300 kV in the UChicago Advanced Electron Microscopy Facility.
Immunoblotting
All uncropped and unprocessed scans of immunoblotting are provided in the Source Data file.
For cell extract immunoblotting, cells were washed with PBS and collected in lysis buffer A [10 mM Tris·HCl (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 0.2% NP-40, 1 mM DTT, protease inhibitor cocktail (Roche)]. Alternatively, for the viral protein synthesis assay, infected cells were collected in RIPA buffer (abcam ab156034) and centrifuged at 1000×g for 5 min to collect lysate. The lysates were run with a Bio-Rad Mini-PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad 1658005) and transferred with a Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad 1703930). The membranes were blocked with non-fat milk (Research Products M172001000) in Tris-buffered saline with Tween 20 (TBST) for 30 min, before incubating with primary antibodies, followed by HRP secondary antibodies. The specificity of antibodies was verified using one or multiple of these methods: (1) cells lacking expression of the protein of interest (e.g., HeLa parental cell line for GFP immunoblotting; mock infected cells for immunoblotting of viral genes); (2) comparison with tagged proteins of interest using antibodies targeting the tags (e.g., overexpressed viral genes for immunoblotting of viral genes); (3) multiple different antibodies targeting the same protein (e.g., two Flag antibodies and two S antibodies).
For virus stock immunoblotting, 3 × 105 plaque-forming units (PFU) of WT-Flag and 7Ala-Flag virus were concentrated with a methanol/chloroform method adapted from previous studies41,50. Briefly, the virus stocks were mixed with 500 μL methanol and 150 μL chloroform and vortexed for 30 s. The mixture was centrifuged at 10,000×g for 10 min before the upper aqueous phase was removed. Another 800 μL of methanol was added, and the mixture was centrifuged at 10,000×g for 10 min. The pellet was dried and dissolved in 15 μL 2× Laemmli Sample Buffer (Bio-Rad 161-0737, with 2-mercaptoethanol added) and boiled at 95 °C for 8 min and used for immunoblotting.
For the virion immunoblotting assay, Vero E6 cells were seeded at 1.0 × 106 cells/well in a 6-well plate, before mock infected (medium alone) or infected with the indicated rSARS-CoV-2 viruses at an MOI of 0.3 for 1 h in 1 mL of DMEM supplemented with 2% CS and 1% penicillin/streptomycin (PS). The cells were then washed with PBS three times to remove extracellular virus that had not entered the cells, before being incubated with 1 mL of Opti-MEM (Life Technologies) for another 23 h (for a total of 24-h of infection). Opti-MEM was used here to facilitate the subsequent concentration and collection of virion proteins due to its reduced serum level. The supernatant, which contained egressed virions, was collected and concentrated with the methanol/chloroform method detailed above for immunoblotting.
NLRP3 inflammasome activation
HeLa cells stably expressing NLRP3-GFP −/+ 3aCoV1-Flag or 3aCoV2-Flag were incubated −/+ nigericin (10 μM) in Opti-MEM (Thermo Fisher Scientific) at 37 °C in an atmosphere with 5% (v/v) CO2 for 80 min. Priming with TLR ligands (e.g., LPS) is not required for NLRP3 activation in this system due to the high expression level of NLRP341,104,105. The cells were then fixed and immunostained for Flag and TGN46. The formation of NLRP3 puncta on the dispersed TGN vesicles indicated NLRP3 activation41.
