Abstract
Impaired adult hippocampal neurogenesis is a key pathological mechanism contributing to memory deficits in Alzheimer’s disease (AD). Recent studies have shown that elevating magnesium levels promotes neurogenesis by enhancing the neuronal differentiation of adult neural progenitor cells in vitro. Therefore, this in vivo study aims to determine if magnesium-L-threonate (MgT) can ameliorate cognitive deficit of AD mice by attenuating adult hippocampal neurogenesis impairment and to reveal the underlying mechanisms. APPswe/PS1dE9 mice were treated with different doses of MgT and ERK inhibitor PD0325901. The memory ability of each mouse was recorded by Morris Water Maze test. After cognitive test, hippocampus tissues were collected to measure the proportion of BrdU/doublecortin double-labeled cells using the flow cytometry test and assess the expression of doublecortin using PCR and Western blot. Furthermore, the activations of CREB, ERK, P38 and JNK were measured by Western blot to identify the involved mechanisms. The cognitive test confirmed that MgT treatment attenuated the memory impairment of APPswe/PS1dE9 mice. Flow cytometry test showed that Brdu/doublecortin labeled newborn neurons gradually increased following MgT administration. In line with the flow cytometry results, Western blot and PCR confirmed that MgT administration significantly increased doublecortin expression levels. Furthermore, the ratios of p-ERK/ERK and p-CREB/CREB increased with MgT elevation. In addition, these effects of MgT treatment were markedly reversed by PD0325901 supplementation. In conclusion, MgT treatment improved cognitive decline by ameliorating adult hippocampal neurogenesis impairment in this AD model, possibly via ERK/CREB activation.
Keywords: Alzheimer’s disease, Neurogenesis, Cognitive decline, Magnesium
INTRODUCTION
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disorder, which often affects the people aged 65 years and over, and is featured clinically by cognitive decline and pathologically by β-amyloid (Aβ) deposition, a considerable neuronal loss and neurofibrillary tangles formation in AD brain [1-4]. Considering the limited effectiveness of current anti-AD drug therapies and the increasing prevalence of this disease, new technology which can effectively delay and suppress the clinical symptoms of AD has become a hot research spot [5, 6]. Although numerous researches demonstrated that Aβ targeting drug could be a potential therapy for AD, Aβ plaque accumulation did not appear sufficient for developing AD-associated cognitive decline and brain atrophy as aggressive anti-Aβ treatment failed to prevent AD progression [7, 8].
Adult hippocampal neurogenesis, originating from neural progenitor cells (NPCs), is a continuous and lifelong process which is affected by various environmental, genetic and pathological factors, resulting in generating newborn neurons in the dentate gyrus area of hippocampus tissues [9, 10]. Numerous research indicated that newborn neurons, which were generated during adult hippocampal neurogenesis process, played a vital role in maintaining the memory ability of brain, therefore dysfunction of adult hippocampal neurogenesis could lead to the cognitive deficit in AD [11-13]. Gradual neuronal loss is an important hallmark of AD progression, which was closely related with the deterioration of cognitive function [14, 15]. However, previous literatures indicated that hippocampal neurogenesis was significantly reduced in AD patients, which would further worsen the neuronal loss and cognitive decline in these patients [16, 17]. Therefore, validated effective methods are expected to promote newborn neurons generation by ameliorating adult hippocampal neurogenesis impairment, which might present a promising strategy to inhibit cognitive deficit in AD.
Magnesium is an essential cation that plays a critical role in various enzymatic reactions necessary for maintaining physiological functions, including memory and physical performance. Moreover, recent research has demonstrated that the elevation of magnesium concentration is effective in regulating the important process of neurogenesis by promoting NPCs to differentiate more into neurons and less into astrocytes via activating ERK/CREB in vitro [9]. However, the promotive effect of magnesium on adult neurogenesis in vivo remains to be elucidated. As impairment in adult hippocampal neurogenesis was confirmed to be a critical factor associated with cognitive decline in AD [18, 19], it has become attractive to determine whether elevating brain magnesium concentration can ameliorate cognitive deficit by regulating hippocampal neurogenesis in AD models.
Although recent study has explored the protective effects of the novel magnesium compound magnesium-L-threonate (MgT), which elevates brain magnesium levels, in inhibiting synaptic loss and preserving cognitive function in an AD mouse model [20], the impact of MgT on neurogenesis remains unknown. Furthermore, accumulating evidences suggested the critical roles for ERK and CREB in the process of neurogenesis, while other pathways including MAPK signaling pathways except for ERK pathway, such as P38 MAPK and JNK pathways, are also needed to be investigated [21-23].
Therefore, this study aimed to explore whether magnesium-L-threonate (MgT), a novel magnesium compound capable of elevating the concentration of brain magnesium via oral administration [24, 25], can ameliorate cognitive deficit by attenuating adult hippocampal neurogenesis impairment in an AD mouse model, and its possible mechanisms.
MATERIALS AND METHODS
Materials
MgT was purchased from Macklin (Shanghai, China). PD0325901 was obtained from APExBIO Technology LLC (Houston, USA). The involved primary and secondary antibodies were acquired from Cell Signaling Technology Company (Danvers, USA) for Western blotting test. In the flow cytometry (FCM) test, reagents were all purchased from Abbkine (Wuhan, China) except the primary antibodies against doublecortin (DCX) and BrdU, which were obtained from Cell Signaling Technology and Abcam (Cambridge, USA). For the real-time PCR (RT-PCR) analysis, the involved primers were made by Genomics Industry (Beijing, China). Other reagents for RT-PCR were obtained from Takara (Tokyo, Japan). All other experimental reagents were purchased from CWBIO (Beijing, China), Thermo-fisher (Waltham, USA), Invitrogen (Carlsbad, USA) and Millipore corporation (Billerica, USA).
