Abstract
Childhood interstitial lung diseases (chILDs) are rare and heterogeneous disorders associated with significant morbidity and mortality. The clinical presentation of chILD typically includes chronic or recurrent respiratory signs and symptoms with diffuse radiographic abnormalities on chest imaging. Diagnosis requires a structured, multi-step approach. Treatment options are limited, with disease-specific therapies available only in selected cases and management relying primarily on supportive care. Awareness of chILDs has been steadily increasing. New diagnoses, advanced diagnostic tests, and novel treatments are emerging each year, highlighting the importance of collaborative, multidisciplinary teams in providing comprehensive care for children and families affected by these complex conditions. On behalf of the European Respiratory Society Clinical Research Collaboration for chILD (ERS CRC chILD-EU), this review provides an updated overview of the diagnostic approach and management strategies for chILDs.
Shareable abstract
Childhood ILDs (chILDs) are rare and heterogeneous disorders associated with significant morbidity and mortality. Clinical presentation of chILD is often nonspecific, so diagnosis is challenging and requires a structured, multi-step approach. https://bit.ly/4bN0ZwQ
Educational aims
To update knowledge on clinical presentation and diagnostic approach of childhood interstitial lung diseases.
To increase healthcare providers’ awareness of current and emerging therapeutic options, emphasising the importance of multidisciplinary collaboration in the management of these complex disorders.
Introduction
Childhood interstitial lung diseases (chILDs) are rare and heterogeneous disorders associated with significant morbidity and mortality [1–4]. The spectrum of interstitial lung disease (ILD) in children differs substantially compared to adults, with certain conditions unique to the paediatric population [5]. Over the past two decades, awareness of these conditions has steadily increased, highlighted by reports of rising prevalence and the growing recognition of rare diseases [6–9]. Since the initial distinction between paediatric and adult ILDs, classification systems have continued to evolve with the growing understanding of chILDs [5, 10–13]. Each year, identification of new underlying disease mechanisms provides opportunities for targeted therapies. However, treatments for chILDs remain largely nonspecific and suboptimal, highlighting the urgent need for novel therapeutic options that can be validated through randomised clinical trials.
Given the continuous progress in this field, regular updates are essential. On behalf of the European Respiratory Society Clinical Research Collaboration for chILD (ERS CRC chILD-EU), this review provides an updated overview of the diagnostic and management strategies for chILDs, reflecting the latest advancements in the field.
Incidence and prevalence
Increased clinical awareness of chILDs has likely contributed to a rise in the reporting of these diseases. Recent epidemiological studies from France and Spain estimated the incidence of chILDs at 4.4 to 8.2 cases per million per year and the prevalence at 44 to 46.4 cases per million respectively, which was substantially higher compared to earlier reports [6, 7, 14, 15].
To date, more than 10 large national and international cohorts have been described, offering valuable insights into the spectrum and characteristics of chILDs (table 1) [6–8, 14, 16–24]. Many of these data originate from national and international registries. Notable examples include the ChILDRN registry established in the United States in 2004, RespiRare in France (2008), the pan-European chILD-EU registry (2013), and national registries such as chILD-Spain, ARNOLD in Australia and chILD-TR in Turkey [6, 7, 12, 16, 21].
TABLE 1.
Childhood interstitial lung disease cohorts with over 50 children, listed in alphabetical order
| Country/area | Patients (n) | Period of time | Incidence/prevalence | Reference |
|---|---|---|---|---|
| Australia and New Zealand | 71 115 |
2009–2014 2003–2013 |
NA NA/1.5 |
Casamento
et al. [16] Saddi et al. [15] |
| China | 133 | 2013–2018 | NA | Tang et al. [17] |
| Denmark | 884 | 1995–2005 | NA | Kornum et al. [18] |
| Europe | 575 | 2013–2016 | NA | Griese et al. [19] |
| France | 205 790 |
2008–2012 2000–2022 |
NA 4.4/44 |
Nathan
et al. [20] Fletcher et al. [6] |
| Germany | 56 | 2005–2006 | 1.32/NA | Griese et al. [14] |
| Spain | 381 | 2018–2019 | 8.18/46.53 | Torrent-Vernetta et al. [7] |
| Turkey | 416 | 2021–2023 | NA | Nayir-Büyükşahin et al. [21] |
| USA | 93 256 683 306 |
1994–2011 2016 2016–2022 2019–2021 |
NA NA NA NA |
Soares
et al. [22] Young et al. [23] Nevel et al. [8] Feld et al. [24] |
Incidence: number per million per year; prevalence: number per million. NA: not available. Reproduced and modified from [6] with permission.
chILD definition and classification
The general diagnostic criteria for chILD remains unchanged since the American Thoracic Society task force in 2013 and include the presence of at least three of the following: 1) respiratory symptoms (cough, rapid and/or difficult breathing, exercise intolerance); 2) respiratory signs (tachypnoea, adventitious sounds, retractions, digital clubbing, failure to thrive, respiratory failure); 3) hypoxaemia; 4) evidence of diffuse parenchymal lung disease on chest radiography or thoracic computed tomography (CT) [3, 25]. A broad differential diagnosis workup is often necessary to exclude lower respiratory tract infections, asthma, recurrent aspiration, congenital heart disease, immunodeficiencies, cystic fibrosis, primary ciliary dyskinesia and bronchopulmonary dysplasia [3, 13, 25, 26].
Respiratory symptoms and clinical signs of chILDs are often nonspecific, varying in severity and presentation across age groups [26]. Neonates and infants typically present with tachypnoea, chest retractions, failure to thrive and hypoxaemia [27, 28]. In older children, in addition to these symptoms, dry cough, haemoptysis, chest pain and exercise intolerance may be more common [17, 29]. On auscultation, crackles, or, less commonly, wheeze may be present, although up to a third of children might have normal findings on auscultation [13]. In advanced stages, with hypoxaemia, digital clubbing may develop. Episodes of desaturation can occur during sleep, feeding or physical activity [25, 26].
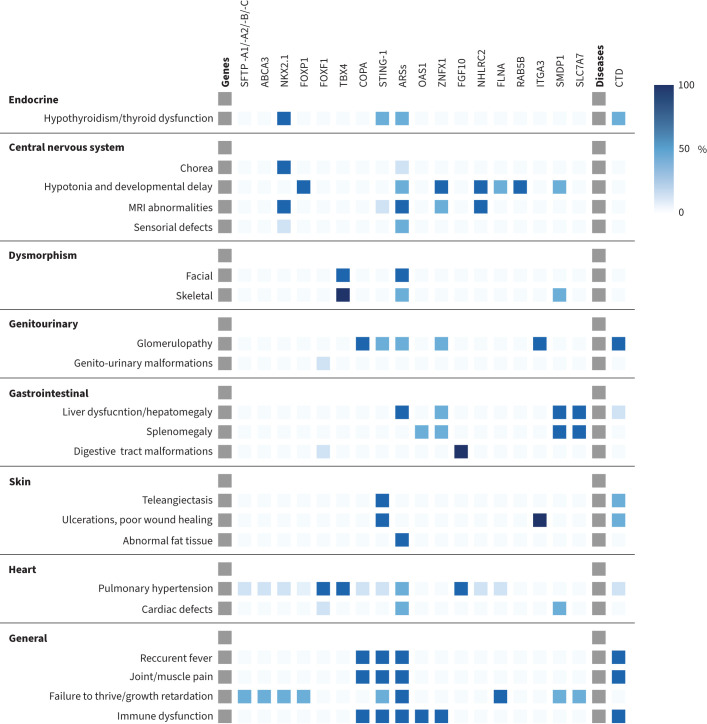
Extrapulmonary symptoms may occur or may even be a presenting symptom. These additional symptoms may provide clues to the underlying aetiology of chILDs (figure 1).
FIGURE 1.
Extrapulmonary symptoms suggestive of specific aetiology (involved gene). The intensity of the colour corresponds to the percentage of symptom occurrence as reported in the literature, with darker shades representing higher frequencies. MRI: magnetic resonance imaging; SFTP -A1/-A2/-B-/C: surfactant protein A1/A2/B/C; ABCA3: ATP binding cassette subfamily A member 3; NKX2.1: thyroid transcription factor; FOXP1: forkhead box P1; FOXF1: forkhead box protein F1; TBX4: T-box transcription factor 4; COPA: coatomer protein complex, subunit alpha; STING-1: stimulator of interferon response cGAMP interactor 1; ARSs: aminoacyl-tRNA synthetases; OAS1: oligoadenylate synthetase 1; ZNFX1: zinc finger nfx1-type domain-containing protein 1; FGF10: fibroblast growth factor 10; NHLRC2: NHL repeat-containing protein 2; FLNA: filamin A; RAB5B: RAS-associated protein; ITGA3: integrin alpha-3; SMDP1: sphingomyelin phosphodiesterase 1; SLC7A7: lysinuric protein intolerance; CTD: connective tissue diseases.
Since the European Respiratory Society (ERS) Task Force's initial classification of chILD in 2004, diagnostic and classification systems have undergone significant evolution [3, 10, 12, 13]. Recently, based on experiences from the chILD-EU register, a European paediatric rare lung disease register and biobank, an age-overarching etiologic classification of diffuse lung diseases was proposed [30]. This classification focuses on four main categories: 1) lung-only, native parenchymal, disorders (e.g. alveolar capillary dysplasia, surfactant metabolism disorders); 2) systemic disease-related disorders (e.g. connective tissue-related disorders, autoinflammatory disorders); 3) exposure-related disorders (e.g. hypersensitivity pneumonitis), and 4) vascular disorders (e.g. vasculitis, lymphangiomatosis).
Diagnostic workup
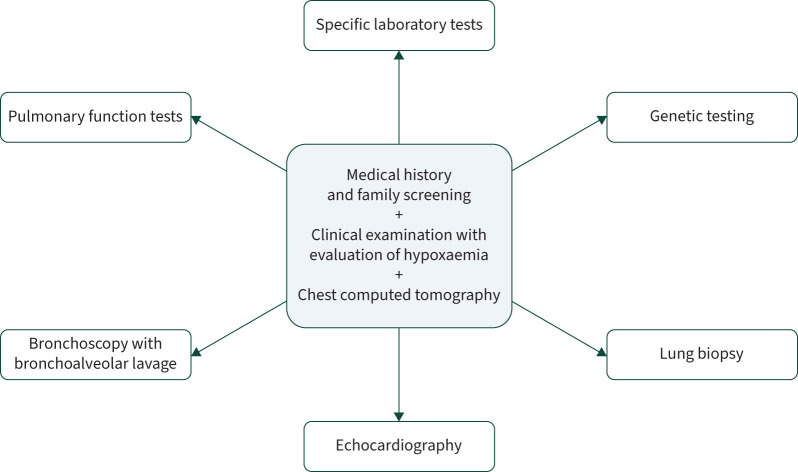
The diagnostic approach for chILDs involve a combination of medical history, family screening, clinical examination, evaluation of hypoxaemia and chest imaging, which form the cornerstone of the diagnosis. Additional diagnostic tools include pulmonary lung function tests, specific laboratory tests, bronchoscopy, echocardiography, genetic testing and, if needed, as a final step, lung biopsy (figure 2). The selection and sequence of additional tests cannot be standardised across the entire chILD group but must be tailored to the specific clinical characteristics of each case and the availability of investigative resources [1].