The in vitro assay was developed in our previous study to examine the activity of NLRP341. Two HEK-293T cell lines were set up that stably expressed NLRP3 (293 NLRP3) and the fusion protein ASCPYD-casp1p20-p10 (293 ASCPYD-casp1p20-p10), respectively. In this study, 3aCoV1 or 3aCoV2 was also stably expressed in 293 NLRP3 cells (‘Activator’). The ‘Activator’ cells were incubated −/+ nigericin (10 μM) at 37 °C in an atmosphere with 5% (v/v) CO2 for 60 min. Priming with TLR ligands (e.g., LPS) is not required for NLRP3 activation in this system due to the high expression level of NLRP341,104,105. The cells were then collected in lysis buffer B [10 mM Tris·HCl (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 0.05% NP-40, protease inhibitor cocktail (Roche)] and centrifuged at 1000×g for 5 min. Ten micrograms of ‘Activator’ cell extracts were then mixed with 106 cells from 293 ASCPYD-casp1p20-p10 (‘Recipient’) semi-permeabilized with 60 ng perfringolysin O (PFO). Additional lysis buffer B was added to reach a final volume of 10 μL. After incubation at 30 °C for 80 min, the reaction mixture was incubated with an additional 0.5% NP-40 on ice for 15 min before centrifugation at 20,000×g for 15 min. The supernatant was boiled in SDS loading buffer and used for caspase-1 immunoblotting to detect caspase-1 cleavage.
RAW 264.7 + ASC + hACE2-Flag cell line was established by stable expression of ASC and hACE2-Flag via lentiviral infection, and the expression of these two proteins was confirmed via ASC and Flag immunostaining. Since RAW 264.7 cells express endogenous NLRP3, priming with LPS (50 ng/mL) for 3 h was used to induce the expression of endogenous NLRP3, before the cells were incubated −/+ nigericin (20 μM) for 60 min. The cells were then fixed and immunostained for spike S2 and ASC.
Generation of recombinant SARS-CoV-2 (rSARS-CoV-2)
The design of rSARS-CoV-2 generated in this study was approved by the Centers for Disease Control and Prevention (CDC) and the Institutional Biosafety Committees at both UChicago and Texas Biomedical Research Institute. rSARS-CoV-2 containing 3aCoV2(WT)-Flag or 3aCoV2(7Ala)-Flag were generated using methods as previously described62–64 as detailed below. The viral genome of SARS-CoV-2 (USA/WA1/2020, GenBank MN985325, the same genome where 3aCoV2 gene in this study is derived) was chemically synthesized (Bio Basic) in five fragments. Flag tag and the indicated mutations were introduced into the 3aCoV2 gene in one of the fragments on the shuttle plasmid pUC57-F1. The whole genome was then assembled with Golden Gate cloning and inserted into the bacterial artificial chromosome (BAC) plasmid pBeloBAC11 (New England Biolabs). Silent mutations were introduced to S and M genes to remove BstBI and Mlul restriction sites, respectively. Confluent monolayers of Vero E6 cells (1.2 × 106 cells/well, 6-well plate format, triplicate) were transfected using Lipofectamine 2000 (Thermo Fisher Scientific 11668019) with 4 μg/well of the pBeloBAC11 plasmid. At 14 h post-transfection, the medium was replaced with infection medium (DMEM with 2% fetal bovine serum (FBS)). At 24 h post-transfection, cells were scaled up into T75 flasks. At 72 h post-transfection, passage (P)0 virus-containing cell culture supernatants were collected and stored at −80 °C. Viral rescues were confirmed by infecting fresh Vero E6 cells (1.2 × 106 cells/well, 6-well plates, triplicate). P0 viruses were passaged twice to P2 with Vero E6-TMPRSS2-T2A-ACE2 cells to generate virus stocks used for this study. rSARS-CoV-2/Δ3a was generated and verified in a previous study21.
rSARS-CoV-2 RNA extraction, sequencing, and analysis
RNA of rSARS-CoV-2 P2 stocks was extracted from a propagation stock following the standard procedure of TRIzol (Invitrogen). The extracted RNA samples were then submitted to the UChicago Genomics Facility. RNA quality and quantity were assessed using the Agilent bio-analyzer. Strand-specific RNA-SEQ libraries were prepared using a TruSeq-based mRNA RNA-SEQ library protocol (Illumina). For this project, mRNA selection was skipped. Library quality and quantity were assessed using the Agilent bio-analyzer, and libraries were sequenced using an Illumina NovaSEQ6000 (Illumina provided reagents and protocols). The RNA sequencing data were then analyzed by the UChicago CRI Bioinformatics Core with the nf-core/sarek pipeline (https://nf-co.re/sarek). Alignment was performed with BWA-mem (0.7.17). Duplicates were marked with MarkDuplicates. Variants were called using GATK HaplotypeCaller and filtered with CNNScoreVariants.