Mice and MgT treatments
Fifteen APPswe/PS1dE9 (APP/PS1) 5-month-old, and 3 age-matched wild-type litter-mate male mice (WT mice) were all obtained from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China) and used throughout this study. The experiment involving mice complied with the protocol of the local committee of ethics for animal experiments.
Previous studies have demonstrated that oral administration of MgT (910 mg/kg/day) effectively increases brain magnesium levels [20], while PD0325901 (10 mg/kg/day) inhibits ERK activation in a mouse model [26]. Based on these findings, the APP/PS1 mice were divided into five groups equally. Four groups received different doses of MgT via drinking water daily and different doses of ERK inhibitor PD0325901 diluted in vehicle solution (0.5% hydroxypropyl methylcellulose+0.2% Tween 80) via oral gavage every four days for three months: LM group (455 mg/kg/day MgT), HM group (910 mg/kg/day MgT), MLP group (910 mg/kg/day MgT and 5 mg/kg/day PD0325901), MHP group (910 mg/kg/day MgT and 10 mg/kg/day PD0325901). The remaining three APP/PS1 mice (referred to as ‘TG group’) received drinking water daily and oral gavage administration of vehicle solution every 4 days for 3 months, served as positive control group. All WT mice (referred to as ‘WT group’) also received drinking water daily and oral gavage administration of vehicle solution every 4 days for 3 months, served as negative control group.
Morris water maze (MWM)
The memory ability of each mouse was tested using the MWM test reported previously [2]. This MWM test contains three protocols: a first-day visible platform trail, a 5-day orientation navigation training and a last-day memory retention test. At the beginning, mice were placed into the pool, guided to and kept on the visible platform for 20 seconds. This trail session was followed by orientation navigation training for 5 days with four trails per day (90 seconds per trail). During the trail, each mouse was put into the water to swim for 90 seconds, at one of four starting quadrants respectively. If the mouse found the hidden platform within 90 seconds and stayed there for 3 seconds, this trail was terminated and the exploration time for locating the platform was recorded as escape latency. The mice would be guided to stay on the hidden platform for 20 seconds when it was failed to discover the target platform within 90 seconds, and the escape latency of 90 seconds was given. For each mouse, the mean escape latencies of four quadrants were calculated. The memory retention test was conducted 24 hours after the end of orientation navigation experiment. During the memory retention test, the platform was removed and the mouse was placed into the pool to swim for 90 seconds. For each mouse, the time spent in the target quadrant (the previous location of the removed platform), the latency to arrive in the removed platform and the numbers of platform crossings were recorded.
FCM analysis
As an essential protein for the migration of neuron, DCX has been widely utilized as the marker of newborn neuron to assess the number of the newborn neurons during the neurogenesis process [27]. Furthermore, BrdU probed newly generated cells and the FCM test for BrdU/DCX labeling was performed to analyze the extent of newborn neuron generation as well as reflect the level of neurogenesis in the hippocampus based on the previously described method [28-30]. In short, each mouse was injected intraperitoneally with BrdU for one day with four injections (50 mg/kg per injection at a 2-hours interval). At 24 hours after the end of BrdU injection, the mouse was anesthetized using 1% pentobarbital sodium, and the removed brain was collected, finally the hippocampus tissues were isolated from the brain. Half the hippocampus was used for the FCM test and the remaining half of the hippocampus was prepared for Western blot analysis and RT-PCR test. In the FCM test, the hippocampus tissues of each mouse were dissociated into single cell suspension, incubated with anti-DCX and anti-BrdU primary antibodies, and then labeled with AlexaFluor 594 and AlexaFluor 488 secondary antibodies prior to the FCM detection of the BrdU/DCX double-labeled cells. Finally, the percentage of BrdU+/DCX+ cells was detected.
Quantitative RT-PCR
Total RNA of the hippocampus tissues in each mouse was extracted to measure the mRNA expression level of DCX (marker of newborn neuron). The quantified cDNA was collected and then RT-PCR was conducted with Roche LightCycler96 PCR Detection System (Basel, Switzerland) using cDNA, SYBR premix Ex Taq and appropriate primers as previously described [23]. The forward and reverse primers for DCX [31] and GAPDH [32] were as follows: DCX (5 ’- CCTTGGATGAGAATGAATGC -3 ’ and 5 ’-TTTGCGTCTTGGTCGTTA-3 ’) and GAPDH (5 ’-CGTGCCGCCTGGAGAAACCTG-3 ’ and 5 ’-AGAGTGGGAGTTGCTGTTGAAGTCG-3 ’). The expression level of the target gene was measured using the previously described method [33].
Western blot analysis
Hippocampus tissues of each brain were homogenized and an equal amount of protein was loaded on polyvinylidene fluoride membrane and probed with a serial of primary antibodies. After incubated with secondary antibodies, the protein band was visualized and the relative density of target protein was calculated using Image J software as previously reported [23].
Statistical analysis
Data are presented as mean±SEM. Comparisons among groups were measured by the one-way ANOVA followed by Fisher’s LSD test using the Prism 6.0 software (San Diego, USA). p<0.05 was indicated significant.