FIGURE 2.
Investigations in the diagnostic approach to childhood interstitial lung disease.
Medical history, family screening and clinical examination
The onset and characteristics of symptoms can sometimes provide valuable clues that point toward a specific diagnosis. A detailed family history is equally important, as familial penetrance and patterns of ILD, autoinflammatory symptoms, or recurrent unusual symptoms within a family may suggest syndromic ILD [1]. Recessive disorders (e.g., ABCA3, SFTPB) are more commonly observed in cases of consanguinity, whereas dominant disorders (e.g., SFTPC, NKX2.1, COPA, STING1) may manifest across generations with variable penetrance and expressivity. Notably, the absence of consanguinity or a family history does not exclude genetic aetiology, as de novo variants that are frequent in dominant diseases, compound heterozygosity, or paucisymptomatic/asymptomatic family members can still be implicated. Recently, the high risk of lung cancer in adults with surfactant metabolism disorders – especially those related to SFTPA1 and SFTPA2 variants – has been observed and a family history of adenocarcinoma of the lung may also be an important family history to be searched for [31]. Additionally, environmental factors such as viral infections, pollution and agricultural exposure (animals, organic dusts) and smoking habits are of interest (exposure-related chILD) [32, 33].
Imaging
Recent improvements in chILD imaging include existing imaging technologies and the adaptation of new techniques [34, 35]. A chest radiograph is commonly performed to evaluate persistent respiratory symptoms. However, its sensitivity and specificity for ILD are limited, and a normal chest radiograph cannot exclude the presence of ILD [25, 34]. Chest computed tomography (CT) remains the cornerstone for ILD evaluation and should be performed in expert centres. The older term “high-resolution CT” (HRCT) is now outdated, with improved spatial resolution with slice thicknesses as thin as 0.25–0.6 mm and reduced radiation dose [36].
Special techniques may be required in selected cases – both inspiratory and expiratory scans for detection of air trapping (bronchiolitis obliterans), intravenous contrast for evaluating lymphadenopathies or vascular abnormalities [34], etc. Alternatives for sedation for the examination in small children are also being developed (milky coma, free-breathing CT techniques) [33].
The most common CT findings associated with chILD include ground-glass opacities (GGOs), nodules and micronodules, consolidations, septal thickening, bronchovascular interstitium thickening, hyperinflation, air trapping, (traction) bronchiectasis and mosaic perfusion. In the majority of cases, radiographic findings are nonspecific; however, in some diseases, imaging findings combined with an appropriate clinical history can confirm the diagnosis. Certain patterns may point to specific diagnoses, such as GGO in the middle lobe, lingula, and parahilar/paramediastinal regions, characteristic of persistent tachypnoea of infancy/neuroendocrine cell hyperplasia of infancy (NEHI), or the crazy paving pattern (GGO with septal thickening) associated with pulmonary alveolar proteinosis (PAP) [1, 37, 38]. Fibrosis is defined by reticular opacities, traction bronchiectasis, architectural distortion, honeycombing and cystic lucency [39].
Lung ultrasound has emerged as a valuable modality for diagnosing and monitoring various pulmonary diseases due to its portability and absence of ionising radiation [40]. While most paediatric studies have focused on common conditions like pulmonary oedema, bronchopulmonary dysplasia and pneumonia, its application in chILD is gaining attention. There have been reports of lung ultrasound being used for screening for ILD in patients with connective tissue diseases, such as systemic sclerosis [41, 42]. Urbankowska et al. [43] demonstrated a significant correlation between lung ultrasound abnormalities in patients with persistent tachypnoea of infancy/neuroendocrine cell hyperplasia of infancy with CT features. The technique is sensitive in assessing interstitial changes and is a valuable tool for monitoring the condition and treatment efficacy.
Magnetic resonance imaging (MRI) also provides a promising, non-ionising modality for lung imaging. Recent technical advancements and refined pulse sequences have significantly improved the diagnostic quality of lung MRI [34]. Compared to CT, 3T lung MRI using standard protocols and sequences can effectively detect abnormalities such as consolidation, parenchymal bands, and fissural thickening in children with ILD. However, its ability to evaluate key features like septal thickening, ground-glass opacities, nodules, and cysts remains limited [44]. Specific MRI techniques may be promising in selected chILD situations, but their use remains restricted to research protocols so far [45, 46].
Pulmonary function tests
Pulmonary function tests (PFTs) are an important component of the diagnostic and follow-up process. The specific tests performed depend on the patient's age and ability to cooperate. In preschool and older children, spirometry, lung volume measurements, and diffusing capacity may be used [47]. Certain forms of chILD are associated with characteristic PFT patterns, such as irreversible obstruction with air trapping in bronchiolitis obliterans, peripheral airway obstruction with air trapping in NEHI, or a restrictive pattern seen in connective tissue disease-associated ILD or surfactant disorders [45–49].
Given that most chILD conditions manifest in age groups where volitional respiratory function testing is challenging, some centres offer infant pulmonary function tests. Infant pulmonary function tests have been shown to provide clinically relevant information in conditions like NEHI and surfactant disorders [50–52]. However, they require specialised equipment and trained personnel, as well as the need for sedation to ensure regular breathing and toleration of a face mask [47, 53, 54].
A 6-min walking test may be a useful additional exploration, especially in chILD follow-up of school-age children.
Genetic tests
Genetic analysis may yield a specific diagnosis and provide information about both prognosis and treatment, as well as family recurrence risk [55–57]. Notably, early identification of a genetic cause may eliminate the need for a lung biopsy. The growing significance of genetic testing is highlighted by recent epidemiological studies. They were performed in up to 58% and 76.9% of cases in the US and the French cohorts respectively, while lung biopsy was conducted in only 39% and 24.4% cases, respectively [6, 8].
Over time, the cost of genetic testing has decreased significantly, and its diagnostic yield has increased (up to 41.8% in the French cohort) [56]. For those who remain inconclusive, it is advisable to preserve DNA for future analysis. Registries and biobanks have an important role in supporting these efforts [19, 58]. There are reports of children whose diagnoses were revisited and revised years after the initial assessment, aided by genetic testing [59, 60].
Since the publication of the initial American Thoracic Society and ERS guidelines, numerous new monogenic causes of lung disease have been identified, significantly expanding the list of ILD genes (table 2) [3, 25]. New forms of chILD continue to be discovered and further described. Recently, Bradford et al. [61] reported three patients with Rubinstein–Taybi syndrome who demonstrated radiographic and histopathologic findings consistent with ILD. Furthermore, two children with chILD who were positive for CHOPS syndrome variations (AFF4 gene) were described [24]. CHOPS is an abbreviation for a list of features of a shared phenotype, including cognitive impairment, coarse facial features, heart defects, obesity, lung involvement, short stature and skeletal abnormalities [62]. Benslimane et al. [63] reported a 10-year-old boy with chILD associated with vascular changes due to severe pulmonary arterial hypertension secondary to chronic thromboembolic disease. Genetic analysis confirmed variations in KDM3B and SIN3A, associated with Diets–Jongmans syndrome and Witteveen–Kolk syndrome, respectively.
TABLE 2.
Known genes associated with childhood interstitial lung diseases (chILDs)
| Protein (gene) | Inheritance | Characteristics |
|---|---|---|
| Inherited surfactant-metabolism disorders | ||
| ATP binding cassette subfamily A member 3 (ABCA3) | AR | NRDS with eventual PHT Infant, childhood or adult ILD |
| Surfactant protein A (SFTPA1, SFTPA2) | AD | Very rarely chILD (most often an adult ILD with lung adenocarcinoma) |
| Surfactant protein B (SFTPB) | AR | NRDS±PHT Some reports of later-onset ILD |
| Surfactant protein C (SFTPC) | AD | NRDS±PHT Infant, childhood or adult ILD |
| Thyroid transcription factor (TTF1 also called NKX2-1) | AD | Brain lung thyroid syndrome (neonatal, childhood or adult ILD; peripheral hypothyroidism; hypotonia and/or benign chorea) |
| Diffuse abnormalities of lung development | ||
| Forkhead box protein F1 (FOXF1) | AD usually with parental imprinting | Alveolar capillary dysplasia with (or without) misalignment of pulmonary veins (usually fatal NRDS+PHT; few survivals in probable non-diffuse forms) |
| T-box transcription factor 4 (TBX4) | AD and AR | Acinar dysplasia (usually fatal NRDS+PHT) Small patella syndrome Later-onset PHT with patellar aplasia or hypoplasia |
| Eukaryotic translation initiation factor 2-alpha kinase 4 (EIF2AK4) | AR | Pulmonary haemangiomatosis Veno-occlusive disease |
| Autoinflammatory or immune dysregulation | ||
| Coatomer protein complex, subunit alpha (COPA) | AD | COPA syndrome: DAH or fibrosing chILD; destructive joint disease, kidney disease |
| SAVI: STING-associated vasculopathy of infancy (STING1 also called TMEM173) | AD | Fibrosing chILD or DAH; systemic inflammation; necrotising skin vasculitis; kidney disease |
| Oligoadenylate synthetase 1 (OAS1) | AD | chILD or PAP Hypogammaglobulinaemia Splenomegaly |
| Zinc finger nfx1-type domain-containing protein 1 (ZNFX1) | AR | chILD with severe viral infections Neurological symptoms Thrombotic microangiopathy |
| Signal transducer and activator of transcription3 (STAT3) | AD | Early-onset, severe interstitial lung and multisystem autoimmunity |
| Pulmonary alveolar proteinosis | ||
| Methionyl-tRNA synthetase (MARS1) | AR | Severe PAP; failure to thrive; hypotonia; psychomotor delay; cholestasis and cirrhosis |
| Colony stimulating factor 2 receptor α (CSF2RA) | Pseudoautosomal recessive | Primary PAP |
| Colony stimulating factor 2 receptor β (CSF2RB) | AR | Primary PAP |
| Lysinuric protein intolerance (SLC7A7) | AR | Secondary PAP Short stature; hepatosplenomegaly; recurrent infection |
| Chemokine, CC motif, receptor 2 (CCR2) deficiency | AR | PAP, progressive polycystic lung disease and recurrent infections |
| GATA-binding protein 2 (GATA2) | AD | Late onset PAP, early onset adult ILD, immunodeficiency |
| Other chILD | ||
| Fibroblast growth factor 10 (FGF10) | AD | Lacrimo-auriculo-dento-digital (LADD) syndrome and aplasia of lacrimal and salivary glands, progressive interstitial lung fibrosis and PHT |
| NHL repeat-containing protein 2 (NHLRC2) | AR | FINCA syndrome (fibrosis, neurodegeneration and cerebral angiomatosis) |
| Filamin A (FLNA) | X-linked recessive | Infant chILD, moderate developmental delay |
| Integrin alpha-3 (ITGA3) | AR | Interstitial lung disease, nephrotic syndrome and epidermolysis bullosa (ILNEB) syndrome |
| Phenylalanine-tRNA synthetase (FARSA, FARSB) | AR | chILD, cerebral aneurism, brain calcifications, facial dysmorphism, developmental delay, failure to thrive |
| Tyrosine-tRNA synthetase (YARS1) | AR | chILD, hypotonia, facial dysmorphism, neurosensorial defect, failure to thrive |
| Isoleucine-tRNA synthetase (IARS1) | AR | chILD, growth retardation, impaired intellectual development, hypotonia and hepatopathy |
| Leucyl-tRNA synthetase (LARS1) | AR | chILD, growth retardation, hypotonia, encephalopathy, facial dysmorphism, hepatopathy, kidney failure |
| Alanyl-tRNA synthetase (AARS1) | AR | chILD, growth retardation, hypotonia, encephalopathy |
| Sphingomyelin phosphodiesterase 1 (SMDP1) | AR | Niemann–Pick disease: progressive chILD, hepatosplenomegaly, neurologic progressive regression and hypotonia |
| Hermansky–Pudlak syndrome (HPS1-10) | AR | Fibrosing ILD, oculocutaneous albinism, platelet storage deficiency, neutropenia, granulomatous colitis |
| Forkhead box P1 (FOXP1) | AD | chILD, intellectual developmental disorder with language impairment with or without autistic features |
| RAS-associated protein (RAB5B) | AD | Progressive chILD, global developmental delay, dysmorphic features |
AD: autosomal dominant; AR: autosomal recessive; NRDS: neonatal respiratory distress syndrome; PHT: pulmonary hypertension; DAH: diffuse alveolar haemorrhage; PAP: pulmonary alveolar proteinosis.