SARS-CoV-2 infection
All SARS-CoV-2 infection experiments were performed according to experimental protocols approved by the Institutional Biosafety Committee at UChicago. SARS-CoV-2 (USA-WA1) was provided by the National Biocontainment Laboratory, Galveston, TX. rSARS-CoV-2 viruses were generated with methods detailed above. SARS-CoV-2 and rSARS-CoV-2 infections were performed in a biosafety level 3 laboratory at the Howard T. Ricketts Regional Biocontainment Laboratory, University of Chicago, located in Argonne National Laboratory.
For primary human cell infection, cells were cultured at 30% confluence on coverslips in a 24-well plate for 24 h prior to infection. On the day of infection, cells were incubated with the indicated rSARS-CoV-2 virus at an MOI of 5 for 1.5 h at 37 °C, before the medium was removed and the cells were incubated with primary cell medium (recipe detailed in Mammalian cell culture section). Cells were incubated at 37 °C for a total of 72 h, before being fixed for imaging.
Plaque assay
Viral titers (in PFU/mL) were determined by plaque assay in Vero E6 cells as described previously106 with methods detailed below. Briefly, Vero E6 cells were seeded into 24-well plates at 95–100% confluency and inoculated with serial 10-fold dilutions of rSARS-CoV-2. Cells were incubated at 37 °C and shaken every 10 min for 1 h before the inoculum was replaced with serum-free MEM, supplemented with 1.25% carboxymethylcellulose. At 72 h post-infection (hpi), cells were fixed with 10% formalin for 15 min and stained with 1% crystal violet in 20% ethanol for 15 min. The wells were then rinsed with water and dried overnight. Plaques were counted, and viral titers were calculated. Plaque size was measured and quantified using Fiji (ImageJ 1.53t). Duplicate plates were performed for each experiment, and representative results from at least three independent experiments were shown. The order of viruses was switched in different experiments to ensure that the differences were not caused by the edge effect of plates.
Measurement of spike-positive cells
Vero E6 cells were seeded at 1.0 × 106 cells/well in a 6-well plate, before being infected with rSARS-CoV-2 (WT-Flag and 7Ala-Flag) at an MOI of 0.1 for 24 h. The virus stock titers were re-measured at the same time as the experiments to confirm that the two viruses were maintained at the same titer. The cells were then digested with trypsin, suspended into single cells, and washed with PBS. The cells were fixed with 10% formalin and permeabilized/blocked with FACS buffer [PBS, 4% FBS, 1 mM EDTA, 0.2% saponin], before being incubated with spike S2 antibody (1:2000) overnight and Alexa Fluor 568 secondary antibody (1:200) for 1 h. The cells were then sorted by flow cytometry (FACSMelody™ Cell Sorter, BD Biosciences). Forward scatter height (FSC-H) vs side scatter height (SSC-H) and SSC-H vs side scatter area (SSC-A) gating strategy was used to remove cell debris and clumps, respectively. Spike-positive cells were analyzed with FlowJo software to measure the percentage of infected cells in each group.