RESULTS
MgT treatment attenuated memory deficit in AD mouse
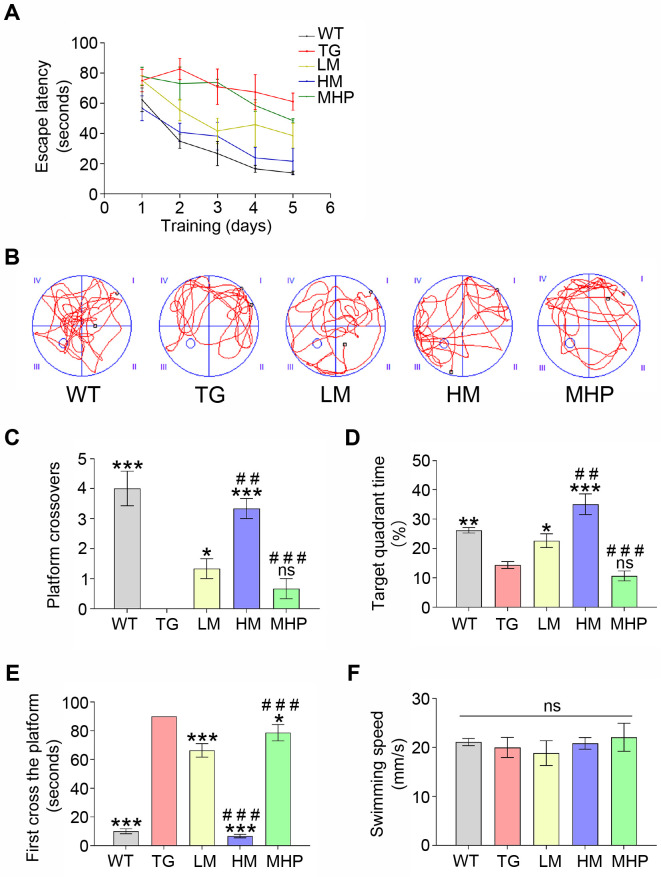
The APP/PS1 mouse was selected as a typical model of AD that exhibited severe deficits in memory ability [34]. We tested whether MgT treatment could prevent cognitive decline in AD mice by the MWM test. No obvious difference was discovered in the average swimming speed among all groups (all p>0.05), indicating that the physical influence on this test was excluded (Fig. 1F). During the orientation navigation experiment, WT group exhibited a gradual decrease in the escape latency over the consecutive 5 days of training. Although TG group exhibited a slight decline in the escape latency over time, it still showed a longer escape latency than WT group. The escape latencies of MgT-treated mice (LM and HM groups) were substantially decreased compared to that of TG group. Also, high-dose MgT treatment was shown to be more efficient to shorten the escape latency than the low-dose administration (Fig. 1A). During the memory retention test, TG group exhibited obvious lower numbers of platform crossing than WT group (p<0.001). Compared with TG group, LM group showed more times crossing the removed platform (p<0.05). The data of platform crossings also indicated significant differences between LM group and HM group (p<0.01) (Fig. 1B, C). Accordingly, TG group spent obvious less time in the target quadrant than WT group (p<0.01), whereas LM group spent more time than TG group (p<0.05). A significant difference was discovered between LM group and HM group in the target quadrant exploration time (p<0.01) (Fig. 1D). Compared with WT group, the latency required to arrive in the removed platform was obviously longer in the TG group, and LM group had a shorter latency than TG group (both p<0.001). A significant difference regarding the latency also existed in LM group versus HM group (p<0.001) (Fig. 1E).
Fig. 1.
MgT treatments ameliorated the cognitive decline of AD mouse. (A) The escape latency of each group. (B) Representative swimming route explored the removed platform of each group. (C) The numbers of platform crossings of each group. (D) The percentage of target quadrant exploration time of each group. (E) The time arrived at the removed platform of each group. (F) The average swimming speed of each group. MgT, magnesium-L-threonate; WT, wild type; TG, transgenic control; LM, low-dose MgT; HM, high-dose MgT; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). Ns represented no statistical difference. *p<0.05, **p<0.01, ***p<0.001 versus TG group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
Effect of MgT administration on DCX expression in AD mouse
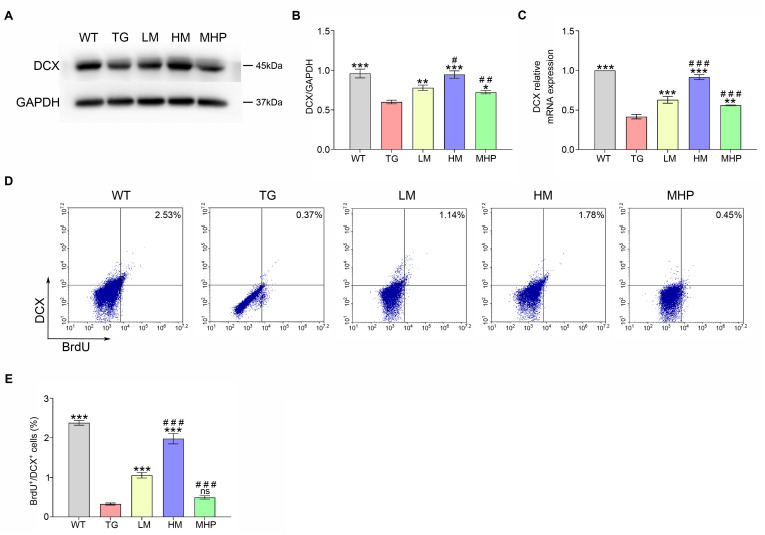
RT-PCR, Western blotting and FCM test were performed on mice treated with different doses of MgT to assess the effects of MgT administration on the expression of DCX (marker of newborn neuron). The Western blot results indicated that TG group had significantly lower expression level of DCX protein than WT group (p<0.001). Treatments with MgT (LM group) exhibited higher protein expression levels of DCX than TG group (p<0.01). A significant difference regarding the expression level of DCX protein also existed in LM group versus HM group (p<0.05) (Fig. 2A, B). In agreement with the Western blot data, the RT-PCR result showed that the expression level of DCX mRNA was lower in the TG group than that in the WT group (p<0.001). The expression level of DCX mRNA was significantly higher in LM group versus TG group (p<0.001). An obvious difference was present between LM group and HM group in DCX mRNA expression (p<0.001) (Fig. 2C). Furthermore, the FCM test indicated that compared with WT group, the percentage of BrdU+/DCX+ cells was obviously decreased in the TG group (p<0.001), and the percentage of these cells gradually increased with MgT treatment (both p<0.001) (Fig. 2D, E).