In addition to this, heterozygous OAS1 gain-of-function (GOF) variants were further evaluated as causing polymorphic autoinflammatory immunodeficiency with recurrent fever, dermatitis, inflammatory bowel disease, PAP and hypogammaglobulinemia [64]. Another rare cause of PAP with progressive polycystic lung disease and recurrent infections is associated with an autosomal recessive complete deficiency of the monocyte chemokine receptor, C-C motif chemokine receptor 2 (CCR2) [65]. Moreover, recent descriptions of the phenotypic spectrum of FINCA (fibrosis, neurodegeneration and cerebral angiomatosis) syndrome (NHLRC2 gene) have revealed that individuals across all age groups may present with a history of chILD, some of whom show improvement over time [60]. The syndrome was first described in 2018 and has been primarily associated with severe lung disease, often leading to early infant death due to respiratory failure caused by progressive ILD.
In addition, age barriers tend to be challenged. As an example, the role of telomere-related genes in paediatric ILD has recently been debated. Two patients with undefined chILD who tested positive for telomere-related variants were studied. These variants include telomerase reverse transcriptase (TERT), regulator of telomere length 1 (RTEL1), poly(A)-specific ribonuclease (PARN), and telomerase RNA component (TERC) [66]. Another example is the recent report of a correlation between specific SP-B mutations and symptom severity, with rare cases of hypomorphic SP-B variants allowing a preserved SP-B function and survival [57]. All the aforementioned studies demonstrate how rapidly knowledge about the genetic basis of chILDs is advancing.
Specific laboratory tests
Laboratory investigations include complete blood count, serum markers for renal, hepatic, and thyroid function, inflammatory markers, and immunologic testing [1]. Serological studies for autoantibodies are particularly important in older children and those with evidence of pulmonary haemorrhage, renal involvement and articular, cutaneous or systemic disease. Similarly, IgG precipitin testing for antigens has a role in those with suspected hypersensitivity pneumonitis.
Specific biomarkers are actively being investigated in chILDs. For example, Krebs von den Lungen-6 (KL-6) protein, a marker associated with the destruction of the alveolar-capillary membrane, has been found to be elevated in children with ILD but not in those with NEHI [67, 68]. This made KL-6 a potential tool for distinguishing NEHI from other ILD [69]. KL-6 levels have also been shown to be elevated in children with connective tissue disease-associated ILD compared to healthy controls [70, 71]. Additionally, Otsubo et al. [72] reported a case of chILD due to a damaging SFTPC variant with elevated serum thymus and activation-regulated chemokine/C-C motif chemokine ligand 17 (TARC/CCL17) levels that decreased after clinical improvement. Despite these promising findings, these biomarkers remain in the research stage and have not yet been integrated into routine clinical practice.
Interferon type I signalling system hyperactivation plays a pivotal role in the pathogenesis of STING-associated vasculopathy with onset in infancy (SAVI) where STING is activated, coatomer protein complex, subunit alpha (COPA) syndrome, and certain connective tissue disorders. Testing for an interferon signature has emerged as a reliable screening tool for these conditions [73]. As therapies targeting interferon signalling pathways are developed and introduced into clinical practice, this diagnostic approach is likely to become increasingly significant [74, 75].
Bronchoscopy with bronchoalveolar lavage
Flexible bronchoscopy with bronchoalveolar lavage (BAL) is a valuable tool for assessing airway anatomy and eliminate concurrent infections. The cellular profile of BAL fluid (BALF) can provide insights into characteristic disease patterns, aiding in the diagnostic process as it happens in pulmonary haemorrhage syndromes, PAP, eosinophilic lung disease and hypersensitivity pneumonitis [1, 3, 25]. However, BALF findings are not definitive, as BALF alone is neither sensitive nor specific for diagnosing chILD. Instead, it serves as a complementary diagnostic tool that guides but does not confirm the final diagnosis [76].
Lung biopsy
Advances in genetic studies have significantly reduced the importance of lung biopsy for diagnosing chILD [1]. Nonspecific features are alveolar epithelial cells hyperplasia, thickened alveolar walls, lung fibrosis, cholesterol clefts and various degrees of alveolar proteinosis [28, 38]. Various diseases may be associated with diffuse developmental disorders of the lung (FOXF1, TBX4, FGF10, but also surfactant disorders), and a same genetic variant may lead to varying biopsy abnormalities among individuals and across the time [28, 77–79]. Finally, abnormal findings may be heterogeneous, and, as illustrated by NEHI, a normal biopsy does not exclude the disease [80, 81].
The current view is that lung biopsy could be reserved for those children with negative or inconclusive genetic studies. However, in critically ill patients who require urgent decisions, lung biopsy may still play a critical role [28, 79, 82]. Unlike genetic testing, it can provide a diagnosis within a few days and in selected age-groups, such as neonates, it can achieve a diagnostic rate in up to 95%, which exceeded that of genetic testing [82]. Deutsch et al. [79] also suggested that lung biopsy could characterise pathological disease processes in patients with known disease with a better definition of the nature and extent of abnormalities, including key processes like inflammation or fibrosis, which can guide therapeutic strategies. This is particularly important as experience with antifibrotic agents and other targeted therapies continues to grow.
The procedure is typically performed using video-assisted thoracoscopy or a mini-thoracotomy [79]. When considering a surgical lung biopsy, it is crucial to weigh its associated risks. A recently-published large population-based study highlighted that postsurgical mortality rates were not negligible, particularly in nonelective procedures [83]. In some centres, transbronchial and cryobiopsy techniques are increasingly being utilised with positive outcomes for specific indications [84–86]. While these methods are valuable in assessing infection or graft rejection in paediatric lung transplant patients, the small biopsy size and susceptibility to crush artifacts significantly limit their diagnostic yield for most chILD disorders [87]. Cryobiopsy, which employs a cryoprobe to freeze and extract a larger tissue sample with minimal crush artifact compared to conventional forceps-based transbronchial lung biopsy, offers a less invasive alternative to surgical lung biopsy while providing valuable diagnostic insights [88, 89, 90]. However, standardised training and further research are needed before it can be routinely used [91].
Treatments
chILD is often a severe condition that highly impairs the child's health-related quality of life as well as the quality of life of their family. The rarity of each chILD entity makes it a real challenge to build clinical trials and requires organised international networking and collaborations together with systematic registries. To date, the majority of chILDs are managed with unspecific treatments, with variable efficacy and non-neglectable side-effects (table 3). Personalised treatments have emerged in the past decade, and their development represents a major goal for the coming years [92, 93].
TABLE 3.
Main nonspecific and specific treatments in childhood interstitial lung diseases (chILDs)
| Treatment | Indication | Specific/nonspecific | Currently used (Curr); clinical trial (CT); research protocol or compassionate use (R); Prospective (P) |
|---|---|---|---|
| Glucocorticoids: methylprednisolone i.v. pulses; oral prednisolone | Almost all chILDs | Nonspecific | Curr |
| NEHI | Nonspecific | CT: NCT06471556 | |
| Long-term azithromycin | Surfactant metabolism disorders | Nonspecific | Curr |
| Hydroxychloroquine | Surfactant metabolism disorders and other chILDs | Nonspecific | Curr/CT: NCT02615938 |
| Immunosuppressive drugs: mycophenolate mofetil, ciclosporin, azathioprine, rituximab, cyclophosphamide, methotrexate | Auto-immune and auto-inflammatory disorders; disorders related to systemic disease processes; sarcoidosis | Nonspecific | Curr |
| Antifibrosing therapies: pirfenidone, nintedanib | Fibrosing surfactant metabolism disorders; undefined fibrosing ILD | Nonspecific | CT: NCT05285982 (nintedanib); NCT04093024 (nintedanib) R (pirfenidone) |
| Janus kinase inhibitors: ruxolitinib, baricitinib, tofacitinib | Auto-inflammatory disorders (SAVI, COPA); | Specific | Curr/CT: NCT04517253 |
| Undefined chILD with elevated IFN signature, FARSA, STAT1 GOF, STAT3 GOF | Nonspecific | R | |
| CFTR modulators | ABCA3 deficiency | Specific | R |
| Cyclosporine A | ABCA3 deficiency | Nonspecific | R |
| Methionine | MARS1-related PAP | Specific | Curr CT: NCT03887169 |
| Amino acids: phenylalanine, isoleucine, tyrosine, leucine, alanine | FARSA, FARSB, YARS1, LARS1, IARS1, AARS | Specific | R |
| Inhaled sargramostim (recombinant human GM-CSF) | Autoimmune PAP | Specific | CT: NCT01511068 R |
| Inhaled sargramostim (recombinant human GM-CSF) | CSF2RA and CSF2RB-related PAP | Specific | CT: NCT01511068 (interrupted, insufficient recruitment) CT: NCT02835742 |
| Whole lung lavages | PAP | Nonspecific | Curr |
| Lung transplantation | End-stage irreversible chILD | Nonspecific | Curr |
| Gene transfer therapy | SP-B deficiency CSF2RA-related PAP Potentially all monogenic chILDs |
Specific | P |
| Mesenchymal stromal cell therapy | CSF2RA-related PAP | CT: NCT01828957 |
NEHI: neuroendocrine cell hyperplasia of infancy; SAVI: STING-associated vasculopathy of infancy; COPA: coatomer protein complex, subunit alpha; IFN: interferon; FARSA: phenylalanine-tRNA synthetase A; STAT: signal transducer and activator of transcription; GOF: gain of function; CFTR: cystic fibrosis transmembrane conductance regulator; ABCA3: ATP binding cassette subfamily A member 3; MARS: methionine-tRNA synthetase; PAP: pulmonary alveolar proteinosis; FARSB: phenylalanine-tRNA synthetase B; YARS1: tyrosyl tRNA synthetase 1; LARS1: leucyl tRNA synthetase 1; IARS1: isoleucyl-tRNA synthetase 1; AARS: alanyl tRNA synthetase; GM-CSF: granulocyte–macrophage colony stimulating factor; CSF2R: colony stimulating factor 2 receptor; SP-B: surfactant protein B.