Measurement of extracellular and cell-associated titers
Vero E6 cells were seeded at 2.5 × 105 cells/well in a 24-well plate at the time of the experiment. The cells were infected with rSARS-CoV-2 (WT-Flag and 7Ala-Flag) at an MOI of 0.1 for 1 h in 300 μL DMEM supplemented with 10% FBS. The virus stock titers were re-measured at the same time as the experiments to confirm that the two viruses were maintained at the same titer. After 1 h of infection, the medium was removed, and the cells were washed with PBS twice to remove residual free virus. The cells were then incubated in 300 μL fresh DMEM supplemented with 10% FBS for another 23 h for a total of 24-h of infection. The supernatant was collected and centrifuged at 1000×g for 3 min to remove residual cells and used for plaque assay with methods detailed above to determine the extracellular viral titers. The cells attached to wells were washed with PBS twice to remove residual free virus, before 300 μL fresh DMEM with 10% FBS was added. The cells were then subjected to three freeze-thaw cycles to completely lyse the cells (confirmed with transmitted light microscopy). The mixture was centrifuged at 1000×g for 3 min to remove cell debris, before being used for plaque assay with methods detailed above to determine the cell-associated viral titers. The plaque assays were performed on the same plate side-by-side. Four titer measurements were performed for each experiment. Representative results from at least four independent experiments are shown.
Real-time quantitative PCR (qPCR)
For Particle-to-PFU ratio measurement, RNA was extracted from virus stocks using NucleoSpin 96 RNA (Macherey-Nagel, 740709), before reverse transcription to cDNA using the GoScriptTM Reverse Transcriptase kit (Promega, A5001) following the manufacturer’s instructions. qPCR was performed using the PowerUpTM SYBRTM Green Master Mix kit (Thermo Fisher Scientific, A25742) with 0.1 μL of cDNA as a template on an Applied Biosystems StepOnePlusTM Real-Time PCR system (Thermo Fisher Scientific). SARS-CoV-2 nucleocapsid (N) RNA was measured using primers 5’-TTACAAACATTGGCCGCAAA-3’ and 5’-GCGCGACATTCCGAAGAA-3’. A standard curve was performed using pCC1-CoV2-F7107, which contains a single copy of the N gene. The viral genome copy was calculated based on the standard curve and normalized to the viral titers to calculate the particle-to-PFU ratio.
For the viral entry assay, Vero E6 cells were seeded at 2.5 × 105 cells/well in a 24-well plate at the time of the experiment. The cells were incubated with the indicated rSARS-CoV-2 virus with the similar virion number (1.37 × 107 virions per 2.5 × 105 cells, equivalent to an MOI of 0.1 for WT-Flag virus) for 1 h in 300 μL DMEM supplemented with 2% CS at 4 °C, which allowed the virus to bind to cells but prevented entry. The cells were then washed with cold PBS three times to remove unbound virus, before being incubated at 37 °C with pre-warmed Opti-MEM to allow viral entry. At 1 h and 2 h post entry (i.e., upon switching to 37 °C), the medium was removed, and cells were lysed for intracellular RNA extraction using TRIzolTM (Invitrogen, 15596018). Mock (medium alone) controls were performed for each time point. Reverse transcription and real-time qPCR were performed as detailed above to quantify the changes in intracellular SARS-CoV-2 N RNA level, with GAPDH as the housekeeping gene control using primers 5’-ATGACATCAAGAAGGTGGTG-3’ and 5’-CATACCAGGAAATGAGCTTG-3’. Because the mock infected samples had undetectable levels of viral N RNA, as expected, they cannot serve as the fold change comparison baseline. Instead, the results of this experiment were presented as fold change to the detection limit of the qPCR program (Ct = 40, i.e., the cycle number of the qPCR program). Viral genome copy numbers were also verified by extracting RNA from virus stocks as described above, and at the same time, the viral entry assay was performed.
For the viral RNA replication assay, Vero E6 cells were seeded at 2.5 × 105 cells/well in a 24-well plate at the time of the experiment. The cells were incubated with the indicated rSARS-CoV-2 viruses at an MOI of 0.05 for 1 h in 300 μL DMEM supplemented with 2% CS and 1% PS at 37 °C. The cells were washed with PBS three times to remove extracellular virus that had not entered the cells. The cells were incubated with fresh Opti-MEM at 37 °C (except for 1 hpi samples, which were collected immediately). At 6 hpi and 24 hpi, cells were lysed for intracellular RNA extraction using TRIzolTM, reverse transcription, and real-time qPCR as detailed above. The results of 6 hpi and 24 hpi were presented as fold change to the 1 hpi sample of WT-Flag virus to reflect the viral RNA replication levels.