Fig. 2.
MgT facilitated the DCX expression and hippocampal newborn neurons generation of AD mouse. (A, B) The DCX protein expression of each group. (C) The DCX mRNA expression of each group. (D, E) The percentage of BrdU+/DCX+ cells of each group. DCX, doublecortin; MgT, magnesium-L-threonate; WT, wild type; TG, transgenic control; LM, low-dose MgT; HM, high-dose MgT; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). Ns represented no statistical difference. *p<0.05, **p<0.01, ***p<0.001 versus TG group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
Effects of MgT treatment on the activation of CREB and MAPK signaling pathway-related major molecules
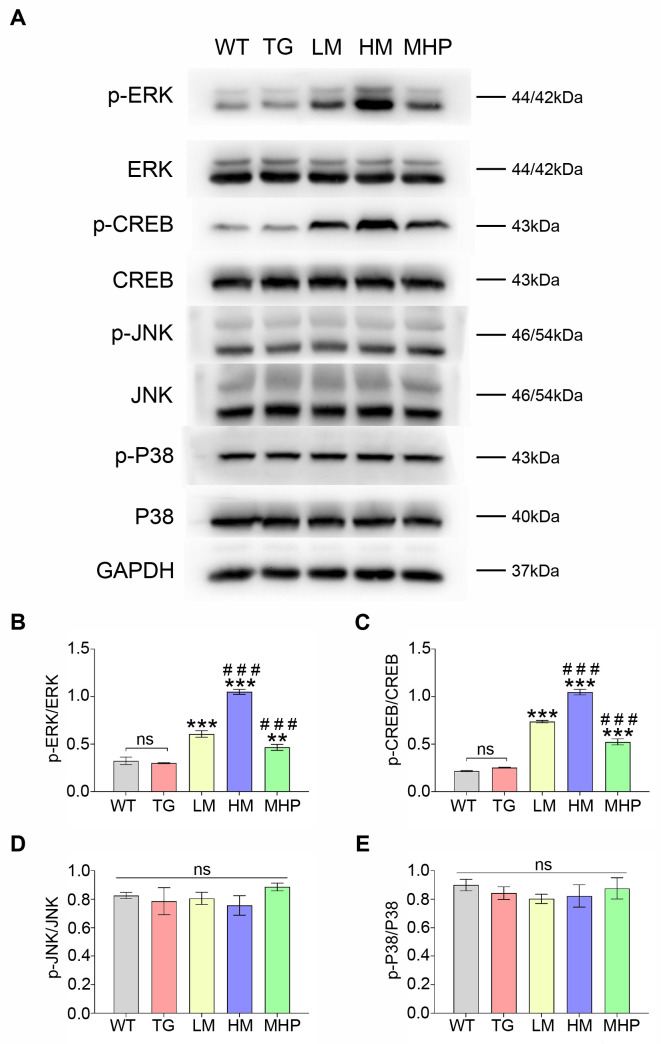
To investigate the anti-AD mechanism of MgT, this study assessed the effects of MgT on the activations of CREB, ERK, P38 and JNK. As shown in Fig. 3A, MgT treatment stimulated the phosphorylation of both ERK and CREB without changing the total protein level of both ERK and CREB in AD mice. The ratios of p-ERK/ERK and p-CREB/CREB were increased in the LM group versus TG group (both p<0.001). The p-ERK/ERK and p-CREB/CREB ratios were significantly different between LM group and HM group (both p<0.001). The p-ERK/ERK and p-CREB/CREB ratios were non-significant between WT and TG groups (p-ERK/ERK, p=0.59; p-CREB/CREB, p=0.23) (Fig. 3B, C). Conversely, no obvious differences regarding the ratios of p-JNK/JNK and p-P38/P38 were found in all groups (all p>0.05) (Fig. 3D, E).
Fig. 3.
MgT treatment promoted the activations of ERK and CREB while didn’t exhibit effects on the activations of P38 and JNK. (A) Western blot data of p-ERK, ERK, p-CREB, CREB, p-JNK, JNK, p-P38 and P38 of each group. (B) The p-ERK/ERK ratio of each group. (C) The p-CREB/CREB ratio of each group. (D) The p-JNK/JNK ratio of each group. (E) The p-P38/P38 ratio of each group. MgT, magnesium-L-threonate; WT, wild type; TG, transgenic control; LM, low-dose MgT; HM, high-dose MgT; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). Ns represented no statistical difference. *p<0.05, **p<0.01, ***p<0.001 versus TG group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
Pivotal role of ERK/CREB activation in MgT-mediated prevention of memory deterioration in AD mouse
To explore the role of ERK/CREB activation in MgT-mediated restoration of adult hippocampal neurogenesis impairment and cognitive decline, experiments were carried out using AD mice treated with ERK inhibitor PD0325901 in the presence of MgT. As shown in Fig. 3A~C, p-ERK/ERK and p-CREB/CREB ratios were lower in MHP group versus HM group (both p<0.001). Consistent with this, MHP group exhibited lower percentage of BrdU+/DCX+ cells and DCX expression than those of HM group (all p<0.01) (Fig. 2). Furthermore, MHP group had longer escape latency, longer latency to the removed platform, less times crossing the removed platform and less target quadrant exploration time than HM group (all p<0.001) (Fig. 1).