Because each chILD entity is rare, and, in most cases the disease-causing pathophysiology remains poorly understood, nonspecific treatment options based on expert advice rather than clinical trials are commonly used [25, 94].
Glucocorticoids represent the most-utilised drugs, with different treatment regimens. While widely prescribed, they have never been evaluated in a controlled trial for use in chILDs. While glucocorticoids can be effective, they display substantial side-effects that need to be closely monitored [95–97]. Currently, its efficacy is being tested in a phase 2 trial in France. In a number of diseases, glucocorticoids are associated with oral hydroxychloroquine and azithromycin. Hydroxychloroquine at a 6–10 mg·kg−1 per day dosage is believed to act as a drug that could eventually allow tapering corticosteroids [98, 99]. The 2015 European recommendations even cite it as a potential first-line treatment in mild and stable forms of chILD [25]. More recently, hydroxychloroquine (6.5 mg·kg−1 per day) effect has been evaluated through a prospective start and stop double-blind, placebo-controlled, multinational clinical trial. The limited number (n=35) and the heterogeneity of the included patients made it difficult to provide definitive answers, but the authors concluded that hydroxychloroquine in this population seems to be well-tolerated and would benefit from further evaluation [100, 101]. The same team focused on the effect of hydroxychloroquine on ABCA3 variants using in vitro cell models, with promising results regarding the evaluation of differential efficacy of the treatments depending on the variants [102]. Conversely, another in vitro model of using patient-specific induced pluripotent stem cells expressing the frequent SFTPC I73T showed that hydroxychloroquine treatment increased proSP-C misfolding, misprocessing, mistrafficking and cell homeostasis [103].
Azithromycin is also largely used in chILDs, especially in surfactant metabolism disorders, despite there being only few case reports upholding its efficacy, supposedly related to a local immunomodulatory response and antimicrobial effects [104]. Not without humour, A. Bush [105] discussed the wide use of azithromycin in a large number of paediatric lung diseases despite a very low degree of scientific evidence and possible side-effects, especially in selecting resistant microorganisms. Immunosuppressive drugs are another therapeutic family in chILDs. They are mostly used in case of systemic-related disease. Their use in chILDs is not protocolised and often adopted from adult diseases. As an example, this is the case for diffuse alveolar haemorrhage, that is also believed to be related to a dysimmune process with the use of mofetil mycophenolate, rituximab or cyclophosphamide, or paediatric sarcoidosis with the use of methotrexate [106, 107]. Using large molecule screening technics, cyclosporine has also emerged as a potential treatment in ABCA3 disorders, with an efficacy that has to be counterbalanced by its potential side-effects [108, 109].
Nonspecific treatments of chILDs have recently been enriched by anti-fibrosing therapies. In a phase 2 clinical trial, nintedanib has shown a good tolerability and an acceptable profile of adverse effects in children aged 6 to 17 years [110, 111]. Adverse events were similar as in adults (mainly diarrhoea) but less frequent and there was a not significant trend toward a stabilisation of forced vital capacity and oxygen saturation. In particular, attention needs to be paid to weight-loss or failure to thrive in children.
Specific treatments are rare in chILDs but their number is increasing and represent very promising perspectives for the involved patients. Janus kinase inhibitors (JAK-I) have recently emerged in chILD with elevated interferon signature [112, 113]. A JAK-1/2-I, baricitinib, was shown to have a beneficial effect in a 52-week phase 2–3 multicentre open-label study in Japanese patients with autoinflammatory disorders that included SAVI (n=9) [114]. It was also reported to be effective in COPA syndrome [115]. Ruxolitinib was also effective in SAVI and COPA syndrome [116, 117]. In a limited number of other chILDs with elevated interferon signature (undefined chILD; FARSA: phenylalanine-tRNA synthetase A variants; STAT1; signal transducer and activator of transcription 1 GOF variants; STAT3 GOF variants), JAK-I were also tested and various degrees of improvement in lung diseases was reported in some of the patients [118–120]. However, these treatments have to be given cautiously and monitored because of potential opportunistic infections. In methionine-tRNA synthetase-related PAP, methionine supplementation has drastically changed the prognosis of the disease [121–123]. Whereas whole lung lavages were the only option in PAP, with significant burden for the patients, this new therapeutic option allows a disease control and regression of lung lesions, with a generally good tolerance profile [124]. In hereditary PAP, an exceptional disease in children, inhaled recombinant granulocyte macrophage colony stimulating factor (GM-CSF) was tried with no success (NCT01511068), whereas anecdotally the statin atorvastatin was helpful, in addition to treatment with whole lung lavages [125, 126].
Inhaled GM-CSF was primarily used successfully in auto-immune PAP in adults, but also in teenagers with significant efficacy [38, 127, 128]. Recently, cystic fibrosis transmembrane conductance regulator (CFTR) modulators in patients with ABCA3 deficiency have been extensively investigated, based on protein homology between the two proteins [92, 129–131]. In vitro investigations using lumacaftor, and even more so ivacaftor, showed promising results and paved the way for testing this treatment in patients [132]. This was recently initiated in a pilot study including three adults who received the triple therapy elexacaftor-tezacaftor-ivacaftor, with an improvement in lung status in all of them.
Gene transfer therapy and mesenchymal stromal cell therapy
In monogenic chILD, gene therapy represents an important hope for curing the patients [133]. Challenges to address are finding a safe and efficient vector, able to target alveolar epithelial cells, ensuring a sufficient level of expression of the corrected gene and providing a long-term efficacy. SP-B deficiency was first targeted because of its loss of function mechanism induced by bi-allelic variants [134–136]. Viral vectors, especially adeno-associated viral vectors have shown promising results in vitro and in mice with a restoration of surfactant production and increased survival. Viral vectors (lentiviral) have also been studied for hereditary PAP related to CSF2RA variants in cell models of macrophages, with functional restoration of GM-CSF signalling [137]. More applications of gene transfer therapy are expected in the coming years, together with the launch of clinical trials in humans.
Mesenchymal stromal cell therapy, delivered intravenously or by intra-tracheal instillation, is another interesting perspective in chILDs owing to the accessibility (they can be isolated from amniotic fluid, bone marrow and various tissues) and plasticity (ability to differentiate in various cells) of mesenchymal stromal cells, along with their longevity and self-renewal potential [138]. Similarly, pulmonary macrophages derived from induced pluripotent stem cells were used in mice models of colony stimulating factor 2 receptor subunit b (CSF2RB)-related PAP with encouraging results [139]. In human paediatric lung disease, they were first tested in bronchopulmonary dysplasia, with a good tolerance but disappointing results, highlighting that this technology requires further evaluation [140].
Lung transplant
Lung transplant remains a limited treatment option for infants and children with incurable severe forms of ILD. The decision to pursue lung transplantation is inherently complex, requiring careful consideration of numerous individual factors. Families require extensive guidance and education before making a decision about whether a lung transplant is appropriate for their child and family. The surgical approach to transplant in ILD is similar to that in other lung transplant candidates, although the limited organ supply in infants can result in prolonged wait times [141].
In the recent study by Carlens et al. [142], the most common chILD diagnoses leading to lung transplant were disorders of the immunocompromised host in children after stem cell transplantation/chemotherapy/radiation followed by growth abnormalities reflecting deficient alveolarisation disorders related to systemic disease processes. Outcomes in chILD patients following lung transplant are comparable to cystic fibrosis and pulmonary hypertension transplanted patients despite a higher mechanical ventilation requirement prior to lung transplant and a longer intensive care unit and overall hospital stay [143]. The median survival rate after lung transplant in children with chILD is comparable to that observed in children with other causes of respiratory failure, approximately 5 years [141, 144].
Supporting care
Oxygen support
Long-term oxygen therapy and, rarely, non-invasive or invasive ventilation may be required in chILDs with respiratory failure. As for other indications, a mean oxygen saturation of 92% with more than 5% of the time below 90% is essential. If chILD is complicated by pulmonary hypertension, the indication for long-term oxygen therapy is even broader (especially if oxygen reactivity of pulmonary hypertension has been confirmed) [145, 146].
Nutrition
Respiratory insufficiency occurs in chILDs in up to 80% of the cases and results in increased energy consumption, which has to be compensated by increased energy intake (around 120% of age-appropriate daily dose). However, patients with chILD also experience oral feeding difficulties and/or gastro-enteral reflux and vomiting that make this goal hard to achieve [147]. Additionally, chILD treatments, especially glucocorticoids, require regimen adaptation and eventual increase in nutrition difficulties [148]. Taking into consideration these aspects while optimising nutrition in chILD is a major part of the management of these children. While most patients will be managed with enriched oral feeding, more invasive management may be needed for others with enteral nutrition via nasogastric tube or gastrostomy [149].
Immunisations
Immunisations schedule should be adapted in chILD patients with respect to possible immunosuppressive treatment and increased risk of respiratory infection. In patients with glucocorticoid pulses or long-term oral corticosteroids, live vaccines should be avoided (or given at least two weeks before starting these treatments). Chickenpox vaccine may be discussed before introducing selective immunosuppressive therapeutics. Additional immunisations include influenza, Streptococcus pneumoniae and respiratory syncytial virus [3, 150].
Adapted physical activity
Respiratory insufficiency and eventual oxygen or ventilatory support impair exercise tolerance. However, maintaining physical activity is essential in chILD management and a personalised programme with an adapted physical activity instructor may be of benefit to the patient's physical and mental health.
Patients’ groups and social support
Patients’ groups are critical in improving patients care. General chILD groups as well as disease-specific groups are increasingly being formed and the information should be systematically delivered to the patients. In line with the needs highlighted by parents and teenagers, optimal information at each step of the disease is still insufficiently provided, despite the increase in specific booklets and websites in many languages.
Social support is also an important part of the management that may notably improve daily life. Depending on local social offers, patients may benefit from various helps (e.g., financial, human, scholarly). A systematic consultation with a social worker may allow optimising these needs, when available.
Monitoring
Once a chILD diagnosis is established and therapies are initiated, ongoing monitoring is essential. This includes evaluating disease progression, assessing treatment efficacy and side-effects, tracking growth and development, detecting the emergence of comorbidities and monitoring health-related quality of life [149, 151, 152]. Regular follow-ups are important to ensure that care remains adapted to the child's changing clinical needs, though no consensus exists on the optimal frequency or standardised investigations for these visits.