Statistics and quantification
Representative results from at least three independent experiments are shown for every figure unless specified otherwise in the figure legends. The sample sizes were selected based on power analysis of results from pilot experiments, which show that the selected sample sizes were not only sufficient to obtain a desirable significance level (<0.01) and power (>90%) but were also able to generate highly reproducible results with biological replicates. Data are presented as mean ± s.d. Statistical analysis was performed using two-sided or one-sided t-test in GraphPad Prism 9.2.0. One-sided t-test was only used when necessary to address the question asked (e.g., whether there was an increase instead of a decrease in areas containing TGN46-positive structures). Statistical significance was determined with the Holm-Sidak method, with α = 0.01. For blinding, imaging was performed before the sample types were mapped to wells or coverslips. For quantification of cells with the phenotype of interest, non-overlapping whole-field images were randomly taken throughout the slide of each sample. Only the DAPI channel was used during the random selection of whole-field images to avoid bias in the selection of cells with particular phenotypes before other channels were imaged. For quantification of TGN or cis/medial-Golgi marker dispersion, cells undergoing mitosis (based on chromosome morphology in the DAPI channel) were excluded from the quantification because these cells had mitotic Golgi disassembly. Forty cells per sample were randomly selected by the DAPI channel. For each cell, a region of interest (ROI) containing the entire TGN (or cis/medial-Golgi)-marker-positive structures was selected in Fiji (ImageJ 1.53t) and measured. Similar methods were used to quantify the ratio of 3DB area to whole-cell area, the number of 3DBs, the diameter of 3DBs, and the ratio of 3DBs loaded with viral structural proteins. For the percentage of cells with NLRP3 puncta formation, the number of cells with the phenotype of interest was recorded from 100 cells (randomly selected by DAPI channel) per sample, and this process was repeated a total of three times for quantification. For colocalization analysis, an ROI was selected to cover 3DBs in Fiji (ImageJ 1.53t), before Pearson’s correlation coefficient (threshold regression: Costes) was calculated using the Coloc 2 plugin.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description Of Additional Supplementary File
Source data
Acknowledgements
We thank Drs. Dominique Missiakas, Glenn Randall, Vlad Nicolaescu, and Derek Elli at the Howard Taylor Ricketts Laboratory, a Regional Biocontainment Laboratory supported by the National Institute of Allergy and Infectious Diseases (award UC7AI180312 to Dominique Missiakas), for support and assistance with BSL-3 research, Dr. Tatyana Golovkina for her support and guidance, Drs. Laimonis Laimins, Balaji Manicassamy for sharing plasmids, Yimei Chen at UChicago Advanced Electron Microscopy, UChicago Genomics Facility, and CRI Bioinformatics Core for technical assistance, and all Chen Lab members for their help and support. This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM151390 (J.C.).
Author contributions
J.C. conceived and directed the project. S.H., L.R., and J.C. designed and performed most of the experiments and analysis. C.Y. and L.M.-S. engineered and provided the recombinant SARS-CoV-2 viruses. S.H., L.R., and J.C. wrote and edited the manuscript.
Peer review
Peer review information
Nature Communications thanks Richard Zhao, Kefeng Lu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-59475-x.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int.
- 2.Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature579, 265–269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese, M. et al. Integrative imaging reveals SARS-CoV-2-induced reshaping of subcellular morphologies. Cell Host Microbe28, 853–866.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff, G., Melia, C. E., Snijder, E. J. & Bárcena, M. Double-membrane vesicles as platforms for viral replication. Trends Microbiol.28, 1022–1033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein, S. et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun.11, 5885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldsmith, C. S. et al. Ultrastructural characterization of SARS coronavirus. Emerg. Infect. Dis.10, 320–326 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snijder, E. J. et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol.80, 5927–5940 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoops, K. et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol.6, e226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.V’Kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol.19, 155–170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendonça, L. et al. Correlative multi-scale cryo-imaging unveils SARS-CoV-2 assembly and egress. Nat. Commun.12, 4629 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke, Z. et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature588, 498–502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui, J., Li, F. & Shi, Z. L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol.17, 181–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escalera, A. et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe30, 373–387.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, J. et al. TMPRSS2 expression dictates the entry route used by SARS‐CoV‐2 to infect host cells. EMBO J.40, e107821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papa, G. et al. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion. PLoS Pathog.17, e1009246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, Y. et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep.39, 110829 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng, C. et al. Neutralization and stability of SARS-CoV-2 omicron variant. Preprint at 10.1101/2021.12.16.472934 (2021).