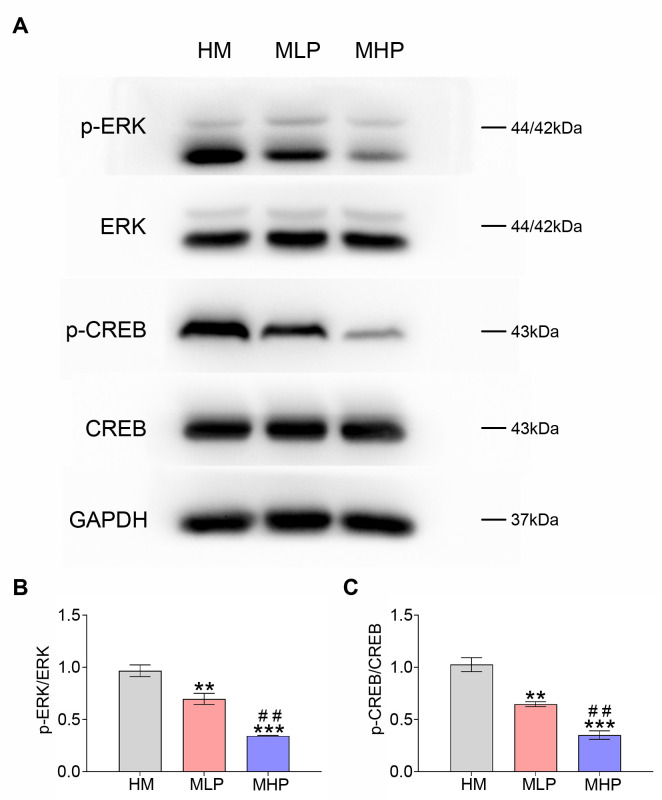
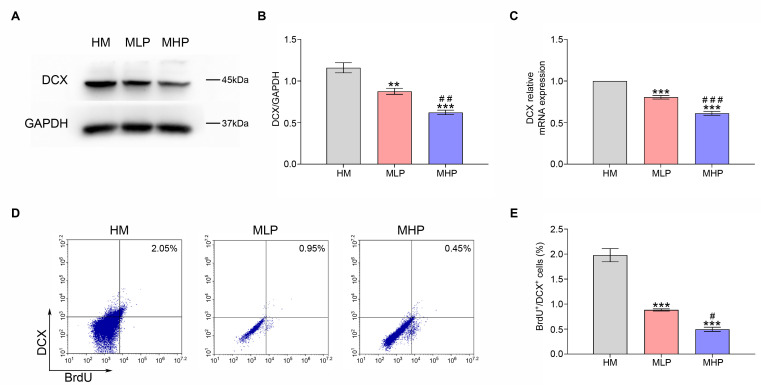
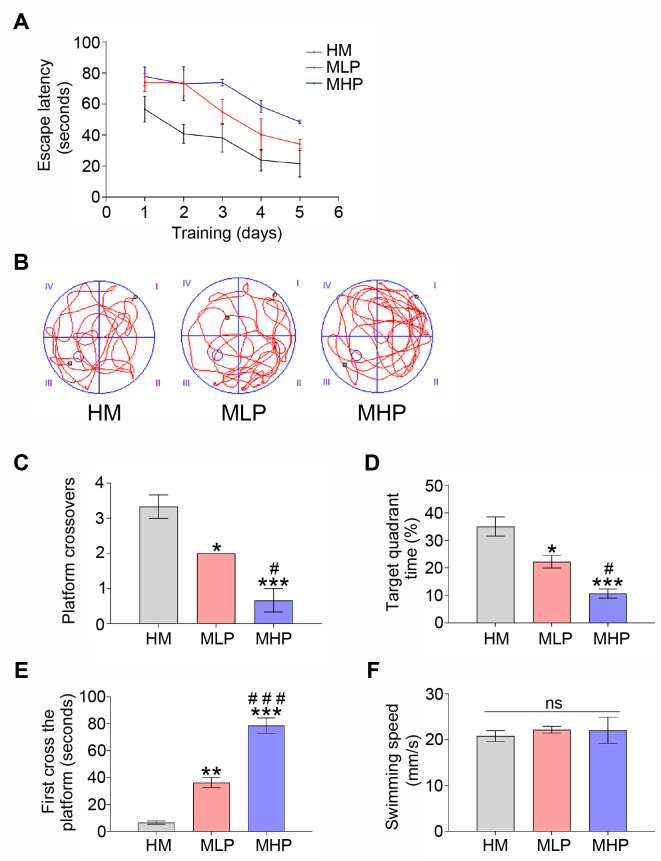
The dose-response experiments of PD0325901 (an ERK inhibitor) were carried out to further demonstrate the vital role of ERK and CREB activations in the protective effects of MgT administration against adult hippocampal neurogenesis impairment and cognitive deficit in this AD model. As shown in Fig. 4A, PD0325901 supplementation was found to inhibit the phosphorylation of ERK and CREB. The ratios of p-ERK/ERK and p-CREB/CREB measured by Western blot were obviously decreased in MLP group when compared to HM group (both p<0.01). Obvious differences were also found between MLP group and MHP group for these ratios (both p<0.01) (Fig. 4B, C). Consistent with this, DCX expression and the percentage of BrdU+/DCX+ cells gradually decreased following PD0325901 treatment (Fig. 5). MWM data demonstrated that the escape latency and the latency to locate the target zone were both gradually increased with the increase in PD0325901 dose (Fig. 6A, E). Treatment with PD0325901 (MLP group) was also found to markedly decrease both the platform crossings and the target quadrant exploration time compared to HM group (both p<0.05). There were also significant differences between MLP group and MHP group in crossings and target quadrant time (both p<0.05) (Fig. 6B~D).
Fig. 4.