To enhance the characterisation of disease progression in chILD, recent efforts have focused on defining exacerbation criteria specific to this population [153, 154]. Moreover, for the first time, minimal important differences have been determined for vital signs and health-related quality of life scores in a large cohort of children with chILDs [155]. These developments offer critical benchmarks for evaluating disease trajectory and the effectiveness of interventions, facilitating improved clinical management and research precision.
Beyond disease monitoring, attention must also focus on the side-effects of therapies. Long-term data on systemic glucocorticoid treatment in chILD patients suggest that the effects on growth, bone mineral density, and body composition are generally within acceptable limits, highlighting the importance of balancing treatment benefits against potential risks [148].
Transition
chILD survivors are frequently managed in adult ILD centres, with only a minority continuing follow-up in paediatric settings [102, 156]. These survivors often experience significant loss of lung function. The multiple aetiologies and diverse therapeutic needs of this population present significant challenges for adult ILD centres [157, 158]. In early 2024, the ERS published a statement highlighting the challenges in transitioning care for children with ILD as they reach adulthood [159]. The authors highlighted the limited availability of healthcare transition programmes specific to this population and called for enhanced efforts to include chILD patients in registries for long-term monitoring, starting in childhood and extending into adulthood.
Multidisciplinary networks
The development of expert networks is crucial in advancing knowledge in rare diseases such as chILD. Multidisciplinary team meetings are valuable in improving diagnosis, treatment and outcomes for these patients. Notable examples include the RespiRare meetings in France, the pan-European chILD-EU collaboration centred in Munich, and the Children's Interstitial Lung Disease Respiratory Network of Australia and New Zealand (chILDRANZ) [19, 160, 161]. Recently, Cassiba et al. [160] highlighted the impact of the RespiRare network meetings in optimising the management of chILD patients. These collaborative forums have been instrumental in refining the understanding of the aetiology of chILDs, tailoring treatment strategies – whether through escalation or de-escalation – and increasing clinician confidence in managing these complex conditions. In addition, they provide unique platforms for conducting research studies that will drive further advances in this challenging field.
Conclusions
In recent years, significant progress has been made in the understanding and management of chILDs. Increased clinical awareness has contributed to a rise in reported prevalence and incidence of these diseases. Genetic testing has become one of the most important tools in the diagnostic approach to chILDs, eliminating the need for lung biopsy in many cases. However, despite a better understanding of disease mechanisms, therapeutic options for chILDs remain largely nonspecific and suboptimal, highlighting the urgent need for novel treatments to be validated through randomised clinical trials.
Key points
Childhood interstitial lung diseases (chILDs) are rare, heterogeneous disorders requiring a structured, multi-step diagnostic approach.
Genetic testing is becoming one of the most important steps in the diagnostic approach to chILDs, replacing the need for a lung biopsy in several cases.
Despite progress, treatments for chILDs remain largely nonspecific and suboptimal, underscoring the urgent need for novel therapeutic options validated through randomised clinical trials.
Multidisciplinary collaboration and regular updates in the field are essential for optimising patient care.
Self-evaluation questions
- What are the key clinical features that should prompt suspicion of chILD in a paediatric patient?
- Cough, rapid and/or difficult breathing
- Tachypnoea, adventitious sounds, retractions
- Hypoxaemia
- Diffuse parenchymal lung disease on chest radiography or thoracic computed tomography
- All of the above
- Which gene is most probably involved in a child presenting with progressive ILD, hypothyroidism and choreoathetoid movements?
- FOXP1
- FOXF1
- NKX2.1
- TBX4
- ZNFX1
- Which of the following statements about the chILD diagnostic approach is accurate?
- Lung biopsy remains the gold standard for diagnosing chILDs.
- Genetic analysis plays a fundamental role in establishing a precise diagnosis in many cases of chILD and can offer key insights into prognosis and guide targeted treatment options.
- Inspiratory and expiratory chest CT imaging are essential for diagnosing all cases of chILD.
- Lung MRI can reliably detect key features of chILD, such as septal thickening, ground-glass opacities, nodules and cysts.
- Bronchoscopy is always a critical diagnostic tool for all cases of chILD.
- What elements should be included in the supportive care of a chILD patient?
- Oxygen monitoring and supplementation when oxygen drops are noticed
- Nutritional monitoring and supplementation if failure to thrive is present
- Up-to-date vaccinations
- Adapted physical activity
- All of the above
Suggested answers
1. e.
2. c.
3. b.
4. e.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- 1.Nathan N, Griese M, Michel K, et al. Diagnostic workup of childhood interstitial lung disease. Eur Respir Rev 2023; 32: 220188. doi: 10.1183/16000617.0188-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clement A, Eber E. Interstitial lung diseases in infants and children. Eur Respir J 2008; 31: 658–666. doi: 10.1183/09031936.00004707 [DOI] [PubMed] [Google Scholar]
- 3.Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013; 188: 376–394. doi: 10.1164/rccm.201305-0923ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griese M. Chronic interstitial lung disease in children. Eur Respir Rev 2018; 27: 170100. doi: 10.1183/16000617.0100-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch GH, Young LR, Deterding RR, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med 2007; 176: 1120–1128. doi: 10.1164/rccm.200703-393OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher C, Hadchouel A, Thumerelle C, et al. Epidemiology of childhood interstitial lung disease in France: the RespiRare cohort. Thorax 2024; 79: 842–852. doi: 10.1136/thorax-2023-221325 [DOI] [PubMed] [Google Scholar]
- 7.Torrent-Vernetta A, Gaboli M, Castillo-Corullón S, et al. Incidence and prevalence of children's diffuse lung disease in Spain. Arch Bronconeumol 2022; 58: 22–29. doi: 10.1016/j.arbres.2021.06.001 [DOI] [PubMed] [Google Scholar]
- 8.Nevel RJ, Deutsch GH, Craven D, et al. The US national registry for childhood interstitial and diffuse lung disease: report of study design and initial enrollment cohort. Pediatr Pulmonol 2024; 59: 2236–2246. doi: 10.1002/ppul.26568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan N. Childhood interstitial lung diseases (chILD) recognition: when epidemiology increases a rare disease incidence. Arch Bronconeumol 2022; 58: 217–218. doi: 10.1016/j.arbres.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 10.Fan LL, Dishop MK, Galambos C, et al. Diffuse lung disease in biopsied children 2 to 18 years of age application of the chILD classification scheme. Ann Am Thorac Soc 2015; 12: 1498–1505. doi: 10.1513/AnnalsATS.201501-064OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thacker PG, Vargas SO, Fishman MP, et al. Current update on interstitial lung disease of infancy: new classification system, diagnostic evaluation, imaging algorithms, imaging findings, and prognosis. Radiol Clin North Am 2016; 54: 1065–1076. doi: 10.1016/j.rcl.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 12.Griese M, Irnstetter A, Hengst M, et al. Categorizing diffuse parenchymal lung disease in children. Orphanet J Rare Dis 2015; 10: 122. doi: 10.1186/s13023-015-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement A, Allen J, Corrin B, et al. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J 2004; 24: 686–697. doi: 10.1183/09031936.04.00089803 [DOI] [PubMed] [Google Scholar]
- 14.Griese M, Haug M, Brasch F, et al. Incidence and classification of pediatric diffuse parenchymal lung diseases in Germany. Orphanet J Rare Dis 2009; 4: 26. doi: 10.1186/1750-1172-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saddi V, Beggs S, Bennetts B, et al. Childhood interstitial lung diseases in immunocompetent children in Australia and New Zealand: a decade's experience. Orphanet J Rare Dis 2017; 12: 133. doi: 10.1186/s13023-017-0637-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casamento K, Laverty A, Wilsher M, et al. Assessing the feasibility of a web-based registry for multiple orphan lung diseases: The Australasian Registry Network for Orphan Lung Disease (ARNOLD) experience. Orphanet J Rare Dis 2016; 11: 42. doi: 10.1186/s13023-016-0389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Li H, Liu H, et al. Etiologic spectrum of interstitial lung diseases in Chinese children older than 2 years of age. Orphanet J Rare Dis 2020; 15: 25. doi: 10.1186/s13023-019-1270-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornum JB, Christensen S, Grijota M, et al. The incidence of interstitial lung disease 1995–2005: A Danish nationwide population-based study. BMC Pulm Med 2008; 8: 24. doi: 10.1186/1471-2466-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griese M, Seidl E, Hengst M, et al. International management platform for children's interstitial lung disease (chILD-EU). Thorax 2018; 73: 231–239. doi: 10.1136/thoraxjnl-2017-210519 [DOI] [PubMed] [Google Scholar]
- 20.Nathan N, Taam RA, Epaud R, et al. A national internet-linked based database for pediatric interstitial lung diseases: The French network. Orphanet J Rare Dis 2012; 7: 40. doi: 10.1186/1750-1172-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayır-Büyükşahin H, Emiralioğlu N, Kılınç AA, et al. Childhood interstitial lung disease in Turkey: first data from the national registry. Eur J Pediatr 2024; 183; 295–304. doi: 10.1007/s00431-023-05290-9 [DOI] [PubMed] [Google Scholar]
- 22.Soares JJ, Deutsch GH, Moore PE, et al. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics 2013; 132: 684–691. doi: 10.1542/peds.2013-1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young L, Nevel R, Casey A, et al. A national registry for childhood interstitial and diffuse lung diseases in the United States. Eur Respir J 2018; 52: Suppl. 62, OA3786. doi: 10.1183/13993003.congress-2018.OA3786 [DOI] [Google Scholar]