- 19.Qu, P. et al. Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 BA.2.86 and FLip variants. Cell187, 585–595.e6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, B. A. et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature591, 293–299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvas, J. A. et al. Contribution of SARS-CoV-2 accessory proteins to viral pathogenicity in K18 human ACE2 transgenic mice. J. Virol.95, e0040221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boni, M. F. et al. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol.5, 1408–1417 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Hu, B., Guo, H., Zhou, P. & Shi, Z. L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol.19, 141–154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieva, J. L., Madan, V. & Carrasco, L. Viroporins: structure and biological functions. Nat. Rev. Microbiol.10, 563–574 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott, C. & Griffin, S. Viroporins: structure, function and potential as antiviral targets. J. Gen. Virol.96, 2000–2027 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Miller, A. N. et al. The SARS-CoV-2 accessory protein Orf3a is not an ion channel, but does interact with trafficking proteins. eLife12, e84477 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern, D. M. et al. Cryo-EM structure of SARS-CoV-2 ORF3a in lipid nanodiscs. Nat. Struct. Mol. Biol.28, 573–582 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su, W., Yu, X. & Zhou, C. SARS-CoV-2 ORF3a induces incomplete autophagy via the unfolded protein response. Viruses13, 2467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, D. et al. ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev. Cell56, 3250–3263.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrido-Huarte, J. L., Fita-Torró, J., Viana, R., Pascual-Ahuir, A. & Proft, M. Severe acute respiratory syndrome coronavirus-2 accessory proteins ORF3a and ORF7a modulate autophagic flux and Ca2+ homeostasis in yeast. Front. Microbiol.14, 1152249 (2023). [DOI] [PMC free article] [PubMed]
- 31.Stephens, E. B. et al. The Role of the Tyrosine-Based Sorting Signals of the ORF3a Protein of SARS-CoV-2 in Intracellular Trafficking and Pathogenesis. Viruses17, 522 (2025). [DOI] [PMC free article] [PubMed]
- 32.Miao, G. et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell56, 427–442.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solvik, T. A. et al. Secretory autophagy maintains proteostasis upon lysosome inhibition. J. Cell Biol.221, e202110151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stukalov, A. et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature594, 246–252 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Miserey-Lenkei, S. et al. A comprehensive library of fluorescent constructs of SARS-CoV-2 proteins and their initial characterisation in different cell types. Biol. Cell113, 311–328 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz-Cosme, R. et al. A novel diG motif in ORF3a protein of SARS-Cov-2 for intracellular transport. Front. Cell Dev. Biol.10, 1011221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart, H. et al. Tetherin antagonism by SARS‐CoV‐2 ORF3a and spike protein enhances virus release. EMBO Rep.24, e57224 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walia, K. et al. SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle. Nat. Commun.15, 2053 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michelucci, A. et al. SARS-CoV-2 ORF3a accessory protein is a water-permeable channel that induces lysosome swelling. Commun. Biol.8, 1–13 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo, Y., Sirkis, D. W. & Schekman, R. Protein sorting at the trans-Golgi network. Annu Rev. Cell Dev. Biol.30, 169–206 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Chen, J. & Chen, Z. J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature564, 71–76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell181, 1036–1045.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherer, K. M. et al. SARS-CoV-2 nucleocapsid protein adheres to replication organelles before viral assembly at the Golgi/ERGIC and lysosome-mediated egress. Sci. Adv.8, eabl4895 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto, T., Ohsaki, Y., Suzuki, M. & Cheng, J. Chapter 13—imaging lipid droplets by electron microscopy. in Methods in Cell Biology, Vol. 116 (eds. Yang, H. & Li, P.), 227–251 (Academic Press, 2013). [DOI] [PubMed]
- 45.Hess, M. W. & Huber, L. A. Chapter 5—measuring lysosomal size and frequency by electron microscopy. in Methods in Cell Biology, Vol. 164 (eds. Kepp, O. & Galluzzi, L.) 47–61 (Academic Press, 2021). [DOI] [PubMed]