PD0325901 treatment reversed the activations of both ERK and CREB induced by MgT administration. (A) Western blot data of p-ERK, ERK, p-CREB and CREB of each group. (B) The p-ERK/ERK ratio of each group. (C) The p-CREB/CREB ratio of each group. MgT, magnesium-L-threonate; HM, high-dose MgT; MLP, high-dose MgT + low-dose PD0325901; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). *p<0.05, **p<0.01, ***p<0.001 versus HM group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
Fig. 5.
PD0325901 treatment reversed the effects of MgT on DCX expression and hippocampal newborn neuron generation of AD mouse. (A, B) The DCX protein expression of each group. (C) The DCX mRNA expression of each group. (D, E) The percentage of BrdU+/DCX+ cells of each group. DCX, doublecortin; MgT, magnesium-L-threonate; HM, high-dose MgT; MLP, high-dose MgT+low-dose PD0325901; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). *p<0.05, **p<0.01, ***p<0.001 versus HM group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
Fig. 6.
PD0325901 treatment reversed the effect of MgT on cognitive deficit of AD mouse. (A) The escape latency of each group. (B) Representative swimming route explored the removed platform of each group. (C) The numbers of platform crossings of each group. (D) The percentage of target quadrant exploration time of each group. (E) The time arrived at the removed platform of each group. (F) The average swimming speed of each group. MgT, magnesium-L-threonate; HM, high-dose MgT; MLP, high-dose MgT+low-dose PD0325901; MHP, high-dose MgT+high-dose PD0325901. Data were given as mean±SEM (n=three per group). Ns represented no statistical difference. *p<0.05, **p<0.01, ***p<0.001 versus HM group; #p<0.05, ##p<0.01, ###p<0.001 versus former group.
DISCUSSION
AD is a chronic neurodegenerative disorder and effective treatment is urgently needed. The etiology of AD has remained largely unexplored, but age-related, lifestyle-related, genetic, and disease-promoting factors may be involved in the impairment of cognitive ability [5]. Accumulating evidences demonstrated that impaired adult hippocampal neurogenesis was an important pathological mechanism of AD, which was closely related with cognitive deficit in this disease [35, 36]. Recent studies have confirmed that newborn neurons originating from hippocampal neurogenesis are vital for maintaining brain’s cognitive ability, therefore impaired adult hippocampal neurogenesis would worsen the neuronal loss and cognitive decline in AD [37, 38]. Therefore, treatment to attenuate adult hippocampal neurogenesis impairment might present a promising method to inhibit cognitive deficit in AD.
Therefore, this study aimed to test if MgT could ameliorate cognitive impairment by attenuating adult hippocampal neurogenesis impairment in an AD mouse model, and to explore the underlying mechanisms. This study chose the APP/PS1 mice as the typical animal model of AD which had been widely used for exploring the mechanism and developing new treatment strategies to prevent AD progression. Recent literatures indicated that this AD mouse model had the advantage of developing AD-like pathologies, such as neuronal loss, hippocampal neurogenesis deficit and AD-associated cognitive impairment during the progression of this disease [3, 39]. In this study, the MWM test helped to confirm the cognitive decline in this AD model. Compared with WT group, the behavioral data showed that the TG group had difficulty in learning to find the timesaving swimming routes to arrive in the target platform, as confirmed by obviously increased escape latency in the 5-day orientation navigation experiment. In addition, TG group also showed deficit in memory retention, as indicated by a worse performance in the memory retention test, when compared to WT group. Furthermore, AD-associated adult hippocampal neurogenesis deficit was well characterized in this model, as assessed by detecting the extent of newborn neuron generation and DCX (marker of newly generated neuron) expression in the hippocampus. According to the results from FCM, quantitative RT-PCR and Western blot, both the proportion of BrdU+/DCX+ cells and DCX expression level were obviously decreased in TG group versus WT group. In line with a previous study [36], these data confirmed the obvious deterioration of adult hippocampal neurogenesis impairment and cognitive deficit in this well-characterized AD mouse model.
A recent study reported that magnesium elevation was capable of regulating the important process of neurogenesis mainly by promoting neuronal differentiation of NPCs in vitro [9], whereas the promotive effect of magnesium in adult neurogenesis still needs to be further confirmed by the in vivo experiments. We then hypothesized that the elevation of brain magnesium by administering MgT could regulate adult hippocampal neurogenesis process and promote the generation of new neurons, which potentially contributed to ameliorating cognitive decline during the progression of AD. To explore the effect of MgT against hippocampal neurogenesis deficit and cognitive decline in AD, different doses of MgT were used and then mice were behaviorally tested using the MWM prior to being sacrificed for further histopathological examinations. Comparison of MWM test performance between TG group and LM group demonstrated that oral administration of MgT for 3 months not only significantly improved spatial learning during the 5-day orientation navigation experiment but also significantly promoted memory consolidation during the memory retention test. Furthermore, it has also been confirmed that the cognitive deficits of AD mice could be improved by MgT treatments and the improvement effects were related to MgT dose, which were demonstrated by comparisons of the behavioral results between LM group and HM group. In accordance with the MWM data, the histopathological results showed that chronic MgT administration significantly upregulated DCX expression levels, as assessed by quantitative RT-PCR and Western blot tests. In addition, the generation of hippocampal newborn neurons were facilitated gradually by the increasing doses of MgT, as assessed by FCM test. These observations led to the conclusion that MgT treatment was effective in ameliorating AD-associated adult hippocampal neurogenesis impairment, and consequently alleviated the cognitive deficit in this AD mouse model.