- 24.Feld L, Voss L, Li ZN, et al. Clinical scope and healthcare utilization in childhood interstitial lung disease at a tertiary center. Pediatr Pulmonol 2024; 59: 2247–2256. doi: 10.1002/ppul.26600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush A, Cunningham S, De Blic J, et al. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax 2015; 70: 1078–1084. doi: 10.1136/thoraxjnl-2015-207349 [DOI] [PubMed] [Google Scholar]
- 26.Nathan N, Berdah L, Delestrain C, et al. Interstitial lung diseases in children. Presse Med 2020; 49: 103909. doi: 10.1016/j.lpm.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Spagnolo P, Bush A. Interstitial lung disease in children younger than 2 years. Pediatrics 2016; 137: e20152725. doi: 10.1542/peds.2015-2725 [DOI] [PubMed] [Google Scholar]
- 28.Bush A. Interstitial lung disease in infancy and early childhood: clinical approach. Pediatr Pulmonol 2025; 60: Suppl. 1, S24–S26. doi: 10.1002/ppul.27254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vece TJ, Fan LL. Interstitial lung disease in children older than 2 years. Pediatr Allergy Immunol Pulmonol 2010; 23: 33–41. doi: 10.1089/ped.2010.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griese M. Etiologic classification of diffuse parenchymal (interstitial) lung diseases. J Clin Med 2022; 11: 1747. doi: 10.3390/jcm11061747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brudon A, Legendre M, Mageau A, et al. High risk of lung cancer in surfactant-related gene variant carriers. Eur Respir J 2024; 63: 2301809. doi: 10.1183/13993003.01809-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurdyś-Bykowska P, Kośmider L, Bykowski W, et al. Epidemiology of traditional cigarette and e-cigarette use among adolescents in Poland: analysis of sociodemographic risk factors. Int J Environ Res Public Health 2024; 21: 1493. doi: 10.3390/ijerph21111493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinbashi M, Rubin BK. Electronic cigarettes and e-cigarette/vaping product use associated lung injury (EVALI). Paediatr Respir Rev 2020; 36: 87–91. doi: 10.1016/j.prrv.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 34.Spielberg DR, Weinman J, DeBoer EM. Advancements in imaging in chILD. Pediatr Pulmonol 2024; 59: 2276–2285. doi: 10.1002/ppul.26487 [DOI] [PubMed] [Google Scholar]
- 35.Miraftabi P, Kirjavainen T, Suominen JS, et al. Children's interstitial lung disease: multidetector computed tomography patterns and correlations between imaging and histopathology. Eur J Radiol 2023; 165: 110886. doi: 10.1016/j.ejrad.2023.110886 [DOI] [PubMed] [Google Scholar]
- 36.Brody AS, Guillerman RP. Ten rules for ordering chest CTs. Pediatr Pulmonol 2021; 56: 1868–1871. doi: 10.1002/ppul.25399 [DOI] [PubMed] [Google Scholar]
- 37.Brody AS, Guillerman RP, Hay TC, et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol 2010; 194: 238–244. doi: 10.2214/AJR.09.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griese M. Pulmonary alveolar proteinosis: a comprehensive clinical perspective. Pediatrics 2017; 140: e20170610. doi: 10.1542/peds.2017-0610 [DOI] [PubMed] [Google Scholar]
- 39.Bhalla D, Jana M, Naranje P, et al. Fibrosing interstitial lung disease in children: an HRCT-based analysis. Indian J Pediatr 2023; 90: 153–159. doi: 10.1007/s12098-021-04004-z [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Lovrenski J, Feletti F. Editorial: Application of lung ultrasound in the management of pediatric lung diseases. Front Pediatr 2023; 11: 1140403.doi: 10.3389/fped.2023.1140403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vicente-Rabaneda EF, Bong DA, Busquets-Pérez N, et al. Ultrasound evaluation of interstitial lung disease in rheumatoid arthritis and autoimmune diseases. Eur J Rheumatol 2024; 11: S316–S322. doi: 10.5152/eurjrheum.2024.20120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godoy-Navarrete F, Jiménez-Núñez FG, Mena-Vázquez N, et al. FRI0039 Lung ultrasound utility in interstitial lung disease detection in rheumatoid arthritis. Ann Rheum Dis 2020; 79: Suppl. 1, 594. doi: 10.1136/annrheumdis-2020-eular.5404 [DOI] [Google Scholar]
- 43.Urbankowska E, Urbankowski T, Drobczyński Ł, et al. Lung ultrasound—a new diagnostic modality in persistent tachypnea of infancy. Pediatr Pulmonol 2020; 55: 1028–1036. doi: 10.1002/ppul.24654 [DOI] [PubMed] [Google Scholar]
- 44.Sodhi KS, Sharma M, Lee EY, et al. Diagnostic utility of 3T lung MRI in children with interstitial lung disease: a prospective pilot study. Acad Radiol 2018; 25: 380–386. doi: 10.1016/j.acra.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 45.Flanagan F, Casey A, Reyes-Múgica M, et al. Post-infectious bronchiolitis obliterans in children. Paediatr Respir Rev 2022; 42: 69–78. doi: 10.1016/j.prrv.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 46.Houin PR, Deterding RR, Young LR. Exacerbations in neuroendocrine cell hyperplasia of infancy are characterized by increased air trapping. Pediatr Pulmonol 2016; 51: E9–E12. doi: 10.1002/ppul.23347 [DOI] [PubMed] [Google Scholar]
- 47.Ring AM, Carlens J, Bush A, et al. Pulmonary function testing in children's interstitial lung disease. Eur Respir Rev 2020; 29: 200019. doi: 10.1183/16000617.0019-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavaliunaite E, Aurora P. Diagnosing and managing bronchiolitis obliterans in children. Expert Rev Respir Med 2019; 13: 481–488. doi: 10.1080/17476348.2019.1586537 [DOI] [PubMed] [Google Scholar]
- 49.Marczak H, Peradzyńska J, Lange J, et al. Pulmonary function in children with persistent tachypnea of infancy. Pediatr Pulmonol 2023; 58: 81–87. doi: 10.1002/ppul.26162 [DOI] [PubMed] [Google Scholar]
- 50.Kerby GS, Wagner BD, Popler J, et al. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol 2013; 48: 1008–1015. doi: 10.1002/ppul.22718 [DOI] [PubMed] [Google Scholar]
- 51.Breuer O, Cohen-Cymberknoh M, Picard E, et al. The use of infant pulmonary function tests in the diagnosis of neuroendocrine cell hyperplasia of infancy. Chest 2021; 160: 1397–1405. doi: 10.1016/j.chest.2021.05.032 [DOI] [PubMed] [Google Scholar]
- 52.Hevroni A, Goldman A, Springer C. Infant pulmonary function testing in chronic pneumonitis of infancy due to surfactant protein C mutation. Pediatr Pulmonol 2015; 50: E17–E23. doi: 10.1002/ppul.23166 [DOI] [PubMed] [Google Scholar]
- 53.Ljungberg H, Gustafsson PM. Infant lung function testing: available and useful methods. Breathe 2004; 1: 13–23. doi: 10.1183/18106838.0101.13 [DOI] [Google Scholar]
- 54.Long FR, Castile RG. Technique and clinical applications of full-inflation and end-exhalation controlled-ventilation chest CT in infants and young children. Pediatr Radiol 2001; 31: 413–422. doi: 10.1007/s002470100462 [DOI] [PubMed] [Google Scholar]
- 55.Nathan N, Borensztajn K, Clement A. Genetic causes and clinical management of pediatric interstitial lung diseases. Curr Opin Pulm Med 2018; 24: 253–259. doi: 10.1097/MCP.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 56.Hamvas A, Chaudhari BP, Nogee LM. Genetic testing for diffuse lung diseases in children. Pediatr Pulmonol 2024; 59: 2286–2297. doi: 10.1002/ppul.26447 [DOI] [PubMed] [Google Scholar]
- 57.Fleury M, Delestrain C, Roditis L, et al. Surfactant protein B deficiency: the RespiRare cohort. Thorax 2025; 80: 109–112. doi: 10.1136/thorax-2024-221947 [DOI] [PubMed] [Google Scholar]
- 58.Krauss E, Tello S, Naumann J, et al. Protocol and research program of the European registry and biobank for interstitial lung diseases (eurILDreg). BMC Pulm Med 2024; 24: 572. doi: 10.1186/s12890-024-03389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nikolic RPA, Moran Toro C. Childhood-onset COPA syndrome recognized retrospectively in the context of polyarticular juvenile idiopathic arthritis and rheumatoid arthritis. Case Rep Rheumatol 2023; 2023: 3240245. doi: 10.1155/2023/3240245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapp CK, Van Dijck I, Laugwitz L, et al. Expanding the phenotypic spectrum of FINCA (fibrosis, neurodegeneration, and cerebral angiomatosis) syndrome beyond infancy. Clin Genet 2021; 100: 453–461. doi: 10.1111/cge.14016 [DOI] [PubMed] [Google Scholar]
- 61.Bradford L, Ross MK, Minso J, et al. Interstitial lung disease in children with Rubinstein-Taybi syndrome. Pediatr Pulmonol 2022; 57: 264–272. doi: 10.1002/ppul.25709 [DOI] [PubMed] [Google Scholar]
- 62.Raible SE, Mehta D, Bettale C, et al. Clinical and molecular spectrum of CHOPS syndrome. Am J Med Genet A 2019; 179: 1126–1138. doi: 10.1002/ajmg.a.61174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benslimane Z, Yavuz S, Francis N. A rare presentation of childhood interstitial lung disease attributed to KDM3B gene mutation: a case report. Pan Afr Med J 2023; 46: 84. doi: 10.11604/pamj.2023.46.84.41457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magg T, Okano T, Koenig LM, et al. Heterozygous OAS1 gain-of-function variants cause an autoinflammatory immunodeficiency. Sci Immunol 2021; 6: eabf9564. doi: 10.1126/sciimmunol.abf9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neehus AL, Carey B, Landekic M, et al. Human inherited CCR2 deficiency underlies progressive polycystic lung disease. Cell 2024; 187: 390–408.e23. doi: 10.1016/j.cell.2023.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nayır Büyükşahin H, Emiralioğlu N, Yalçın E, et al. Two cases with undefined childhood interstitial lung disease: can it be related to telomere variants? J Paediatr Child Health 2024; 60: 754–756. doi: 10.1111/jpc.16666 [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Xu L, Sui P, et al. Excess neuropeptides in lung signal through endothelial cells to impair gas exchange. Dev Cell 2022; 57: 839–853.e6. doi: 10.1016/j.devcel.2022.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marczak H, Peradzyńska J, Paplińska-Goryca M, et al. Serum biomarkers in neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol 2024; 59: 2885–2890. doi: 10.1002/ppul.27148 [DOI] [PubMed] [Google Scholar]
- 69.Al-Salmi QA, Walter JN, Colasurdo GN, et al. Serum KL-6 and surfactant proteins A and D in pediatric interstitial lung disease. Chest 2005; 127: 403–407. doi: 10.1378/chest.127.1.403 [DOI] [PubMed] [Google Scholar]
- 70.Kilinc AA, Arslan A, Yildiz M, et al. Serum KL-6 level as a biomarker of interstitial lung disease in childhood connective tissue diseases: a pilot study. Rheumatol Int 2020; 40: 1701–1706. doi: 10.1007/s00296-019-04485-4 [DOI] [PubMed] [Google Scholar]