- 46.Von Bartheld, C. S. & Altick, A. L. Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog. Neurobiol.93, 313–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy17, 1–382 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balla, T. & Varnai, P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE2002, pl3 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Pelegrin, P., Barroso-Gutierrez, C. & Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol.180, 7147–7157 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Gross, O. Measuring the inflammasome. Methods Mol. Biol.844, 199–222 (2012). [DOI] [PubMed] [Google Scholar]
- 51.He, W. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res.25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell156, 1193–1206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell156, 1207–1222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramakrishna, S., Padhi, S. & Priyakumar, U. D. Modeling the structure of SARS 3a transmembrane protein using a minimum unfavorable contact approach. J. Chem. Sci.127, 2159–2169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu, P. et al. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog.16, e1008421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, T., Wu, Q. & Zhang, Z. Probable Pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol.30, 1346–1351.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam, T. T. et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature583, 282–285 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science302, 276–278 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Jordan, I., Horn, D., Oehmke, S., Leendertz, F. H. & Sandig, V. Cell lines from the Egyptian fruit bat are permissive for modified vaccinia Ankara. Virus Res.145, 54–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu, B. et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog.13, e1006698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guidance on the Regulation of SARS-CoV/SARS-CoV-2 Chimeric Viruses. Compliance|Federal select agent program. https://www.selectagents.gov/compliance/guidance/chimeric/index.htm (2023).
- 62.Chiem, K., Ye, C. & Martinez-Sobrido, L. Generation of recombinant SARS-CoV-2 using a bacterial artificial chromosome. Curr. Protoc. Microbiol.59, e126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiem, K. et al. Generation and characterization of recombinant SARS-CoV-2 expressing reporter genes. J. Virol.10.1128/JVI.02209-20 (2021). [DOI] [PMC free article] [PubMed]
- 64.Ye, C. et al. Rescue of SARS-CoV-2 from a single bacterial artificial chromosome. mBio11, e02168–e021620 (2020). [DOI] [PMC free article] [PubMed]
- 65.Hackstadt, T. et al. Disruption of the golgi apparatus and contribution of the endoplasmic reticulum to the SARS-CoV-2 replication complex. Viruses13, 1798 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobayashi, T. et al. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell11, 1829–1843 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathieu, M. et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun.12, 4389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelli, R. K. et al. Enhanced apoptosis as a possible mechanism to self-limit SARS-CoV-2 replication in porcine primary respiratory epithelial cells in contrast to human cells. Cell Death Discov.7, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dolan, K. A. et al. Structure of SARS-CoV-2 M protein in lipid nanodiscs. Elife11, e81702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng, Y. et al. Structures of the SARS‐CoV‐2 nucleocapsid and their perspectives for drug design. EMBO J.39, e105938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li, Y. et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci.118, e2022643118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu, Y. et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature602, 294–299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiliaev, N. et al. Natural and recombinant SARS-CoV-2 isolates rapidly evolve in vitro to higher infectivity through more efficient binding to heparan sulfate and reduced S1/S2 cleavage. J. Virol. 10.1128/jvi.01357-21 (2021). [DOI] [PMC free article] [PubMed]
- 74.Pei, D. et al. Contribution of mitophagy to cell-mediated mineralization: revisiting a 50-year-old conundrum. Adv. Sci.5, 1800873 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter, M. & Shieh, J. Chapter 5—microscopy. in Guide to Research Techniques in Neuroscience (Second Edition) (eds. Carter, M. & Shieh, J.) 117–144 10.1016/B978-0-12-800511-8.00005-8 (Academic Press, San Diego, 2015).