In light of the vital role of hippocampal neurogenesis deficit in the deterioration of memory function during AD progression, this study sought to explore the underlying mechanisms by which MgT treatment ameliorated AD-associated adult hippocampal neurogenesis impairment in this disease model. Several lines of evidence had emphasized the potential roles of ERK and CREB as key regulators that influenced the process of neurogenesis by regulating the neuronal differentiation fate of NPCs [9, 40]. Furthermore, the effects of MgT on other major MAPK pathways, for instance, P38 and JNK pathways [23], also remained to be determined. To explore the mechanism underling the amelioration of adult hippocampal neurogenesis impairment and cognitive deficit in AD mice by MgT administration, this study detected the effects of MgT on the activations of CREB, ERK, P38, and JNK using the Western blotting method. The activations of ERK and CREB were demonstrated to be enhanced by MgT and reversed by ERK inhibitor, whereas the P38 and JNK pathways did not show any response to the MgT treatments. Accordingly, the activations of both ERK and CREB, the generation of newborn neuron, the upregulation of DCX expression and the improvement of cognitive performance induced by MgT were all reversed by ERK inhibitors. In summary, these results confirmed the critical role of ERK/CREB activation in MgT-mediated amelioration of adult hippocampal neurogenesis impairment and memory deficit in this AD mouse model.
Based on the above novel mechanistic findings, it might be beneficial to monitor the effect of MgT treatment in combating the onset of AD via facilitating the activations of both ERK and CREB in AD patients. In addition, further research of MgT treatment in other types of AD models and clinical trials would be required to validate this conclusion. Overall, this study provides a new therapeutic target for developing new treatments for memory deficit in AD.
ACKNOWLEDGEMENTS
This study was supported by Science and Technology Projects in Guangzhou (Grant No. 2024A04J4012), Guangzhou General Science and Technology Project of Health and Family Planning (Grant No. 20251A011018), Doctoral Research Foundation of Guangzhou First People’s Hospital (Grant No. BSKY20220030), Guangzhou General Science and Technology Project of Health and Family Planning (Grant No. 20221A011081), Guangdong Medical Research Foundation (Grant No. A2023213), Natural Science Foundation of Guangdong Province (Grant No. 2414050005763), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2022A1515110288) and Natural Science Foundation of Guangzhou (Grant No. SL2022A04J00442).
References
- 1.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 2.Fan S, Zheng Y, Liu X, Fang W, Chen X, Liao W, Jing X, Lei M, Tao E, Ma Q, Zhang X, Guo R, Liu J. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer's disease. Drug Deliv. 2018;25:1091–1102. doi: 10.1080/10717544.2018.1461955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang W, Liao W, Zheng Y, Huang X, Weng X, Fan S, Chen X, Zhang X, Chen J, Xiao S, Thea A, Luan P, Liu J. Neurotropin reduces memory impairment and neuroinflammation via BDNF/NF-κB in a transgenic mouse model of Alzheimer's disease. Am J Transl Res. 2019;11:1541–1554. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaji S, Berghoff SA, Spieth L, Schlaphoff L, Sasmita AO, Vitale S, Büschgens L, Kedia S, Zirngibl M, Nazarenko T, Damkou A, Hosang L, Depp C, Kamp F, Scholz P, Ewers D, Giera M, Ischebeck T, Wurst W, Wefers B, Schifferer M, Willem M, Nave KA, Haass C, Arzberger T, Jäkel S, Wirths O, Saher G, Simons M. Apolipoprotein E aggregation in microglia initiates Alzheimer's disease pathology by seeding β-amyloidosis. Immunity. 2024;57:2651–2668.e12. doi: 10.1016/j.immuni.2024.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Recent advances in Alzheimer's disease: mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024;9:211. doi: 10.1038/s41392-024-01911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 8.Yeapuri P, Machhi J, Lu Y, Abdelmoaty MM, Kadry R, Patel M, Bhattarai S, Lu E, Namminga KL, Olson KE, Foster EG, Mosley RL, Gendelman HE. Amyloid-β specific regulatory T cells attenuate Alzheimer's disease pathobiology in APP/PS1 mice. Mol Neurodegener. 2023;18:97. doi: 10.1186/s13024-023-00692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao W, Jiang M, Li M, Jin C, Xiao S, Fan S, Fang W, Zheng Y, Liu J. Magnesium elevation promotes neuronal differentiation while suppressing glial differentiation of primary cultured adult mouse neural progenitor cells through ERK/CREB activation. Front Neurosci. 2017;11:87. doi: 10.3389/fnins.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Qin Q, Huang P, Cao F, Yin M, Xie Y, Wang W. Chronic pain accelerates cognitive impairment by reducing hippocampal neurogenesis may via CCL2/CCR2 signaling in APP/PS1 mice. Brain Res Bull. 2023;205:110801. doi: 10.1016/j.brainresbull.2023.110801. [DOI] [PubMed] [Google Scholar]