- 71.Lee JS, Lee EY, Ha YJ, et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 2019; 21: 58. doi: 10.1186/s13075-019-1835-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otsubo Y, Fujita Y, Ando Y, et al. Elevated serum TARC/CCL17 levels associated with childhood interstitial lung disease with SFTPC gene mutation. Pediatr Pulmonol 2022; 57: 1820–1822. doi: 10.1002/ppul.25950 [DOI] [PubMed] [Google Scholar]
- 73.Rice GI, Melki I, Frémond ML, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J Clin Immunol 2017; 37: 123–132. doi: 10.1007/s10875-016-0359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lepelley A, Martin-Niclós MJ, Le Bihan M, et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J Exp Med 2020; 217: e20200600. doi: 10.1084/jem.20200600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ladoux C, Pasquet M, Crow YJ, et al. STING-associated vasculopathy with onset in infancy (SAVI) presenting as massive intra alveolar hemorrhage. J Clin Immunol 2023; 43: 699–702. doi: 10.1007/s10875-023-01431-9 [DOI] [PubMed] [Google Scholar]
- 76.Chaya S, Schütz K, Kaiser H, et al. The diagnostic utility of BAL in children with interstitial lung disease. Eur Respir J 2024; 64: Suppl. 68, OA4695. doi: 10.1183/13993003.congress-2024.OA4695 [DOI] [Google Scholar]
- 77.Canakis AM, Cutz E, Manson D, et al. Pulmonary interstitial glycogenosis: a new variant of neonatal interstitial lung disease. Am J Respir Crit Care Med 2002; 165: 1557–1565. doi: 10.1164/rccm.2105139 [DOI] [PubMed] [Google Scholar]
- 78.Chan CD, Niyogi A, Jaffray B, et al. Lung biopsy in children: when is it useful? Arch Dis Child 2021; 106: 291–293. doi: 10.1136/archdischild-2019-318443 [DOI] [PubMed] [Google Scholar]
- 79.Deutsch GH, Young LR. Lung biopsy in the diagnosis and management of chILD. Pediatr Pulmonol 2024; 59: 2298–2312. doi: 10.1002/ppul.26454 [DOI] [PubMed] [Google Scholar]
- 80.Griese M, Seidl E. Persistent tachypnea of infancy, neuroendocrine cell hyperplasia of infancy, and pulmonary interstitial glycogenosis: “A3-Specific conditions of undefined etiology”. Pediatr Pulmonol 2024; 59: 2702–2707. doi: 10.1002/ppul.27102 [DOI] [PubMed] [Google Scholar]
- 81.Miraftabi P, Kirjavainen T, Lohi J, et al. The original histopathologic description of neuroendocrine cell hyperplasia of infancy is not applicable to every patient with the disease. Pediatr Pulmonol 2024; 59: 3016–3019. doi: 10.1002/ppul.27118 [DOI] [PubMed] [Google Scholar]
- 82.Levy Y, Bitton L, Sileo C, et al. Lung biopsies in infants and children in critical care situation. Pediatr Pulmonol 2024; 59: 907–914. doi: 10.1002/ppul.26845 [DOI] [PubMed] [Google Scholar]
- 83.Wee WB, Shapera S, To T, et al. Mortality of pediatric surgical lung biopsies in Ontario, Canada, 2000–2019. Ann Am Thorac Soc 2024; 21: 767–773. doi: 10.1513/AnnalsATS.202304-306OC [DOI] [PubMed] [Google Scholar]
- 84.Rodrigues I, Gomes RE, Coutinho LM, et al. Diagnostic yield and safety of transbronchial lung cryobiopsy and surgical lung biopsy in interstitial lung diseases: a systematic review and meta-analysis. Eur Respir Rev 2022; 31: 210280. doi: 10.1183/16000617.0280-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joseph T, Agrawal S, Nair S, et al. Novel technique of performing transbronchial lung cryobiopsy (TBLC) for diagnosing diffuse parenchymal lung diseases (DPLD) in infants. Respirol Case Rep 2023; 11: e01096. doi: 10.1002/rcr2.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandra T, Srikanta JT, Madhusudan M, et al. Safety, utility and clinical efficacy of cryobiopsy of lung in paediatric population–A single centre experience. Lung India 2023; 40: 418–422. doi: 10.4103/lungindia.lungindia_217_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Visner GA, Faro A, Zander DS. Role of transbronchial biopsies in pediatric lung diseases. Chest 2004; 126: 273–280. doi: 10.1378/chest.126.1.273 [DOI] [PubMed] [Google Scholar]
- 88.Korevaar DA, Colella S, Fally M, et al. European Respiratory Society guidelines on transbronchial lung cryobiopsy in the diagnosis of interstitial lung diseases. Eur Respir J 2022; 60: 2200425. doi: 10.1183/13993003.00425-2022 [DOI] [PubMed] [Google Scholar]
- 89.Dhochak N, Mittal S, Mohan A, et al. Transbronchial lung cryobiopsy for diffuse lung diseases in children: a case series. Pediatr Pulmonol 2022; 57: 2851–2854. doi: 10.1002/ppul.26074 [DOI] [PubMed] [Google Scholar]
- 90.Srikanta JT, Swarna S, Shylendra DS, et al. Transbronchial lung cryobiopsy for diagnosis of pediatric interstitial lung disease. Indian Pediatr 2018; 55: 519–520. doi: 10.1007/s13312-018-1344-y [DOI] [PubMed] [Google Scholar]
- 91.Schramm D, Vicencio A. Pediatric cryobiopsy. Pediatr Pulmonol 2023; 58: 16–17. doi: 10.1002/ppul.26186 [DOI] [PubMed] [Google Scholar]
- 92.Bush A. Learning from cystic fibrosis: how can we start to personalise treatment of children's interstitial lung disease (chILD)? Paediatr Respir Rev 2024; 50: 46–53. doi: 10.1016/j.prrv.2023.11.001 [DOI] [PubMed] [Google Scholar]
- 93.Hurley K, Ozaki M, Philippot Q, et al. A roadmap to precision treatments for familial pulmonary fibrosis. EBioMedicine 2024; 104: 105135. doi: 10.1016/j.ebiom.2024.105135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.del Álamo M, Bührer C, Fisher D, et al. Identifying obstacles hindering the conduct of academic-sponsored trials for drug repurposing on rare-diseases: an analysis of six use cases. Trials 2022; 23: 783. doi: 10.1186/s13063-022-06713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desmarquest P, Tamalet A, Fauroux B, et al. Chronic interstitial lung disease in children: response to high-dose intravenous methylprednisolone pulses. Pediatr Pulmonol 1998; 26: 332–338. doi: [DOI] [PubMed] [Google Scholar]
- 96.De Benedictis FM, Bush A. Corticosteroids in respiratory diseases in children. Am J Respir Crit Care Med 2012; 185: 12–23. doi: 10.1164/rccm.201107-1174CI [DOI] [PubMed] [Google Scholar]
- 97.Breuer O, Schultz A. Side effects of medications used to treat childhood interstitial lung disease. Paediatr Respir Rev 2018; 28: 68–79. doi: 10.1016/j.prrv.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 98.Braun S, Ferner M, Kronfeld K, et al. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr Pulmonol 2015; 50: 410–419. doi: 10.1002/ppul.23133 [DOI] [PubMed] [Google Scholar]
- 99.Modjtahedi BS, Movassagh N, Gandhi N, et al. Screening for hydroxychloroquine toxicity in children. Cutan Ocul Toxicol 2013; 32: 344. doi: 10.3109/15569527.2013.781619 [DOI] [PubMed] [Google Scholar]
- 100.Griese M, Köhler M, Witt S, et al. Prospective evaluation of hydroxychloroquine in pediatric interstitial lung diseases: study protocol for an investigator-initiated, randomized controlled, parallel-group clinical trial. Trials 2020; 21: 307. doi: 10.1186/s13063-020-4188-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griese M, Kappler M, Stehling F, et al. Randomized controlled phase 2 trial of hydroxychloroquine in childhood interstitial lung disease. Orphanet J Rare Dis 2022; 17: 289. doi: 10.1186/s13023-022-02399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Seidl E, Knoflach K, et al. ABCA3-related interstitial lung disease beyond infancy. Thorax 2023; 78; 587–595. doi: 10.1136/thorax-2022-219434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alysandratos KD, Russo SJ, Petcherski A, et al. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep 2021; 36: 109636. doi: 10.1016/j.celrep.2021.109636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thouvenin G, Nathan N, Epaud R, et al. Diffuse parenchymal lung disease caused by surfactant deficiency: dramatic improvement by azithromycin. BMJ Case Rep 2013; 2013: bcr2013009988. doi: 10.1136/bcr-2013-009988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bush A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? Paediatr Respir Rev 2020; 34: 67–74. doi: 10.1016/j.prrv.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 106.Ring AM, Schwerk N, Kiper N, et al. Diffuse alveolar haemorrhage in children: an international multicentre study. ERJ Open Res 2023; 9: 00733-2022. doi: 10.1183/23120541.00733-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nathan N, Sileo C, Calender A, et al. Paediatric sarcoidosis. Paediatr Respir Rev 2019; 29: 53–59. doi: 10.1016/j.prrv.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 108.Forstner M, Lin S, Yang X, et al. High-content screening identifies cyclosporin a as a novel ABCA3-specific molecular corrector. Am J Respir Cell Mol Biol 2022; 66: 382–390. doi: 10.1165/rcmb.2021-0223OC [DOI] [PubMed] [Google Scholar]
- 109.Yang X, Forstner M, Rothenaigner I, et al. Cyclosporine A in children with ABCA3 deficiency. Pediatr Pulmonol 2024; 1: 3221.–. doi: 10.1002/ppul.27178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deterding R, Young LR, DeBoer EM, et al. Nintedanib in children and adolescents with fibrosing interstitial lung diseases. Eur Respir J 2023; 61: 2201512. doi: 10.1183/13993003.01512-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gozal D, Kolb M. Nintedanib in chILD: a small step, yes… but at least a step forward in a marathon! Eur Respir J 2023; 61: 2201797. doi: 10.1183/13993003.01797-2022 [DOI] [PubMed] [Google Scholar]
- 112.Hadjadj J, Frémond ML, Neven B. Emerging place of JAK inhibitors in the treatment of inborn errors of immunity. Front Immunol 2021; 12: 717388. doi: 10.3389/fimmu.2021.717388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crow YJ, Neven B, Frémond ML. JAK inhibition in the type I interferonopathies. J Allergy Clin Immunol 2021; 148: 991–993. doi: 10.1016/j.jaci.2021.07.028 [DOI] [PubMed] [Google Scholar]
- 114.Kanazawa N, Ishii T, Takita Y, et al. Efficacy and safety of baricitinib in Japanese patients with autoinflammatory type I interferonopathies (NNS/CANDLE, SAVI, And AGS). Pediatr Rheumatol 2023; 21: 38. doi: 10.1186/s12969-023-00817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krutzke S, Rietschel C, Horneff G. Baricitinib in therapy of COPA syndrome in a 15-year-old girl. Eur J Rheumatol 2020; 7: Suppl. 1, S78–S81. doi: 10.5152/eurjrheum.2019.18177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frémond ML, Nathan N. COPA syndrome, 5 years after: where are we? Joint Bone Spine 2021; 88: 105070. doi: 10.1016/j.jbspin.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 117.Frémond ML, Legendre M, Fayon M, et al. Use of ruxolitinib in COPA syndrome manifesting as life-threatening alveolar haemorrhage. Thorax 2020; 75: 92–95. doi: 10.1136/thoraxjnl-2019-213892 [DOI] [PubMed] [Google Scholar]