- 76.Ghosh, S. et al. Beta-coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell183, 1520–1535.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen, I. Y., Moriyama, M., Chang, M. F. & Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol.10, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu, H. et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology568, 13–22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guarnieri, J. W. et al. SARS-COV-2 viroporins activate the NLRP3-inflammasome by the mitochondrial permeability transition pore. Front. Immunol.14, 1064293 (2023). [DOI] [PMC free article] [PubMed]
- 80.Rodrigues, T. S. et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med.218, e20201707 (2021). [DOI] [PMC free article] [PubMed]
- 81.Meizlish, M. L. et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv.5, 1164–1177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeng, J. et al. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine75, 103803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen, E. et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis.20, e238–e244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang, S. et al. Sequential glycosylations at the multibasic cleavage site of SARS-CoV-2 spike protein regulate viral activity. Nat. Commun.15, 4162 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas, G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol.3, 753–766 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong, Y., Qin, S., Dai, L. & Tian, Z. The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct. Target Ther.6, 396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang, Q. et al. Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. Elife9, e61552 (2020). [DOI] [PMC free article] [PubMed]
- 88.Shajahan, A., Pepi, L. E., Rouhani, D. S., Heiss, C. & Azadi, P. Glycosylation of SARS-CoV-2: structural and functional insights. Anal. Bioanal. Chem.413, 7179–7193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol.3, e61552 (2011). [DOI] [PMC free article] [PubMed]
- 90.Moremen, K. W., Tiemeyer, M. & Nairn, A. V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol.13, 448–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardenbrook, N. J. & Zhang, P. A structural view of the SARS-CoV-2 virus and its assembly. Curr. Opin. Virol.52, 123–134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai, Y. et al. Distinct conformational states of SARS-CoV-2 spike protein. Science369, 1586–1592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao, H. et al. Molecular architecture of the SARS-CoV-2 Virus. Cell183, 730–738.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turoňová, B. et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science370, 203–208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu, C. et al. The architecture of inactivated SARS-CoV-2 with postfusion spikes revealed by Cryo-EM and Cryo-ET. Structure28, 1218–1224.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ni, T. et al. ChAdOx1 COVID vaccines express RBD open prefusion SARS-CoV-2 spikes on the cell surface. iScience26, 107882 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bangaru, S. et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science370, 1089–1094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogando, N. S. et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol.101, 925–940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hossain, A., Akter, S., Rashid, A. A., Khair, S. & Alam, A. S. M. R. U. Unique mutations in SARS-CoV-2 Omicron subvariants’ non-spike proteins: potential impacts on viral pathogenesis and host immune evasion. Micro. Pathog.170, 105699 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hassan, Sk, S. et al. The importance of accessory protein variants in the pathogenicity of SARS-CoV-2. Arch. Biochem. Biophys.717, 109124 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzou, P. L., Tao, K., Pond, S. L. K. & Shafer, R. W. Coronavirus resistance database (CoV-RDB): SARS-CoV-2 susceptibility to monoclonal antibodies, convalescent plasma, and plasma from vaccinated persons. PLoS One17, e0261045 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA114, E4612–E4620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davis, M. Application note: Nikon NIS-elements Denoise.ai software: utilizing deep learning to denoise confocal data. Nat. Res. Customhttps://www.nature.com/articles/d42473-019-00355-6 (2019).
- 104.Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem.287, 36617–36622 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression1. J. Immunol.183, 787–791 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drayman, N. et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science373, 931–936 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie, X. et al. Engineering SARS-CoV-2 using a reverse genetic system. Nat. Protoc.16, 1761–1784 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description Of Additional Supplementary File
Data Availability Statement
Source data are provided with this paper.