- 11.Kiyota T, Morrison CM, Tu G, Dyavarshetty B, Weir RA, Zhang G, Xiong H, Gendelman HE. Presenilin-1 familial Alzheimer's disease mutation alters hippocampal neurogenesis and memory function in CCL2 null mice. Brain Behav Immun. 2015;49:311–321. doi: 10.1016/j.bbi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollands C, Bartolotti N, Lazarov O. Alzheimer's disease and hippocampal adult neurogenesis; exploring shared mechanisms. Front Neurosci. 2016;10:178. doi: 10.3389/fnins.2016.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spicer MM, Yang J, Fu D, DeVore AN, Lauffer M, Atasoy NS, Atasoy D, Fisher RA. Regulator of G protein signaling 6 mediates exercise-induced recovery of hippocampal neurogenesis, learning, and memory in a mouse model of Alzheimer's disease. Neural Regen Res. 2025;20:2969–2981. doi: 10.4103/NRR.NRR-D-23-01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta C, Anderson HD, Anderson CM. Astrocyte dysfunction in Alzheimer disease. J Neurosci Res. 2017;95:2430–2447. doi: 10.1002/jnr.24075. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Liu P, Bian H, Jin S, Liu J, Yu N, Cui H, Sun F, Qian X, Qiu W, Ma C. Reduced neurogenesis in human hippocampus with Alzheimer's disease. Brain Pathol. 2024;34:e13225. doi: 10.1111/bpa.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavoshinezhad S, Mohseni Kouchesfahani H, Ahmadiani A, Dargahi L. Interferon beta ameliorates cognitive dysfunction in a rat model of Alzheimer's disease: modulation of hippocampal neurogenesis and apoptosis as underlying mechanism. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109661. doi: 10.1016/j.pnpbp.2019.109661. [DOI] [PubMed] [Google Scholar]
- 19.Geigenmüller JN, Tari AR, Wisloff U, Walker TL. The relationship between adult hippocampal neurogenesis and cognitive impairment in Alzheimer's disease. Alzheimers Dement. 2024;20:7369–7383. doi: 10.1002/alz.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Yu J, Liu Y, Huang X, Abumaria N, Zhu Y, Huang X, Xiong W, Ren C, Liu XG, Chui D, Liu G. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer's disease mouse model. Mol Brain. 2014;7:65. doi: 10.1186/s13041-014-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini Farahabadi SS, Ghaedi K, Ghazvini Zadegan F, Karbalaie K, Rabiee F, Nematollahi M, Baharvand H, Nasr-Esfahani MH. ERK1/2 is a key regulator of Fndc5 and PGC1α expression during neural differentiation of mESCs. Neuroscience. 2015;297:252–261. doi: 10.1016/j.neuroscience.2015.03.069. [DOI] [PubMed] [Google Scholar]
- 22.Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015;8:46. doi: 10.3389/fnmol.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, Fang W, Fan S, Liao W, Xiong Y, Liao S, Li Y, Xiao S, Liu J. Neurotropin inhibits neuroinflammation via suppressing NF-κB and MAPKs signaling pathways in lipopolysaccharide-stimulated BV2 cells. J Pharmacol Sci. 2018;136:242–248. doi: 10.1016/j.jphs.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Abumaria N, Yin B, Zhang L, Li XY, Chen T, Descalzi G, Zhao L, Ahn M, Luo L, Ran C, Zhuo M, Liu G. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci. 2011;31:14871–14881. doi: 10.1523/JNEUROSCI.3782-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao W, Wei J, Liu C, Luo H, Ruan Y, Mai Y, Yu Q, Cao Z, Xu J, Zheng D, Sheng Z, Zhou X, Liu J. Magnesium-L-threonate treats Alzheimer's disease by modulating the microbiota-gut-brain axis. Neural Regen Res. 2024;19:2281–2289. doi: 10.4103/1673-5374.391310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Hoss J, Kolind M, Jackson MT, Deo N, Mikulec K, McDonald MM, Little CB, Little DG, Schindeler A. Modulation of endochondral ossification by MEK inhibitors PD0325901 and AZD6244 (Selumetinib) Bone. 2014;59:151–161. doi: 10.1016/j.bone.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816X.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Kim HD, Park YJ, Ohn YH, Park TK. Time-dependent changes of cell proliferation after laser photocoagulation in mouse chorioretinal tissue. Invest Ophthalmol Vis Sci. 2015;56:2696–2708. doi: 10.1167/iovs.14-16112. [DOI] [PubMed] [Google Scholar]
- 29.Huang YL, Zeng NX, Chen J, Niu J, Luo WL, Liu P, Yan C, Wu LL. Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR-124 expression in rats with depression induced by chronic unpredictable mild stress. Neural Regen Res. 2020;15:1150–1159. doi: 10.4103/1673-5374.270414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Chen H, Zheng JF, Guo ZD, Huang ZJ, Wu Y, Zhong JJ, Sun XC, Cheng CJ. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen Res. 2020;15:2318–2326. doi: 10.4103/1673-5374.285001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshavarzi M, Khoshnoud MJ, Ghaffarian Bahraman A, Mohammadi-Bardbori A. An endogenous ligand of aryl hydrocarbon receptor 6-formylindolo[3,2-b]carbazole (FICZ) is a signaling molecule in neurogenesis of adult hippocampal neurons. J Mol Neurosci. 2020;70:806–817. doi: 10.1007/s12031-020-01506-x. [DOI] [PubMed] [Google Scholar]
- 32.Lu W, Zhao Z, Zhao Y, Yu S, Zhao Y, Fan B, Kacskovics I, Hammarström L, Li N. Over-expression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology. 2007;122:401–408. doi: 10.1111/j.1365-2567.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 35.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richetin K, Moulis M, Millet A, Arràzola MS, Andraini T, Hua J, Davezac N, Roybon L, Belenguer P, Miquel MC, Rampon C. Amplifying mitochondrial function rescues adult neurogenesis in a mouse model of Alzheimer's disease. Neurobiol Dis. 2017;102:113–124. doi: 10.1016/j.nbd.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227:497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu XF, Wang YC, Zong L, Chen ZY, Li Y. Elevating integrin-linked kinase expression has rescued hippocampal neurogenesis and memory deficits in an AD animal model. Brain Res. 2018;1695:65–77. doi: 10.1016/j.brainres.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Mattar P, Dixit R, Lawn SO, Wilkinson G, Kinch C, Eisenstat D, Kurrasch DM, Chan JA, Schuurmans C. RAS/ERK signaling controls proneural genetic programs in cortical development and gliomagenesis. J Neurosci. 2014;34:2169–2190. doi: 10.1523/JNEUROSCI.4077-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]