- 118.Charbit-Henrion F, Goguyer-Deschaumes R, Borensztajn K, et al. Systemic inflammatory syndrome in children with FARSA deficiency. Clin Genet 2022; 101: 552–558. doi: 10.1111/cge.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deyà-Martínez A, Rivière JG, Roxo-Junior P, et al. Impact of JAK inhibitors in pediatric patients with STAT1 gain of function (GOF) mutations—10 children and review of the literature. J Clin Immunol 2022; 42: 1071–1082. doi: 10.1007/s10875-022-01257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Atschekzei F, Traidl S, Carlens J, et al. JAK inhibitors to treat STAT3 gain-of-function: a single-center report and literature review. Front Immunol 2024; 15: 1400348. doi: 10.3389/fimmu.2024.1400348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hadchouel A, Drummond D, Pontoizeau C, et al. Methionine supplementation for multi-organ dysfunction in MetRS-related pulmonary alveolar proteinosis. Eur Respir J 2022; 59: 2101554. doi: 10.1183/13993003.01554-2021 [DOI] [PubMed] [Google Scholar]
- 122.Hadchouel A, Wieland T, Griese M, et al. Biallelic mutations of methionyl-tRNA synthetase cause a specific type of pulmonary alveolar proteinosis prevalent on Réunion Island. Am J Hum Genet 2015; 96: 826–831. doi: 10.1016/j.ajhg.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lenz D, Stahl M, Seidl E, et al. Rescue of respiratory failure in pulmonary alveolar proteinosis due to pathogenic MARS1 variants. Pediatr Pulmonol 2020; 55: 3057–3066. doi: 10.1002/ppul.25031 [DOI] [PubMed] [Google Scholar]
- 124.Reiter K, Schoen C, Griese M, et al. Whole-lung lavage in infants and children with pulmonary alveolar proteinosis. Paediatr Anaesth 2010; 20: 1118–1123. doi: 10.1111/j.1460-9592.2010.03442.x [DOI] [PubMed] [Google Scholar]
- 125.Tazawa R, Ueda T, Abe M, et al. Inhaled GM-CSF for pulmonary alveolar proteinosis. N Engl J Med 2019; 381: 923–932. doi: 10.1056/NEJMoa1816216 [DOI] [PubMed] [Google Scholar]
- 126.Nayır Büyükşahin H, Yalçın E, Özdemir A, et al. Successful atorvastatin treatment of pulmonary alveolar proteinosis in a child with GM-CSF receptor deficiency. Pediatr Pulmonol 2024; 59: 1777–1780. doi: 10.1056/NEJMoa1816216 [DOI] [PubMed] [Google Scholar]
- 127.McCarthy C, Bonella F, O'Callaghan M, et al. European Respiratory Society guidelines for the diagnosis and management of pulmonary alveolar proteinosis. Eur Respir J 2024; 64: 2400725. doi: 10.1183/13993003.00725-2024 [DOI] [PubMed] [Google Scholar]
- 128.Can Oksay S, Onay ZR, Bilgin G, et al. Inhaled treatment for dyspnea in a rare childhood disease. Pediatr Pulmonol 2024; 59: 3692–3698. doi: 10.1002/ppul.27208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Regard L, Martin C, Da Silva J, et al. CFTR modulators: current status and evolving knowledge. Semin Respir Crit Care Med 2023; 44: 186–195. doi: 10.1055/s-0042-1758851 [DOI] [PubMed] [Google Scholar]
- 130.Kinting S, Höppner S, Schindlbeck U, et al. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Hum Mol Genet 2018; 27: 943–953. doi: 10.1093/hmg/ddy011 [DOI] [PubMed] [Google Scholar]
- 131.Kinting S, Li Y, Forstner M, et al. Potentiation of ABCA3 lipid transport function by ivacaftor and genistein. J Cell Mol Med 2019; 23: 5225–5234. doi: 10.1111/jcmm.14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le Brun M, Nathan N, Louvrier C, et al. Efficacy and safety of CFTR modulators in patients with interstitial lung disease caused by ABCA3 transporter deficiency. ERJ Open Res 2024; 10: 00701-2024. doi: 10.1183/23120541.00701-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cooney AL, Wambach JA, Sinn PL, et al. Gene therapy potential for genetic disorders of surfactant dysfunction. Front Genome Ed 2021; 3: 785829. doi: 10.3389/fgeed.2021.785829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahiny AJ, Dewerth A, Mays LE, et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol 2015; 33: 584–586. doi: 10.1038/nbt.3241 [DOI] [PubMed] [Google Scholar]
- 135.Kang MH, van Lieshout LP, Xu L, et al. A lung tropic AAV vector improves survival in a mouse model of surfactant B deficiency. Nat Commun 2020; 11: 3929. doi: 10.1038/s41467-020-17577-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Munis AM, Hyde SC, Gill DR. A human surfactant B deficiency air–liquid interface cell culture model suitable for gene therapy applications. Mol Ther Methods Clin Dev 2021; 20: 237–246. doi: 10.1016/j.omtm.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hetzel M, Suzuki T, Hashtchin AR, et al. Function and safety of lentivirus-mediated gene transfer for CSF2RA-deficiency. Hum Gene Ther Methods 2017; 28: 318–329. doi: 10.1089/hgtb.2017.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pelizzo G, Silvestro S, Avanzini MA, et al. Mesenchymal stromal cells for the treatment of interstitial lung disease in children: A look from pediatric and pediatric surgeon viewpoints. Cells 2021; 10: 3270. doi: 10.3390/cells10123270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mucci A, Lopez-Rodriguez E, Hetzel M, et al. iPSC-Derived macrophages effectively treat pulmonary alveolar proteinosis in Csf2rb-deficient mice. Stem Cell Reports 2018; 11: 696–710. doi: 10.1016/j.stemcr.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl Med 2021; 10: 1129–1137. doi: 10.1002/sctm.20-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuklinski CA, Blatter JA. Interstitial lung disease as an indication for pediatric lung transplant. Pediatr Pulmonol 2024; 59: 2313–2320. doi: 10.1002/ppul.26812 [DOI] [PubMed] [Google Scholar]
- 142.Carlens J, Schwerk N, Müller C, et al. Paediatric lung transplantation for children with interstitial lung disease: A 12-year single-center analysis of underlying ChILD diagnoses and outcome. Eur Respir J 2023; 62: Suppl. 67, OA2508. doi: 10.1183/13993003.congress-2023.OA2508 [DOI] [Google Scholar]
- 143.Schneider H, Länger F, Ius F, et al. Paediatric lung transplantation for childhood interstitial lung disease shows favorable outcome compared with LuTx for cystic fibrosis or pulmonary hypertension. J Heart Lung Transplant 2024; 43: Suppl., S626. doi: 10.1016/j.healun.2024.02.977 [DOI] [Google Scholar]
- 144.Eldridge WB, Zhang Q, Faro A, et al. Outcomes of lung transplantation for infants and children with genetic disorders of surfactant metabolism. J Pediatr 2017; 184: 157–164.e2. doi: 10.1016/j.jpeds.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hayes D, Wilson KC, Krivchenia K, et al. Home oxygen therapy for children an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 199: e5–e23. doi: 10.1164/rccm.201812-2276ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Balfour-Lynn IM, Field DJ, Gringras P, et al. BTS guidelines for home oxygen in children. Thorax 2009; 64: Suppl. 2, ii1–ii26. doi: 10.1136/thx.2009.116020 [DOI] [PubMed] [Google Scholar]
- 147.Dziekiewicz M, Marczak H, Banasiuk M, et al. Characteristics of gastroesophageal reflux disease in children with interstitial lung disease. Pediatr Pulmonol 2023; 58: 171–177. doi: 10.1002/ppul.26176 [DOI] [PubMed] [Google Scholar]
- 148.Ring AM, Buchvald FF, Main KM, et al. Long-term effects of high-dose systemic corticosteroids on growth and bone mineral density in patients treated for childhood interstitial lung disease (chILD). Pediatr Pulmonol 2024; 59: 964–973. doi: 10.1002/ppul.26858 [DOI] [PubMed] [Google Scholar]
- 149.Emiralioglu N, Kiper N. Do we neglect nutrition in childhood interstitial lung disease? Eur J Clin Nutr 2024; 78: 1023–1024. doi: 10.1038/s41430-024-01485-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boesing M, Albrich W, Bridevaux PO, et al. Vaccination in adult patients with chronic lung diseases. Praxis (Bern 1994) 2024; 113: 297–305. doi: 10.23785/PRAXIS.2024.11.003 [DOI] [PubMed] [Google Scholar]
- 151.Seidl E, Niemitz M, Stöhr S, et al. Health related quality of life in childhood interstitial lung disease – an ERS Clinical Research Collaboration. Eur Respir J 2023; 62: Suppl. 67, PA424. doi: 10.1183/13993003.congress-2023.PA424 [DOI] [Google Scholar]
- 152.Nathan N, Lauby C, Taam RA, et al. Health-related quality of life in children interstitial lung disease (abstract). Eur Respir J 2019; 54: Suppl. 63, PA5184. doi: 10.1183/13993003.congress-2019.PA5184 [DOI] [Google Scholar]
- 153.Clement A, De Blic J, Epaud R, et al. Management of children with interstitial lung diseases: the difficult issue of acute exacerbations. Eur Respir J 2016; 48: 1559–1563. doi: 10.1183/13993003.01900-2016 [DOI] [PubMed] [Google Scholar]
- 154.Seidl E, Schwerk N, Carlens J, et al. Acute exacerbations in children's interstitial lung disease. Thorax 2022; 77: 799–804. doi: 10.1136/thoraxjnl-2021-217941 [DOI] [PubMed] [Google Scholar]
- 155.Griese M, Schwerk N, Carlens J, et al. Minimal important difference in childhood interstitial lung diseases. Thorax 2023; 78: 476–483. doi: 10.1136/thorax-2022-219206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cho JG, Thakkar D, Buchanan P, et al. ABCA3 deficiency from birth to adulthood presenting as paediatric interstitial lung disease. Respirol Case Rep 2020; 8: e00633. doi: 10.1002/rcr2.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manali ED, Griese M, Nathan N, et al. Childhood interstitial lung disease survivors in adulthood: a European collaborative study. Eur Respir J 2025; 65: 2400680. doi: 10.1183/13993003.00680-2024 [DOI] [PubMed] [Google Scholar]
- 158.Gilbert C, Bennett KM, Brown C, et al. Experiences of UK-based adult transition services for interstitial lung disease in childhood: “There's a lot less cushioning.”. Pediatr Pulmonol 2023; 58: 1993–1999. doi: 10.1002/ppul.26423 [DOI] [PubMed] [Google Scholar]
- 159.Pohunek P, Manali E, Vijverberg S, et al. ERS statement on transition of care in childhood interstitial lung diseases. Eur Respir J 2024; 64: 2302160. doi: 10.1183/13993003.02160-2023 [DOI] [PubMed] [Google Scholar]
- 160.Cassibba J, Epaud R, Berteloot L, et al. The significance of multidisciplinary team meetings in diagnosing and managing childhood interstitial lung disease within the RespiRare network. Pediatr Pulmonol 2024; 59: 417–425. doi: 10.1002/ppul.26765 [DOI] [PubMed] [Google Scholar]
- 161.McKnight L, Schultz A, Vidic N, et al. Learning to make a difference for chILD: value creation through network collaboration and team science. Pediatr Pulmonol 2024; 59: 2257–2266. doi: 10.1002/ppul.26377 [DOI] [PubMed] [Google Scholar]